Abstract
Background
Increasing habitual exercise has been associated with improved cardiopulmonary exercise testing (CPET) performance, specifically maximal oxygen consumption in children with operatively corrected congenital heart disease. This has not been studied in children following Fontan palliation, a population in whom CPET performance is dramatically diminished.
Methods
A single-center cross-sectional study with prospective and retrospective data collection was performed that assessed habitual exercise preceding a clinically indicated CPET in children and adolescents with Fontan palliation, transposition of the great arteries following arterial switch operation (TGA), and normal cardiac anatomy without prior operation. Data from contemporaneous clinical reports and imaging studies were collected. The association between percent predicted VO2max and habitual exercise duration adjusted for known covariates was tested.
Results
A total of 175 subjects (75 post Fontan, 20 with TGA, and 80 with normal cardiac anatomy) were enrolled. VO2max was lower in the Fontan group than patients with normal cardiac anatomy (p<0.0001) or TGA (p<0.0001). In Fontan subjects, both univariate and multivariate analysis failed to demonstrate a significant association between habitual exercise and VO2max (p=0.6), in sharp contrast to cardiac normal subjects. In multivariate analysis, increasing age was the only independent risk factor associated with decreasing VO2max in the Fontan group (p=0.003).
Discussion
Habitual exercise was not associated with VO2max in subjects with a Fontan as compared to biventricular circulation. Further research is necessary to understand why their habitual exercise is ineffective and/or what aspects of the Fontan circulation disrupt this association.
Keywords: Hypoplastic left heart syndrome, Transposition of the great arteries, Pediatric cardiology, Exercise physiology, Outcomes research
Background
Cardiopulmonary exercise testing (CPET) quantifies an individual's response to exertion. Maximal oxygen consumption (VO2max) has been used as both an endpoint in research studies and clinical care as a surrogate for the ability to participate in age-appropriate activities. Survivors of congenital heart surgery consistently demonstrate reduced VO2max[1–7]. Supervised exercise regimens have been associated with improvements in VO2max in children and young adults after congenital heart surgery[8–13]. Whether conditioning or training effects are achieved through daily recreational activity that is more typical of childhood has not been studied to the same degree. In children with repaired conotruncal anomalies, habitual exercise, assessed by questionnaire, correlated more strongly with VO2max than CMR measurements of ventricular function. Similar studies assessing the relationship between habitual exercise and exercise performance have not, to our knowledge, been performed in a uniform cohort of patients in children after single ventricle palliation.
We hypothesized that in children with Fontan physiology, increasing habitual exercise would be associated with increased VO2max, but that the relationship would be less robust than in children with biventricular circulation. To this end, we performed a cross-sectional study with prospective and retrospective data collection to assess this association, measuring habitual exercise and CPET performance in school age children and adolescents with Fontans and comparing them to subjects with 1) transposition of the great arteries (TGA) after an arterial switch operation and 2) children with structurally normal hearts.
METHODS
Study Population
Children and adolescents between the ages 8 and 17.5 years referred for CPET at The Children's Hospital of Philadelphia between March 1, 2012 and December 31, 2013 were approached for enrollment and included if they had: 1) hypoplastic left heart syndrome or other variants of single ventricle heart disease who had undergone staged palliation culminating in a Fontan operation, 2) TGA and an arterial switch operation, or 3) normal cardiac anatomy. This generated three internally homogenous cohorts representing 1) “single ventricle” heart disease, 2) “repaired” biventricular heart disease, and 3) normal cardiac anatomy, no cardiac surgery, and normal ventricular function. Children with other cardiac anatomic anomalies and/or surgeries were excluded. Potential subjects receiving beta-blockers were also excluded because of blunting of chronotropic response to exertion and potential exercise impairment. Prospective subjects who did not complete the exercise questionnaire were excluded. At our institution, CPET are performed for Fontan and TGA patients[14], so the study population should be a representative sample. The indications for control subjects are listed (Table 2).
Table 2.
Characteristics of subgroups by diagnosis
| Fontan (n=75) | |
| Anatomic diagnosis | |
| HLHS (MS/AS) | 20% (15) |
| HLHS (MS/AA) | 8% (6) |
| HLHS (MA/AS) | 3% (2) |
| HLHS (MA/AA) | 11% (8) |
| Double outlet right ventricle | 7% (5) |
| Tricuspid atresia | 9% (7) |
| Complete common atrioventricular canal unbalanced to the right | 12% (9) |
| Transposition of the great arteries with VSD and pulmonic stenosis | 7% (5) |
| Double inlet left ventricle | 11% (8) |
| Pulmonary atresia intact ventricular septum | 4% (3) |
| Other | 9% (7) |
| Systemic ventricle | |
| Right | 69% (52) |
| Left | 27% (20) |
| Both | 4% (3) |
| Heterotaxy syndrome | 11% (8) |
| Restrictive atrial septum | 3% (2) |
| Pulmonary vein anomaly | 8% (6) |
| Stage 1 operation | |
| Norwood with modified Blalock-Taussig shunt | 53% (40) |
| Norwood with RV to PA conduit | 5% (4) |
| Shunt without arch reconstruction | 27% (20) |
| No operation | 1% (1) |
| Fontan | |
| Extra-cardiac Fontan | 59% (44) |
| Lateral tunnel Fontan | 37% (28) |
| Hepatic vein inclusion | 4% (3) |
| Protein losing enteropathy | 7% (5) |
| Pacemaker | 6% (4) |
| TGA (n=20) | |
| Ventricular septal defect | 25% (5) |
| Pre-operative right ventricular outflow tract obstruction | 5% (1) |
| Pre-operative balloon atrial septostomy | 30% (6) |
| Pre-operative left ventricular outflow tract obstruction | 0% (0) |
| Pre-operative coarctation | 0% (0) |
| Cardiac normal patients (n=80) | |
| Indications for cardiopulmonary exercise test | |
| Assessment for long QT syndrome | 20% (16) |
| Chest pain | 16% (13) |
| Family history of heart disease or sudden death | 10% (8) |
| Palpitations | 16% (13) |
| Syncope and pre-syncope | 15% (12) |
| Wolff-Parkinson-White syndrome | 15% (12) |
| Other * | 21% (17) |
Abbreviations: AA aortic atresia, AS aortic stenosis, DORV double outlet right ventricle, MA mitral atresia, MS mitral stenosis, VSD ventricular septal defect
includes dizziness(n=2), dyspnea (n=1), history of Kawasaki disease (n=4) left ventricular hypertrophy on EKG (n=2), low resting heart rate, non-specific ST segment changes on EKG, premature ventricular contractions (n=3), status post ablation procedure (n=2)
Study Procedures
Medical records were reviewed to determine cardiac anatomy, medical history, and procedural/surgical history. Data from CPET were extracted from clinical reports, including a habitual exercise questionnaire[15], administered to all patients undergoing CPET. This questionnaire asks patients and their families to recall aspects of their daily activity over the past three months to measure their habitual exercise. The specific measures are the specific activities, the duration (hours/week) of each activity, and degree of exercise restriction, as well as categorizing the aerobic intensity of the most intense activity (grouped into 4 categories or “exercise classes” of ascending intensity)[15]. Other CPET parameters collected were VO2 at maximal exercise, VO2 at anaerobic threshold, maximal work, and respiratory exchange ratio at maximal exertion. To assess for chronotropic incompetence, heart rate at rest and at maximal exertion were measured. Chronotropic index was calculated according to the formula (heart rate at maximal exercise − heart rate at rest) / (220-age in years- heart rate at rest), as previously described[5]. Data from trans-thoracic echocardiograms (TTE) and CMRI studies within 6 months of CPET were reviewed, unless a transcatheter or operative intervention was performed between the CPET and TTE/CMR. Imaging studies were performed at rest following clinical protocols, and data extracted were based on the interpretation of staff cardiologists, who blind to subject clinical status, habitual exercise, or CPET performance. For Fontan subjects, TTE data recorded included systemic ventricular function and degree of atrioventricular (AV) valve regurgitation. For TGA subjects, left ventricular ejection fraction and shortening fraction were recorded. For Fontans with CMR studies, ejection fraction, systemic ventricular stroke volume, and cardiac index were recorded.
Statistical Analysis
Descriptive statistics were calculated. Continuous variables are expressed as mean ± standard deviation or median (range: and interquartile range) as appropriate. For categorical variables percentages and counts are presented. The baseline characteristics of the three subgroups were compared using analysis of variance, Kruskal-Wallis, and chi-square tests to identify aspects of their baseline characteristics that were significantly different. This comparison identified characteristics that potentially would benefit from statistical adjustment and to insure that there was appropriate overlap to make these adjustments.
For our primary analysis, the exposure was hours of habitual exercise over the 3 months prior to the CPET, which were calculated as previously described[15]. The primary outcome was oxygen consumption at maximal exercise, expressed as percent expected for age, sex, and weight[16]. This outcome was chosen because it is an integrative measure of exercise performance with well-established age- and sex-adjusted normal values, allowing for comparison across the range of ages in our study population. Secondary outcomes were limited to oxygen consumption at anaerobic threshold (VO2-AT) also expressed as percent expected for age, sex, and weight, which provides an effort independent measure of capacity. Assessment of parameters measuring other aspects of exercise performance were not performed to avoid erroneous conclusions from multiple comparisons and because of differences in physiologic response to exercise in subjects with univentricular versus biventricular physiology. Univariate linear regression was performed to assess the association between exposure (habitual exercise duration) and outcome (CPET measures of exercise capacity). Multivariable linear regression was performed where possible to adjust for other pre-specified covariates. For Fontans the covariates screened were age, sex, activity class, exercise restriction, systemic ventricular morphology, variation in surgical strategy at first stage and Fontan operation, presence of restrictive atrial septum, pulmonary venous anomaly, protein losing enteropathy, pacemaker, and heterotaxy syndrome. For cardiac normal patients, covariates were age, sex, race, and exercise class. Bivariate screening of covariates for inclusion was performed. All variables with p<0.20 in the bivariate screen were included in a multivariate model with no attempts at further model refinement to avoid bias. A sensitivity analysis with a more stringent threshold (p<0.10) for inclusion of covariates was performed for both subgroups. There were insufficient subjects to perform multivariate analysis for TGA subjects. Other secondary analyses included 1) subgroup analyses of subjects with concomitant MRI or TTE were performed to measure the association between ventricular function and CPET performance and 2) subgroup analysis of subjects with maximal exertion tests (RER ≥1.10) to assess whether effort level or chronotropic impairment biased results. In the Fontan cohort, a post hoc secondary analysis assessing the association of age and chronotropic index was performed to identify progressive chronotropic impairment that might have resulted in worsening VO2max.
Prior to enrolling patients, power calculations were performed for our primary analysis based on a range of Pearson's correlation coefficient with beta (0.2) and alpha (0.05) fixed. A study sample of 21 subjects were required for a minimally detectable difference of r=0.5. Similarly, the required sample size for r=0.3 was 64, and r=0.2 was 150. These guided our enrollment goals of 64 subjects in each subgroup. Because of slow accrual of TGA subjects, enrollment was stopped before the goal enrollment was achieved.
The threshold for statistical significance was p<0.05. No compensation for multiple comparisons was made. The primary analysis is identified and other analyses should be considered exploratory. Case restriction (without any imputation) was applied to account for missing data. All analyses were performed using StataMP 13 (College Station TX, USA).
RESULTS
Study population
A total of 190 subjects were recruited. Fifteen subjects (5 Fontan, 3 TGA, and 7 normal cardiac anatomy) were excluded because they did complete the exercise questionnaire, leaving a study population of 175 subjects (75 Fontan, 20 TGA, and 80 normal cardiac anatomy). Data were not collected on eligible subjects who declined to participate. Baseline characteristics of the study population are summarized in Table 1 and Table 2.
Table 1.
Characteristics of the study population
| Fontan (n=75) | TGA (n=20) | Cardiac normal (n=80) | p | |
|---|---|---|---|---|
| Age (years) | 12.2±3.4 | 12.5±2.6 | 14.1±2.2 | <0.0001 |
| Male sex % (n) | 53% (40) | 60% (12) | 66% (53) | 0.26 |
| Race % (n) | ||||
| White | 76% (55) | 85% (17) | 58% (46) | 0.005 |
| Black or African-American | 12% (9) | 5% (1) | 8% (6) | |
| Other or chose not to answer | 12% (9) | 10% (2) | 35% (28) | |
| Height (cm) | 149±16 | 151±17 | 163±12 | <0.0001 |
| Weight (kg) | 43.0±14.6 | 43.0±15.1 | 57.2±16.5 | <0.0001 |
| Body mass index | 18.8±3.6 | 18.3±3.3 | 21.1±4.2 | 0.0002 |
| Habitual exercise (hours/week) | 6.8 (IQR: 4.4–10.9, Range: 0.5– 31.3) | 8 (IQR: 6.1–11.8, Range: 1.8–18.1) | 6.2 (IQR: 4.0–9.9, Range: 0.1–24.3) | 0.26 |
| Maximal exercise class % (n) | 0.06 | |||
| I | 0% (0) | 0% (0) | 0% (0) | |
| II | 7% (5) | 10% (2) | 9% (7) | |
| III | 28% (21) | 20% (4) | 10% (8) | |
| IV | 65% (49) | 70% (14) | 81% (65) | |
| Restriction (by cardiologist) % (n) | ||||
| Unrestricted | 27% (20) | 55% (11) | 83% (66) | <0.001 |
| Moderately strenuous activity | 55% (41) | 35% (7) | 9% (7) | |
| Mildly strenuous activity | 19% (14) | 5% (1) | 1% (1) | |
| No activity | 0% (0) | 5% (1) | 8% (6) | |
| Parent assessment of fitness % (n) | ||||
| 1 (Very poor) | 0% (0) | 0% (0) | 0% (0) | <0.001 |
| 2 | 11% (8) | 10% (2) | 1% (1) | |
| 3 | 33% (25) | 30% (6) | 21% (16) | |
| 4 | 43% (32) | 30% (6) | 32% (25) | |
| 5 (Very fit) | 13% (10) | 30% (6) | 45% (35) |
Abbreviations: IQR interquartile range, TGA transposition of the great arteries
There were several significant differences between the cohorts. The cardiac normal subjects were older, taller, and heavier (p<0.001 for all), as well as having a higher BMI (p=0.002). They also had a lower proportion of white subjects than either the TGA or Fontan groups (p=0.005). In terms of habitual exercise, all of the groups had similar habitual exercise duration (Figure 1) with median habitual exercise of 6.2 hours per week for cardiac normal subjects, 8 hours per week for TGA subjects, and 6.8 hours per week for Fontans. The intensity of activities was not significantly different between the three cohorts (p=0.06). Fontan and TGA groups were more likely to have exercise restriction imposed by their cardiologist (p<0.001). Qualitative assessment of fitness by subjects and their families was also significantly worse in Fontan subjects than TGA and cardiac normal subjects (p<0.001).
Figure 1. Habitual exercise in the study population.
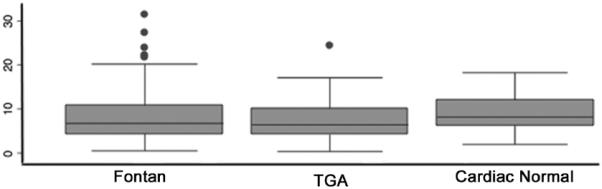
Box plot of habitual exercise in Fontan, transposition of the great arteries, and cardiac normal subgroups. The box plot demonstrates median, interquartile ranges, and outliers.
Exercise capacity
The performance of the three cohorts on exercise testing is summarized (Table 3). Expressed as percent expected for age and sex, VO2max in Fontans (82±27% predicted for age and sex) was significantly lower than that in TGA (109±39%, p=0.0001) and cardiac normal subjects (103±18, p<0.0001). Similarly, oxygen consumption at anaerobic threshold (VO2AT) and indexed maximum work were lower in Fontans relative to cardiac normal subjects (p<0.0001 for both). The TGA cohort had higher indexed work than those the Fontan cohort (p<0.0001) but there was no significant difference in VO2AT (p=0.10). There was no significant difference in VO2max (p=0.21), indexed maximum work (p=0.07), and VO2AT (p=0.73) between the TGA and cardiac normal cohorts. There were no statistically significant differences in heart rate at rest between the three cohorts (p=0.06). Heart rate at maximal exercise was significantly lower for Fontans (p<0.0001), as was chronotropic index (p<0.0001). These differences remained significant when the analysis was restricted to subjects achieving a maximal test (data not shown).
Table 3.
Performance on cardiopulmonary exercise testing
| Fontan (n=75) | TGA (n=20) | Cardiac normal (n=80) | p | |
|---|---|---|---|---|
| VO2 at maximal exercise (mL/min/kg) | 34.4±10.2 | 43.9±6.3 | 44.1±8.9 | <0.001 |
| VO2 at maximal exercise (% predicted) | 84±27 | 109±23 | 103±18 | <0.001 |
| Maximum work (watts) | 95±24 | 123±39 | 171±53 | <0.001 |
| Indexed maximum work (watts/kg) | 2.1±0.4 | 2.7±0.4 | 3.0±0.7 | <0.001 |
| Respiratory exchange ratio at maximal exertion | 1.2 (IQR: 1.1–1.3, Range: 0.9–1.5) | 1.3 (IQR: 1.2–1.3, Range: 1.0–1.4) | 1.2 (IQR: 1.2–1.3, Range: 1.0–1.5) | 0.001 |
| Achieved maximal exercise test % (n) | 80% (60) | 85% (17) | 93% (74) | 0.08 |
| VO2 at anaerobic threshold (mL/min/sqm) | 21.0±7.9 | 24.7±4.9 | 24.7±6.1 | 0.003 |
| VO2 at anaerobic threshold (% predicted) | 91±36 | 105±24 | 103±23 | 0.03 |
| Heart rate at rest (beats minute−1) | 74 (IQR: 61–85, Range: 49–117) | 67 (IQR: 57–75, Range: 50–97) | 70 (IQR: 60–76, Range: 49–117) | 0.06 |
| Heart rate at maximal exercise (beats minute−1) | 169 (IQR: 160–187, Range: 116–203) | 193 (IQR: 182–198, Range: 164–203) | 193 (IQR: 187–200, Range: 164–214) | <0.0001 |
| Chronotropic index | 0.70±0.15 | 0.87±0.08 | 0.90±0.08 | <0.0001 |
Abbreviations: TGA: transposition of the great arteries, VO2 oxygen consumption
TTE and CMR data
In the Fontan group, 92% of subjects (n=69/75) had TTE and 11% had MRI (8/75) within 6 months of CPET (Table 4). 95% had normal or mildly diminished systolic function; 69% had either mild or no systemic atrioventricular valve regurgitation. Of the 8 Fontans with CMR data, only 2 (25%) had an abnormal ejection fraction, and these (46% and 52%) were only mildly diminished. Of TGA's, 60% (12/20) subjects had eligible TTE, of which 92% (11/12) had a normal LV ejection fraction (>55%).
Table 4.
Data from Imaging Studies
| TGA | Fontan | |
|---|---|---|
| Transthoracic echocardiogram | n=16 | n=69 |
| LV ejection fraction (n=11) | 65 (IQR: 63–68, Range: 60–74) | n/a |
| LV shortening fraction (n=16) | 35 (34–37, Range: 32–38) | n/a |
| Systemic ventricular function (n=19) | ||
| Normal | n/a | 86% (59) |
| Trivial/Mildly diminished | n/a | 9% (6) |
| Moderately diminished | n/a | 6% (4) |
| Severely diminished | n/a | 0% (0) |
| Systemic AV valve regurgitation | ||
| None/Trivial | n/a | 30% (21) |
| Mild | n/a | 39% (27) |
| Moderate | n/a | 29% (20) |
| Severe | n/a | 1% (1) |
| Cardiac MRI | n=0 | n=8 |
| Ejection fraction (%) | n/a | 63% (IQR: 59–65, Range: 46–69) |
| Indexed systemic ventricular stroke volume (ml/sqm) | n/a | 33 (IQR: 27–42, Range: 22–62) |
| Cardiac index (L/min/sqm) | n/a | 2.7 (IQR: 2.2–3.1, Range: 1.9–4.0) |
Abbreviations: AV: atrioventricular, LV: Left ventricular
Factors associated with changes in exercise capacity
In univariate analysis of the Fontan cohort, there was no significant association between habitual exercise and VO2max (p=0.22, r2: 0.02, Table 5A). Increasing age was associated with decreasing VO2max (beta: −4.4% per year, p=<0.001). The presence of a systemic left ventricle (beta: 15.5%, p=0.03), and an initial operation of a shunt without arch reconstruction (beta: 19.1%, p=0.0009) were both associated with increased VO2max, while a lateral tunnel Fontan (beta: −12.7%, p=0.47) was associated with lower VO2max. Other potential covariates (presence of pulmonary vein anomaly, heterotaxy, Norwood operation with placement of a right ventricle to pulmonary artery conduit, and protein losing enteropathy) had large magnitude differences but their association was not statistically significant. Intensity of exercise (exercise class) did not have a significant association with VO2max. In multivariate analysis (Table 5B), habitual exercise duration was still not significantly associated with VO2max (p=0.60). The only factor that was independently associated with VO2max was increasing age, which was associated with decreasing VO2max (beta: −4.0, p=0.003, r2 for multivariate model 0.32). Sensitivity analysis using a more stringent selection criteria for covariates did not affect the observed associations (Supplementary Table 1). A post-hoc analysis was performed to determine if chronotropic impairment was more pronounced in older Fontan subjects. There was no association between increasing age and chronotropic index (p=0.66), nor did it affect the association between age and VO2max (data not shown).
Table 5a.
Univariate analysis of factors associated with VO2max in subjects with Fontan physiology
| Beta | 95% CI | p | |
|---|---|---|---|
| Habitual activity (per 10 hours/week) | 6.0 | −3.6 to 15.7 | 0.22 |
| Age | −4.4 | −6.3 to −2.5 | <0.001 |
| Male sex | −10.3 | −22.3 to 1.78 | 0.09 |
| Systemic ventricle | |||
| Right | 1 | n/a | n/a |
| Left | 15.5 | 2.0 to 28.9 | 0.03 |
| Both | 21.7 | −8.8 to 52.1 | 0.16 |
| Restrictive atrial septum | −9.8 | −48.0 to 28.2 | 0.61 |
| Pulmonary vein anomaly | −10.2 | −32.7 to 12.3 | 0.37 |
| Heterotaxy | −13.6 | −33.3 to 6.0 | 0.17 |
| Stage 1 operation | |||
| Norwood with modified BTS | 1 | n/a | n/a |
| Norwood with RV to PA conduit | 15.5 | −11.5 to 42.6 | 0.26 |
| Shunt without arch reconstruction | 19.1 | 5.05 to 33.3 | 0.009 |
| No operation | 6.5 | −11.8 to 24.7 | 0.40 |
| Other | 8.7 | −43.5 to 61.0 | 0.74 |
| Fontan operation | |||
| Extra-cardiac Fontan | 1 | n/a | n/a |
| Lateral tunnel Fontan | −12.7 | −25.2 to −0.2 | 0.047 |
| Hepatic vein inclusion | −23.2 | −54.1 to 7.6 | 0.14 |
| Protein losing enteropathy | −21.0 | −45.2 to 3.1 | 0.09 |
| Pacemaker | −4.2 | −31.5 to 23.2 | 0.76 |
| Exercise class | |||
| Low impact | 1 | n/a | n/a |
| Moderate impact | −7.8 | −28.0 to 12.5 | 0.45 |
| High impact | −6.7 | −25.8 to 12.5 | 0.49 |
| Restriction | |||
| No restriction | 1 | n/a | n/a |
| Moderate exertion | 11.2 | −2.9 to 25.4 | 0.12 |
| Mild exertion | −4.5 | −22.6 to 13.5 | 0.62 |
| Transthoracic echocardiogram (n=69) | |||
| Systolic function | |||
| Normal | 1 | n/a | n/a |
| Mildly diminished | 3.3 | −13.7 to 20.2 | 0.70 |
| Moderately diminished | −27.8 | −48.2 to −7.4 | 0.008 |
| Atrioventricular valve regurgitation | |||
| None to trivial | 1 | n/a | n/a |
| Mild | 1.1 | −10.0 to 12.2 | 0.84 |
| Moderate | −13.3 | −25.2 to −1.4 | 0.03 |
| Severe | −53.5 | −92.5 to −14.6 | 0.008 |
| Cardiac MRI (n=8) | |||
| Ejection fraction | 1.4 | −1.2 to 4.1 | 0.24 |
| Indexed stroke volume | 0.5 | −1.2 to 2.2 | 0.51 |
| Cardiac index | 5.4 | −28.5 to 39.2 | 0.71 |
Table 5b.
Multivariate analysis of factors associated with VO2max in subjects with Fontan physiology
| n=75 | Beta | 95% CI | p |
|---|---|---|---|
| Habitual activity (per 10 hours) | −2.8 | −13.5 to 7.8 | 0.60 |
| Age (per year) | −4.0 | −6.6 to −1.4 | 0.003 |
| Male sex | −6.6 | −18.9 to 5.6 | 0.28 |
| Systemic ventricle | |||
| Right | 1 | n/a | n/a |
| Left | 6.6 | −10.1 to 23.4 | 0.43 |
| Both | −0.9 | −33.6 to 31.7 | 0.95 |
| Heterotaxy | −14.7 | −41.9 to 12.5 | 0.28 |
| Stage 1 operation | |||
| Norwood with modified BT shunt | 1 | n/a | n/a |
| Norwood with RV to PA conduit | −3.5 | −31.6 to 24.5 | 0.80 |
| Shunt without arch reconstruction | 7.1 | −10.7 to 24.9 | 0.43 |
| No operation | 2.4 | −11.5 to 16.2 | 0.74 |
| Other | −11.4 | −64.1 to 41.4 | 0.67 |
| Fontan operation | |||
| Extra-cardiac Fontan | 1 | n/a | n/a |
| Lateral tunnel Fontan | −2.7 | −16.3 to 10.9 | 0.70 |
| Hepatic vein inclusion | 8.5 | −31.0 to 48.1 | 0.67 |
| Protein losing enteropathy | −9.0 | −32.9 to 14.9 | 0.46 |
Abbreviations: BT shunt: Blalock-Taussig shunt, RV to PA: right ventricle to pulmonary artery
In the subset of Fontans who underwent TTE, moderately diminished systemic ventricular systolic function was associated with decreased VO2max (beta: −27.8, p=0.008). Moderate and severe atrioventricular valve regurgitation were also associated with significantly lower VO2max (p=0.03 and 0.008 respectively). A very small minority of subjects underwent CMR studies within 6 months of CPET so additional analyses were not performed.
For TGA subjects, univariate analysis demonstrated a suggestive but not significant association between activity and VO2max (beta: 20.9% per 10 hours/week, p=0.09, r2: 0.15) (Table 6). Male sex was associated with a significant decrease in VO2max (beta: −32.0, p=0.001). Other factors were not associated with significant differences in VO2max. In the subgroup with contemporaneous TTE and CPET, ejection fraction and shortening fraction at rest did not have a significant association with VO2max.
Table 6.
Univariate analysis of factors affecting VO2max in subjects with TGA
| N=20 | Beta | 95% CI | p |
|---|---|---|---|
| Habitual activity (per 10 hours/week) | 20.9 | −3.5 to 45.3 | 0.09 |
| Age | −2.2 | −6.4 to 2.0 | 0.28 |
| Male sex | −32.0 | −48.4 to −15.6 | 0.001 |
| Balloon atrial septostomy | 14.9 | −8.4 to 38.1 | 0.20 |
| VSD | 0.4 | −25.4 to 26.2 | 0.98 |
| Activity class | |||
| Low impact | 1 | n/a | n/a |
| Moderate impact | −2.9 | −42.2 to 36.4 | 0.88 |
| High impact | 21.5 | −12.8 to 55.8 | 0.20 |
| Restriction | |||
| No restriction | 1 | n/a | n/a |
| Moderate exertion | −19.2 | −42.3 to 3.9 | 0.10 |
| Mild exertion | −19.6 | −69.4 to 30.2 | 0.42 |
| No activity | −27.9 | −77.7 to 21.9 | 0.25 |
| TTE (n=12) | |||
| LV ejection fraction (n=12) | −0.4 | −2.1 to 1.4 | 0.67 |
| LV shortening fraction (n=15) | 0.6 | −6.6 to 7.8 | 0.86 |
In subjects with normal cardiac anatomy, univariate analysis demonstrated that increasing habitual exercise was associated with increased VO2max (beta: 9.5% per 10 hours/week, p=0.03, r2: 0.2). Male sex (beta: −10.7%, p=0.008) and black race (beta: −26.8%, p<0.001) were associated with decreased VO2max (Table 7A). In multivariate analysis, habitual exercise remained independently associated with increased VO2max (beta: 10.1% per 10 hours/week, p=0.02, r2 for the entire model: 0.28). Increasing age, male sex, and black race were associated with decreased VO2max (Table 7B). Sensitivity analysis using p<0.1 as the threshold for including covariates in the multivariable model did not alter observed results (Supplementary Table 2).
Table 7a.
Univariate analysis of factors affecting VO2max in subjects with normal cardiac anatomy
| n=80 | Beta | 95% CI | p |
|---|---|---|---|
| Habitual exercise (per 10 hours/week) | 9.5 | 8.4–18.1 | 0.03 |
| Age | −0.01 | −3.1 to 4.4 | 0.14 |
| Male sex | −10.7 | −18.6 to −2.9 | 0.008 |
| Race | |||
| White | 1 | n/a | n/a |
| Black | −26.8 | −40.5 to −13.1 | <0.001 |
| Other | −6.7 | −14.5 to 1.0 | 0.09 |
| Level of restriction | |||
| No restriction | 1 | n/a | n/a |
| Moderate exertion | −4.2 | −18.5 to 10.2 | 0.57 |
| Mild exertion | −16.0 | −52.5 to 20.4 | 0.39 |
| No activity | −5.4 | −18.9 to 8.1 | 0.43 |
| Activity class | |||
| Low impact activity | 1 | n/a | n/a |
| Moderate impact activity | 4.0 | −14.8 to 22.8 | 0.68 |
| High impact activity | 1.3 | −12.7 to 15.4 | 0.85 |
Table 7b.
Multivariate analysis of factors affecting VO2max in subjects with normal cardiac anatomy
| n=80 | Beta | 95% CI | p |
|---|---|---|---|
| Habitual exercise (per 10 hours/week) | 10.1 | 1.9 to 18.4 | 0.02 |
| Age | −2.0 | −3.7 to −0.4 | 0.02 |
| Male sex | −11.2 | −20.0 to −2.8 | 0.01 |
| Race | |||
| White | 1 | n/a | n/a |
| Black | −17.1 | −31.8 to −2.5 | 0.02 |
| Other | −7.8 | −15.8 to 0.3 | 0.06 |
Secondary analyses of VO2AT were performed (Supplementary Tables 3–5). Subgroup analysis of subjects with maximal exercise tests (RER ≥ 1.10) was performed and there was no change in results (data not shown).
Discussion
In this cross-sectional study, we studied the relationship between reported habitual exercise in children and adolescents with Fontan physiology and compared it to that those with normal cardiac anatomy and those with operatively corrected biventricular circulation. Fontans had worse exercise performance than the other two groups. The expected association between increasing habitual exercise and improved exercise performance was seen in cardiac normal subjects, suggested in TGA's, but not seen in Fontan subjects.
In previous studies, increasing habitual exercise has been associated with improved exercise performance in congenital heart patients with biventricular circulation[15, 17–19]. This association has not been assessed previously in patients with Fontan physiology. Poor exercise performance in Fontan patients is widely documented[2–6], but the mechanisms for it have not been fully explained. The Pediatric Heart Network Fontan Cross-Sectional Study demonstrated diminished VO2max in Fontan patients between the 6 and 18 years of age, but with wide variability[5]. The determinants of this variability are poorly understood. This is important because several studies (including the current one), suggest that CPET performance in Fontan patients progressively deteriorates over late childhood and adolescence[20, 21]. This decline coincides with a period of clinical decline in a proportion of the population. Interventions that slowed or even preserved exercise performance might potentially improve quality of life by allowing for continued participation in age appropriate activities or even slow the clinical decline that often leads to heart transplantation or death. In addition, a previous cross-sectional study of older Fontans demonstrated decreased duration of moderate and greater activity compared to younger patients[22].
Demonstrating a causal connection between the observed declines in activity level, CPET performance, and clinical status is hard to assess. However, intensive monitored weight training was accompanied by improvements in CPET performance and lean muscle mass in one cohort of Fontan patients. The mechanism that connects training to exercise performance (independent of ventricular function) has not been defined but may be due to changes in peripheral vasculature and musculoskeletal systems improving the efficiency of oxygen extraction, in contrast to changes in ventricular function that improve oxygen delivery[23]. We hoped that habitual exercise would have a similar effect to supervised training, with increased activity associated with improved VO2max.
A significant association between habitual exercise and VO2max was not seen in the Fontan cohort. It is possible that one of several factors (ventricular dysfunction, insufficient activity, or chronotropic impairment) might obscure this association in Fontan subjects. However, the subjects in our Fontan cohort generally had normal or only mildly diminished ventricular dysfunction, little AV valve regurgitation, and limited co-morbidities. We also performed a secondary analysis of subjects who achieved a maximal exercise test to determine if sinus node dysfunction, effort, or other factors resulted in an exaggeratedly diminished performance for the Fontan cohort and repeated our analysis using VO2AT to demonstrate that the observations about peak exercise performance were reproduced at submaximal exertion. Our Fontan cohort had similar habitual exercise duration and intensity relative to our two other cohorts, and a level of activity that exceeded those reported previously in children with repaired conotruncal anomalies[15].
Further research is necessary to clarify whether disruption of the expected association is due to a biological difference in response to exercise or whether there are unmeasured confounding variables (e.g. duration and exercise class may not capture all aspects of activity). It is possible that aerobic training in isolation is less effective at increasing VO2max in Fontans relative to patients with biventricular circulation. While supervised weight training has demonstrated benefits in exercise capacity[8], observed aerobic training programs with demonstrable benefit in biventricular circulation have failed to show a similar benefit in Fontan patients[9]. It may be that dynamic exercise and the accrual of lean muscle mass (especially in the lower extremities) influences exercise performance in Fontan's disproportionately, or that aerobic exercise is ineffective in them. In either case, separate consideration of static and dynamic components of exercise and their effect on body composition (such as lean body mass) may provide a more useful evaluation of habitual exercise in Fontan patients than in patients with bi-ventricular circulation. This is especially important given recent observations about changes in body composition, specifically lean body mass and bone density in Fontan patients[24, 25].
The impact of restriction on exercise participation in Fontan patients (and congenital heart patients in general) is another potentially important consideration. Published recommendations advocate limiting Fontan patients to low static intensity activities and at most moderate dynamic competitive physical activity[26, 27]. In our cohort, Fontan subjects were subject to more restriction, but the vast majority continued to engage in strenuous non-competitive activity (93% engaged in activities in exercise class 3 or 4) with similar durations to the other cohorts. The classification of exercise in the Bethesda Conference guidelines does not perfectly correlate with the exercise classes used in this study, but all of the activities in exercise class 3 and 4 of this study are more intense (if competitive) than what is recommended for Fontan patients. It is possible that restrictions influence how vigorously Fontan patients engage in recreational activities (as opposed to changing the types of activities engaged in). This would be an unmeasured confounder in our study, which relies on self-report of activity type and duration only. Continuing re-evaluation of exercise recommendations is important as further studies refine the understanding of both the benefits and risk of exercise (and indeed the effect of restriction on both). This is especially important if there are disproportionate benefits between primarily aerobic exercise and strength training. Cardiologists continue to have an important role in communicating these risks and benefits to patients and their families.
In addition to habitual exercise, we hypothesized that several patient-level factors might influence VO2max in Fontan patients. A systemic left ventricle and an initial operation without a Norwood arch reconstruction were both associated with significantly better VO2max, while increasing age was associated with worsening VO2max. Other possible covariates (e.g. pulmonary vein anomalies, heterotaxy, and protein losing enteropathy) did not have significant associations with decreased VO2max. These factors were individually relatively rare in the study sample, limiting statistical power. We performed a multivariate analysis to assess whether together these factors were influential, but in that analysis, patient age was the covariate that remained significant. Though conventions about the number of covariates by population size were followed, it is possible that the model was over-specified. Research in larger cohort studies is the only means of overcoming this limitation.
It is beyond the scope of this study to define the mechanism of the association between age and decreased CPET performance. In this series, the capacity to increase heart rate with exercise was clearly reduced in Fontan subjects, which is consistent with previous reports[3–5]. Chronotropic impairment does have the potential to impair exercise performance. We sought to assess whether chronotropic impairment was more pronounced in older Fontans. In the current study, though there was a significant association between increasing age and worsening VO2max, no such association between age and chronotropic index was found. Nor did the addition of chronotropic index to our model alter the association between age and VO2max. This supports the contention that the limitation of exercise performance in Fontan's is not due to chronotropic impairment [5, 23]. Instead progression of other central (e.g. systolic ventricular dysfunction or limitations in the capacity of the neo-portal circulation to accept an increased cardiac output due to impaired diastolic relaxation or resistance in the pulmonary vascular bed) and/or peripheral factors (e.g. lean muscle mass and peripheral vascular changes) may influence changes in exercise capacity over time.
The absence of an association between habitual exercise and VO2max in Fontans differs from the results in cardiac normal subjects. Previous studies have demonstrated that training is associated with increased VO2max in both healthy adults and those with heart failure[10, 13, 28–30], so it is not surprising that this effect was robust in subjects with normal cardiac anatomy. Enrollment of TGA subjects was limited, resulting in limited statistical power for all comparisons. TGA subjects were very active and had robust exercise performance, which is at odds with previously published series[31, 32]. In a limited size sample, it is not possible to speculate why this was so. Despite this, there is a large magnitude but not significant association between habitual exercise and VO2max. Along with the previous studies in patients with conotruncal anomalies[15], these findings suggest that there is a robust association between habitual exercise and VO2max for children with biventricular circulation. The limited association of VO2max and habitual activity in Fontan subjects may reflect intrinsic differences between the Fontan population and those with biventricular circulations.
There are several limitations to this study. There are inherent difficulties in measuring habitual activity. Exercise is comprised of multiple measurable domains, including duration, intensity, and specific biomechanical (or muscular) work in each activity. The challenge of measurement is compounded in sports or recreational play, which combine a number of complicated motor tasks[33]. We chose to use duration of activity as a global measure, sacrificing detail but avoiding inaccuracy introduced by aspects of activity that are challenging to record. We acknowledge that prospective measurement of activity with pedometers or accelerometers could potentially increase the precision of activity duration, and future studies would benefit from comparing reported and measured activity. On the other hand, though self-report of exercise is prone to error (i.e. recall bias), it is unlikely that this error is systematically worse in Fontans and their families. Regardless of technique, measuring habitual exercise prior to CPET also has the potential to alter exercise habits introducing similar bias. Ultimately, the benefits of retrospective recall of duration of exercise outweighed the cited limitations. Finally, in a cross-sectional study, one cannot test whether increased activity “causes” VO2max to improve. This is only possible in a longitudinal design. Though we restricted analysis in this case to oxygen consumption, future analyses of the relationship of habitual exercise to other CPET variables (e.g. ventilatory efficiency or oxygen pulse) may be valuable in elucidating the mechanisms of this relationship.
In spite of these limitations, duration of habitual exercise was not associated with improved maximal exercise performance in Fontan patients, which was in contrast to those with bi-ventricular circulation. Further research is necessary to understand whether there are physiological differences in the response to habitual exercise between patients with single or bi-ventricular circulations or whether other aspects of habitual exercise are more influential in the exercise capacity of Fontans.
Supplementary Material
ACKNOWLEDGEMENTS
The authors acknowledge Elizabeth Ford, Shannon O'Malley, Sarah DiMaio, and Annie Linton who administered the CPET's for this study and worked with the study team to recruit and enroll subjects. They also acknowledge Sharon Edman for her work as the database manager and study coordinator for the project.
Dr. O'Byrne received research support from the National Institutes of Health(NIH) [T32 HL007915] and is a recipient of the Entelligence Young Investigator Grant. He currently receives support from the NIH and National Heart Lung and Blood Institute [K23 HL130420-01]. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health. The supporting agencies had no role in the design, conduct, interpretation, or decision to publish the data in this manuscript.
REFERENCE
- 1.Diller GP. Exercise Intolerance in Adult Congenital Heart Disease: Comparative Severity, Correlates, and Prognostic Implication. Circulation. 2005;112:828–835. doi: 10.1161/CIRCULATIONAHA.104.529800. doi: 10.1161/CIRCULATIONAHA.104.529800. [DOI] [PubMed] [Google Scholar]
- 2.Driscoll DJ, Danielson GK, Puga FJ, et al. Exercise tolerance and cardiorespiratory response to exercise after the fontan operation for tricuspid atresia or functional single ventricle. J Am Coll Cardiol. 1986;7:1087–1094. doi: 10.1016/s0735-1097(86)80227-3. doi: 10.1016/S0735-1097(86)80227-3. [DOI] [PubMed] [Google Scholar]
- 3.Driscoll DJ, Durongpisitkul K. Exercise testing after the Fontan operation. Pediatr Cardiol. 1999;20:57–9. doi: 10.1007/s002469900397. discussion 60. [DOI] [PubMed] [Google Scholar]
- 4.Durongpisitkul K, Driscoll DJ, Mahoney DW, et al. Cardiorespiratory response to exercise after modified Fontan operation: determinants of performance. J Am Coll Cardiol. 1997;29:785–790. doi: 10.1016/s0735-1097(96)00568-2. [DOI] [PubMed] [Google Scholar]
- 5.Paridon SM, Mitchell PD, Colan SD, et al. A Cross-Sectional Study of Exercise Performance During the First 2 Decades of Life After the Fontan Operation. J Am Coll Cardiol. 2008;52:99–107. doi: 10.1016/j.jacc.2008.02.081. doi: 10.1016/j.jacc.2008.02.081. [DOI] [PubMed] [Google Scholar]
- 6.Harrison DA, Liu P, Walters JE, et al. Cardiopulmonary function in adult patients late after Fontan repair. J Am Coll Cardiol. 1995;26:1016–1021. doi: 10.1016/0735-1097(95)00242-7. doi: 10.1016/0735-1097(95)00242-7. [DOI] [PubMed] [Google Scholar]
- 7.Fernandes SM, McElhinney DB, Khairy P, et al. Serial Cardiopulmonary Exercise Testing in Patients with Previous Fontan Surgery. Pediatr Cardiol. 2009;31:175–180. doi: 10.1007/s00246-009-9580-5. doi: 10.1007/s00246-009-9580-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cordina RL, O'Meagher S, Karmali A, et al. Resistance training improves cardiac output, exercise capacity and tolerance to positive airway pressure in Fontan physiology. Int J Cardiol. 2013;168:780–788. doi: 10.1016/j.ijcard.2012.10.012. doi: 10.1016/j.ijcard.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Duppen N, Etnel JR, Spaans L, et al. Does exercise training improve cardiopulmonary fitness and daily physical activity in children and young adults with corrected tetralogy of Fallot or Fontan circulation? A randomized controlled trial. Am Heart J. 2015;170:606–614. doi: 10.1016/j.ahj.2015.06.018. doi: 10.1016/j.ahj.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 10.Rhodes J, Curran TJ, Camil L, et al. Impact of cardiac rehabilitation on the exercise function of children with serious congenital heart disease. Pediatrics. 2005;116:1339–1345. doi: 10.1542/peds.2004-2697. doi: 10.1542/peds.2004-2697. [DOI] [PubMed] [Google Scholar]
- 11.Duppen N, Takken T, Hopman MTE, et al. Systematic review of the effects of physical exercise training programmes in children and young adults with congenital heart disease. Int J Cardiol. 2013;168:1779–1787. doi: 10.1016/j.ijcard.2013.05.086. doi: 10.1016/j.ijcard.2013.05.086. [DOI] [PubMed] [Google Scholar]
- 12.Duppen N, Kapusta L, de Rijke YB, et al. The effect of exercise training on cardiac remodelling in children and young adults with corrected tetralogy of Fallot or Fontan circulation: a randomized controlled trial. Int J Cardiol. 2015;179:97–104. doi: 10.1016/j.ijcard.2014.10.031. doi: 10.1016/j.ijcard.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 13.Winter MM, van der Bom T, de Vries LCS, et al. Exercise training improves exercise capacity in adult patients with a systemic right ventricle: a randomized clinical trial. Eur Heart J. 2012;33:1378–1385. doi: 10.1093/eurheartj/ehr396. doi: 10.1093/eurheartj/ehr396. [DOI] [PubMed] [Google Scholar]
- 14.Wernovsky G, Rome JJ, Tabbutt S, et al. Guidelines for the outpatient management of complex congenital heart disease. Cong heart dis. 2006;1:10–26. doi: 10.1111/j.1747-0803.2006.00002.x. doi: 10.1111/j.1747-0803.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- 15.O'Byrne ML, Mercer-Rosa L, Ingall E, et al. Habitual exercise correlates with exercise performance in patients with conotruncal abnormalities. Pediatr Cardiol. 2013;34:853–860. doi: 10.1007/s00246-012-0556-5. doi: 10.1007/s00246-012-0556-5. [DOI] [PubMed] [Google Scholar]
- 16.Cooper D, Weiler-Ravell D. Gas exchange response to exercise in children. American Review of Respiratory Disease. 1984;129(supplement):S47–S48. doi: 10.1164/arrd.1984.129.2P2.S47. [DOI] [PubMed] [Google Scholar]
- 17.Muller J, Christov F, Schreiber C, et al. Exercise capacity, quality of life, and daily activity in the long-term follow-up of patients with univentricular heart and total cavopulmonary connection. Eur Heart J. 2009;30:2915–2920. doi: 10.1093/eurheartj/ehp305. doi: 10.1093/eurheartj/ehp305. [DOI] [PubMed] [Google Scholar]
- 18.Müller J, Hess J, Hager A. Daily physical activity in adults with congenital heart disease is positively correlated with exercise capacity but not with quality of life. Clin Res Cardiol. 2011;101:55–61. doi: 10.1007/s00392-011-0364-6. doi: 10.1007/s00392-011-0364-6. [DOI] [PubMed] [Google Scholar]
- 19.Winter MM, Bouma BJ, van Dijk APJ, et al. Relation of Physical Activity, Cardiac Function, Exercise Capacity, and Quality of Life in Patients With a Systemic Right Ventricle. Am J Cardiol. 2008;102:1258–1262. doi: 10.1016/j.amjcard.2008.06.053. doi: 10.1016/j.amjcard.2008.06.053. [DOI] [PubMed] [Google Scholar]
- 20.Giardini A, Hager A, Pace Napoleone C, Picchio FM. Natural history of exercise capacity after the Fontan operation: a longitudinal study. Ann Thoracic Surg. 2008;85:818–821. doi: 10.1016/j.athoracsur.2007.11.009. doi: 10.1016/j.athoracsur.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Fernandes SM, McElhinney DB, Khairy P, et al. Serial cardiopulmonary exercise testing in patients with previous Fontan surgery. Pediatr Cardiol. 2010;31:175–180. doi: 10.1007/s00246-009-9580-5. doi: 10.1007/s00246-009-9580-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCrindle BW, Williams RV, Mital S, et al. Physical activity levels in children and adolescents are reduced after the Fontan procedure, independent of exercise capacity, and are associated with lower perceived general health. Arch Dis Child. 2007;92:509–514. doi: 10.1136/adc.2006.105239. doi: 10.1136/adc.2006.105239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldberg DJ, Avitabile CM, McBride MG, Paridon SM. Exercise capacity in the Fontan circulation. Cardiology in the Young. 2013;23:823–829. doi: 10.1017/S1047951113001649. doi: 10.1017/S1047951113001649. [DOI] [PubMed] [Google Scholar]
- 24.Avitabile CM, Goldberg DJ, Zemel BS, et al. Deficits in bone density and structure in children and young adults following Fontan palliation. Bone. 2015;77:12–16. doi: 10.1016/j.bone.2015.04.012. doi: 10.1016/j.bone.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Avitabile CM, Leonard MB, Zemel BS, et al. Lean mass deficits, vitamin D status and exercise capacity in children and young adults after Fontan palliation. Heart. 2014;100:1702–1707. doi: 10.1136/heartjnl-2014-305723. doi: 10.1136/heartjnl-2014-305723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graham TP, Driscoll DJ, Gersony WM, et al. Task Force 2: congenital heart disease. J Am Coll Cardiol. 2005:1326–1333. doi: 10.1016/j.jacc.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Takken T, Giardini A, Reybrouck T, et al. Recommendations for physical activity, recreation sport, and exercise training in paediatric patients with congenital heart disease: a report from the Exercise, Basic & Translational Research Section of the European Association of Cardiovascular Prevention and Rehabilitation, the European Congenital Heart and Lung Exercise Group, and the Association for European Paediatric Cardiology. Eur J Prevent Cardiol. 2012;19:1034–1065. doi: 10.1177/1741826711420000. doi: 10.1177/1741826711420000. [DOI] [PubMed] [Google Scholar]
- 28.Ekblom B. Effect of physical training on on oxygen transport system in man. Acta Physiologica Scandinavica Supplementum. 1968;328:1–45. [PubMed] [Google Scholar]
- 29.Karlsson J, Åstrand P, Ekblom B. Training of the oxygen transport system in man. J Appl Physiol. 1967;22:1061–1065. doi: 10.1152/jappl.1967.22.6.1061. [DOI] [PubMed] [Google Scholar]
- 30.Haykowsky MJ, Brubaker PH, Stewart KP, et al. Effect of Endurance Training on the Determinants of Peak Exercise Oxygen Consumption in Elderly Patients With Stable Compensated Heart Failure and Preserved Ejection Fraction. J Am Coll Cardiol. 2012;60:120–128. doi: 10.1016/j.jacc.2012.02.055. doi: 10.1016/j.jacc.2012.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giardini A, Khambadkone S, Rizzo N, et al. Determinants of Exercise Capacity After Arterial Switch Operation for Transposition of the Great Arteries. Am J Cardiol. 2009;104:1007–1012. doi: 10.1016/j.amjcard.2009.05.046. doi: 10.1016/j.amjcard.2009.05.046. [DOI] [PubMed] [Google Scholar]
- 32.Pasquali SK, Marino BS, McBride MG, et al. Coronary artery pattern and age impact exercise performance late after the arterial switch operation. J Thorac Cardiovasc Surg. 2007;134:1207–1212. doi: 10.1016/j.jtcvs.2007.06.022. doi: 10.1016/j.jtcvs.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 33.Shephard RJ. Limits to the measurement of habitual physical activity by questionnaires. Br J Sports Med. 2003;37:197–206. doi: 10.1136/bjsm.37.3.197. discussion 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


