Abstract
Objective
Determine the efficacy and safety of high dose vitamin D supplementation for ARI prevention in older long-term care residents.
Design, Setting, and Participants
Randomized controlled trial investigating high dose vs standard dose vitamin D conducted from 2010–2014. Participants were older residents (≥60 years) of Colorado long-term care facilities.
Interventions
1) The high dose group received monthly supplement of 100,000 IU vitamin D3; 2) The standard dose group received either a monthly placebo (for participants taking 400–1,000 IU/day as part of usual care) or a monthly supplement of 12,000 IU of vitamin D3 (for participants taking <400 IU/day as part of usual care).
Main Outcomes
Incidence of ARI during the 12-month intervention. Secondary outcomes included falls/fractures, 25-hydroxyvitamin D levels, hypercalcemia, and kidney stones.
Results
We randomized 107 participants (55 high dose, 52 standard dose) and included all in the final analysis. The high dose group had 0.67 ARIs per person-year compared to 1.11 in the standard dose group (incidence rate ratio [IRR] 0.60; 95%CI 0.38–0.94; p= 0.02). Falls were more common in the high dose group (1.47 per person-year) compared to 0.63 in the standard dose group (IRR 2.33; 95%CI 1.49–3.63; p<0.001). Fractures were uncommon and similar in both groups (high dose 0.10 vs standard dose 0.19 per person-year; p=0.31). The mean 25-hydroxyvitamin D level during the trial was 32.6 ng/mL in the high dose group and 25.1 ng/mL in the standard dose group. There was no hypercalcemia or kidney stones in either group.
Conclusion
Monthly high dose vitamin D3 supplementation reduced the incidence of ARI in older long-term care residents but was associated with a higher rate of falls without an increase in fractures.
Keywords: Vitamin D, Nursing Home, Respiratory Infection, Falls, Immunosenescence
INTRODUCTION
Acute respiratory infection (ARI) is common in older adults and results in important morbidity, healthcare utilization, and functional decline.1,2 Older long-term care (LTC) residents are particularly vulnerable to increased ARI due to senescent immune responses and decreased functional reserve.3,4 Strategies to reduce the incidence and severity of ARI in this population are limited, and effective vaccines are lacking for most common respiratory pathogens.
Micronutrient deficiencies are common in LTC residents5,6 and can exacerbate age-related changes in immune function.7,8 Multivitamin supplementation appears to improve indices of immune function,9 but clinical impact on ARI and other infections has been modest.10,11 Vitamin D has an important role in many aspects of immune function, particularly innate immunity.12 Older adults are at high risk for vitamin D deficiency,13 and epidemiologic studies demonstrate a consistent association between vitamin D deficiency and ARIs.14,15 However, clinical trials of vitamin D supplementation for ARI prevention have been mixed, with meta-analyses suggesting modest benefit but substantial heterogeneity.15,16 Existing trials are limited by the use of relatively low doses for short durations in predominantly healthy populations.
Therefore, we conducted a randomized, controlled trial to evaluate the efficacy and safety of high dose vitamin D3 supplementation for 12 months to reduce the incidence and severity of ARI in older LTC residents.
METHODS
Study Design
This study was a double-blinded, parallel group, randomized controlled phase II trial of oral high vs. standard dose vitamin D3 supplementation administered monthly for 12 months. The trial was conducted from June 2010 to January 2014. The protocol was approved by the Colorado Multiple Institutional Review Board and supervised by an independent Data Safety and Monitoring Board. Written informed consent was obtained from each participant or their legally authorized representative (LAR). The trial was registered at Clinicaltrials.gov NCT01102374 (see Online Supplement for full protocol).
Eligibility Criteria
Older residents (age ≥60 years) of 25 selected Colorado LTC facilities (skilled nursing or assisted living facilities) were eligible for participation. Exclusion criteria included: 1) Terminal illness; 2) Anticipated facility discharge within 12 months; 3) Inability to take whole/crushed tablets; 4) Active cancer, except squamous/basal cell carcinoma; 5) Underweight (body mass index <18 kg/m2); 6) Current immunosuppressive medications; 7) Renal failure (eGFR<15 mL/min/1.73m2); 8) Currently taking >1,000 IU/day vitamin D; 9) Personal (or strong family) history of kidney stones; 10) History of sarcoidosis or other granulomatous disorders; 11) Baseline hypercalcemia (albumin-adjusted calcium >10.5 mg/dL); 12) Baseline serum 25-hydroxyvitamin D (25OHD) level ≥ 40 ng/mL (to convert to nmol/L, multiple by 2.496); 13) Inability of participant or LAR to speak/understand English and no available interpreter; 14) Inability to provide informed consent and no available healthcare LAR.
Intervention
We randomized participants to one of two vitamin D dose groups: high dose (equivalent to 3,000–4,000 IU/day) or standard dose (equivalent to 400–1,000 IU/day). The participant’s total vitamin D dose included any study drug supplementation plus vitamin D taken as part of usual care (0–1,000 IU/day). Because the facilities were not formally ‘engaged’ in the research, the study team administered study drug monthly and did not interfere in their usual clinical care. Based on Institutional Review Board and Data Safety and Monitoring Board recommendations, we ensured that all trial participants received at least 400 IU/day equivalent of vitamin D to meet the Institute of Medicine Dietary Reference Intake (including typical dietary intake).
Participants already taking 400 – 1,000 IU/day vitamin D as usual care were randomized to one of two study drugs: 1) oral 100,000 IU vitamin D3 monthly or 2) matched oral placebo monthly, while continuing their usual vitamin D regimen. Participants taking <400 IU/day vitamin D supplementation as usual care were also randomized to one of two study drugs: 1) oral 100,000 IU vitamin D3 monthly or 2) oral 12,000 IU vitamin D3 monthly, while continuing any usual vitamin D regimen. The 12,000 IU/month supplementation ensured at least 400 IU/day.
Study drugs were provided by Pencol Compounding Pharmacy (Denver, CO), who performed routine testing of study drug content throughout the trial to verify study drug content. Vitamin D and placebo were identical in size, weight, color, smell, texture, and taste. If necessary, the capsule was opened and sprinkled on food for administration. The research pharmacy provided study medication to the research staff in numbered blister packs, and the sequence was concealed until interventions were assigned. The protocol and study drugs were also under the oversight of the Food and Drug Administration as an Investigational New Drug.
Randomization
The research pharmacy (Veterans Affairs Eastern Colorado Research Pharmacy; Denver, CO) performed 1:1 randomization in permuted block sizes of 4–8, stratified by site and baseline vitamin D supplementation (<400 IU/day or 400–1,000 IU/day). Study personnel, outcome assessors, study participants, and treating clinicians were blinded to study group assignment, allocation sequence, and baseline 25OHD level (a third party provided dichotomous data on eligibility as <40 ng/mL or ≥40 ng/mL). Unblinding was not required for any participant during the course of the trial.
Outcomes
The primary outcome was total number of incident ARIs during the 12-month follow-up period. We measured both upper (common colds, sinusitis, pharyngitis, otitis media) and lower (acute bronchitis, influenza, pneumonia) ARIs that required medical attention (nurse or physician assessment and/or new prescribed treatment) by a chart review method validated in the LTC setting,17 with additional active surveillance during monthly study visits.
The infection-related secondary outcomes included severity of ARIs, as measured by emergency department visits or hospitalization for ARIs, time to first ARI, and incidence of other infections (categorized as urinary tract, skin/soft tissue, other) during the 12 month follow-up period. We also assessed efficacy of the intervention by change in 25OHD levels at 3, 7, and 11 months, compared to baseline. Collection of samples for trough 25OHD levels occurred just prior to the next monthly dose, and analysis was performed at the University of Washington using the liquid chromatography-tandem mass spectrometry method and accounting for the C-3 epimer of 25OHD. Cryopreserved samples were batch tested after trial participation to avoid unblinding and reduce measurement variability.
The primary safety outcome was incident hypercalcemia defined as albumin-adjusted serum calcium >10.5 mg/dL, measured at 3, 7, and 11 months. We also measured incident falls, fractures, kidney stones, all-cause hospitalizations, and all-cause death by chart review. At monthly medication administration visits, research assistants queried participants and clinical staff for new adverse events during. We classified these events using the Medical Dictionary for Regulatory Activities (MedDRA) hierarchy.
Data Collection
Baseline data collected from chart review and interviews with participants, legally authorized representatives, and clinical providers, included: 1) demographics; 2) facility length of stay; 3) co-morbid conditions; 4) advanced directives; 5) vaccination status (seasonal and H1N1 influenza, pneumococcal); 6) smoking history; 7) body mass index; 8) physical activity; and 9) current medications, including vitamin D and calcium supplementation. We collected outcome and adverse event data at monthly medication administration visits, with expanded data collection, chart review, and blood draws at 3, 7, and 11 months.
Statistical Analysis
The primary intention-to-treat analysis measured the effect of randomized treatment group (high dose vs. standard dose vitamin D) on the number of incident ARIs observed during the follow-up period. We estimated the treatment effect using Poisson regression as performed with the PROC GENMOD procedure using SAS® software (SAS 9.3, Cary, NC), expressed as the percentage change of the estimated mean number of ARIs for the high dose group compared with the standard dose group. Some participants had less than 12 months of observation time for reasons such as drop out, death, or lost-to-follow-up. The logarithm of the participant time active in the study was included in the model as an offset in order to account for the different observation times. We handled secondary analyses of count data in a manner similar to the primary outcome of ARI. All outcomes were pre-specified in the study protocol. Pre-specified subgroup analyses included age, sex, residence, co-morbidities, baseline vitamin D supplementation, BMI, renal function, and completers (defined as ≥11 out of 12 possible doses of study medication).
Our sample size calculation assumed a control group rate of 1.4 ARIs per person-year and an estimated 35% reduction in the high dose vitamin D intervention group. We anticipated that 80% of the total possible follow-up time would be obtained (due to censoring). With a 2-sided type I error rate of 0.05 and 80% power, we planned for a total sample size of 200 randomized participants (100 per group). In consultation with the DSMB and NIH, recruitment for the trial ended at 107 randomized participants, due to the lack of available local participants and insufficient resources to expand recruitment beyond the local geographic area.
RESULTS
Participants
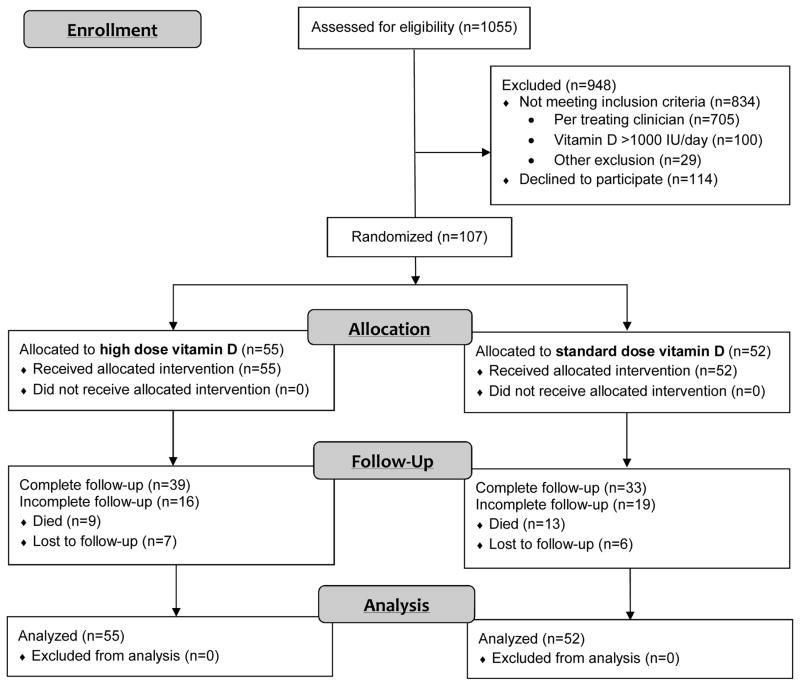
From the 1055 LTC residents screened, 107 were eligible and randomized (55 high dose and 52 standard dose). The primary reason for exclusion was clinician discretion based on their knowledge of exclusion criteria and study intervention (Figure 1). The intention-to-treat analysis included all 107 randomized participants.
Figure 1.
CONSORT diagram for flow of participants in trial
Baseline characteristics of the participants are presented in Table 1. There were modest differences in some baseline characteristics with the high dose group having higher mean BMI and rates of chronic obstructive pulmonary disease (COPD) and diabetes but lower rates of current smoking, asthma, coronary artery disease, and dementia. Baseline vitamin D supplementation and serum 25OHD levels were similar between the two groups.
Table 1.
Baseline characteristics of randomized study participants
| Characteristic | High Dose (n=55) n (%) or mean (SD) |
Standard Dose (n=52) n (%) or mean (SD) |
|---|---|---|
| Demographics | ||
| Age, years | 80 (10) | 82 (10) |
| Female sex | 33 (60.0%) | 29 (55.8%) |
| Non-Hispanic white | 48 (87.3%) | 48 (92.3%) |
| Facility length of stay, months | 24 (24) | 28 (34) |
| Skilled nursing facility | 15 (27.3%) | 13 (25.0%) |
| Required surrogate for consent | 19 (34.5%) | 25 (48.1%) |
| Do not hospitalize order | 1 (1.8%) | 3 (5.8%) |
| Co-morbidities | ||
| Body mass index, kg/m2 | 28.1 (6.8) | 26.0 (5.4) |
| Smoking history | ||
| Current smoker | 6 (10.9%) | 9 (17.3%) |
| Ex-smoker | 20 (36.4%) | 11 (21.2%) |
| Never smoker | 28 (50.9%) | 31 (59.6%) |
| Asthma | 1 (1.8%) | 5 (9.6%) |
| Chronic obstructive pulmonary disease | 17 (30.9%) | 14 (26.9%) |
| Congestive heart failure | 12 (21.8%) | 15 (28.8%) |
| Coronary artery disease | 8 (14.5%) | 14 (26.9%) |
| Diabetes | 21 (38.2%) | 14 (26.9%) |
| Dementia | 16 (29.1%) | 25 (48.1%) |
| Depression | 30 (54.5%) | 28 (53.8%) |
| History of cancer | 7 (12.7%) | 5 (9.6%) |
| Osteoporosis | 2 (3.6%) | 4 (7.7%) |
| Documented influenza vaccine in past 12 months | 30 (54.5%) | 32 (61.5%) |
| Outdoor physical activity in past month | ||
| None | 24 (43.6%) | 23 (44.2%) |
| At least monthly | 4 (7.3%) | 4 (7.7%) |
| At least weekly | 19 (34.5 %) | 15 (28.8%) |
| Daily | 8 (14.5%) | 10 (19.2%) |
| Vitamin D-related | ||
| Vitamin D supplementation dose (IU/day) | 226 (279) | 232 (304) |
| 400–1000 IU/day | 23 (41.8%) | 20 (38.5%) |
| Serum 25-hydroxyvitamin D, ng/mL | 23.0 (8.4) | 23.0 (9.9) |
| <20 ng/mL | 18 (32.7%) | 19 (36.5%) |
| Serum albumin-adjusted calcium, mg/dl | 9.1 (0.3) | 9.1 (0.4) |
| Serum phosphorus, mg/dl | 3.5 (0.7) | 3.5 (0.7) |
| Estimated GFR, mL/min/1.73m2 | 69.7 (23.2) | 70.2 (30.0) |
IU, international units; GFR, glomerular filtration rate
Vitamin D Intervention
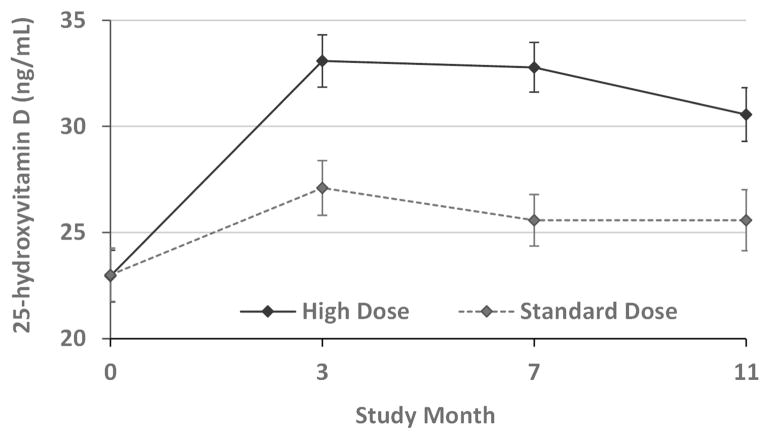
The number of monthly study drug doses received during the 12 months was similar between the two treatment groups (high dose group: median 11 [IQR 8–12]; standard dose group: median 11 [IQR 6–12]). The mean 25OHD levels increased in both the high dose and standard dose vitamin D groups (Figure 2). The mean trough 25OHD levels in the high dose group remained greater than the target 30 ng/mL throughout the trial and were significantly higher than the standard dose group at every timepoint (p<0.001).
Figure 2. Change in 25-hydroxyvitamin D levels in two randomized groups.
Treatment groups: high dose vitamin D (solid line), standard dose vitamin D (dotted line)
Primary Outcome
The incidence of ARI was lower in the high dose group compared to the standard dose group (incidence rate ratio [IRR] 0.60; 95%CI 0.38–0.94; p= 0.02). There were 17 (31%) participants in the high dose group and 24 (46%) in the standard dose group who had at least one ARI (p=0.10). The time to first ARI analysis (Supplementary Figure S1) demonstrates a similar effect size as the primary analysis (hazard ratio 0.59; 95%CI 0.32–1.09; p= 0.09).
Secondary Outcomes
The high dose group had a lower incidence of upper ARI (IRR 0.52; 95%CI 0.31–0.90; p= 0.02) and skin and soft tissue infections (0.32; 95%CI 0.13–0.80; p= 0.02), compared to the standard dose group (Table 2). There were no differences in the incidence of lower ARI, hospitalizations for ARI, urinary tract infections, or other infections.
Table 2.
Incidence of efficacy and safety outcomes in high dose vs. standard dose vitamin D groups
| Outcome Measures | High Dose (n=55) | Standard dose (n=52) | IRR (95%CI) | P value | ||
|---|---|---|---|---|---|---|
| Events | Incidence | Events | Incidence | |||
| (n with ≥1 event) | (n with ≥1 event) | |||||
| 47.8 person-years* | 42.8 person-years* | |||||
| Efficacy | ||||||
| Acute respiratory infection+ | 32 (17) | 0.67 | 48 (24) | 1.12 | 0.60 (0.38–0.94) | 0.02 |
| Upper ARI | 21 (13) | 0.44 | 36 (20) | 0.84 | 0.52 (0.31–0.90) | 0.02 |
| Lower ARI | 11 (7) | 0.23 | 12 (9) | 0.28 | 0.82 (0.36–1.86) | 0.64 |
| Hospitalizations for ARI | 3 (2) | 0.06 | 6 (5) | 0.14 | 0.45 (0.11–1.79) | 0.26 |
| Skin/soft tissue infections | 6 (6) | 0.13 | 17 (11) | 0.40 | 0.32 (0.13–0.80) | 0.02 |
| Urinary tract infections | 38 (19) | 0.80 | 22 (14) | 0.51 | 1.55 (0.92–2.62) | 0.10 |
| Other infections | 27 (16) | 0.57 | 26 (18) | 0.61 | 0.93 (0.54–1.60) | 0.80 |
| Safety | ||||||
| Falls | 70 (20) | 1.47 | 27 (15) | 0.63 | 2.33 (1.49–3.63) | <0.001 |
| Fractures | 5 (4) | 0.10 | 8 (8) | 0.19 | 0.56 (0.18–1.71) | 0.31 |
| All-cause hospitalizations | 33 (22) | 0.69 | 33 (24) | 0.77 | 0.90 (0.55–1.45) | 0.66 |
| Deaths | 9 | 0.19 | 13 | 0.30 | 0.62 (0.27–1.45) | 0.27 |
| Incident kidney stone | 0 | 0 | 0 | 0 | -- | NA |
| Incident hypercalcemia | 0 | 0 | 0 | 0 | -- | NA |
| 25OHD level ≥80 ng/mL | 0 | 0 | 0 | 0 | -- | NA |
Represents person time of follow-up available
Primary outcome
IRR, incidence rate ratio; ARI, acute respiratory infection; 25OHD, 25-hydroxyvitamin D
No pre-specified vitamin D-related safety outcomes was observed in either group (hypercalcemia, kidney stones, hypervitaminosis D). The overall proportion of participants with all-cause hospitalizations (46% high dose vs. 43% standard dose) and death (22% vs. 21%, respectively) was high in both groups of LTC residents, but not different between groups.
The high dose group had a higher incidence of falls, compared to the standard dose group (IRR 2.33; 95%CI 1.49–3.63; p<0.001). At least 1 fall during the follow-up period was recorded for 20 (36%) of participants in the high dose group and 15 (29%) in the standard dose group (p=0.41). Similarly, the time to first fall analysis (Supplementary Figure S2) demonstrated no marked difference between groups (hazard ratio 1.34; 95%CI 0.68–2.59; p= 0.41). Thus, the overall difference in fall incidence appeared to be driven by participants with multiple recorded falls.
The overall incidence of fractures was low and did not differ between groups (IRR 0.56; 95%CI 0.18–1.71; p= 0.31) with one or more fractures in 4 (7%) of high dose group participants and 8 (15%) of standard dose group participants (p=0.18).
Adverse Events
There were no significant between-group differences in the recorded adverse events overall or by MedDRA groups (Supplementary Table S1).
Subgroup Analyses
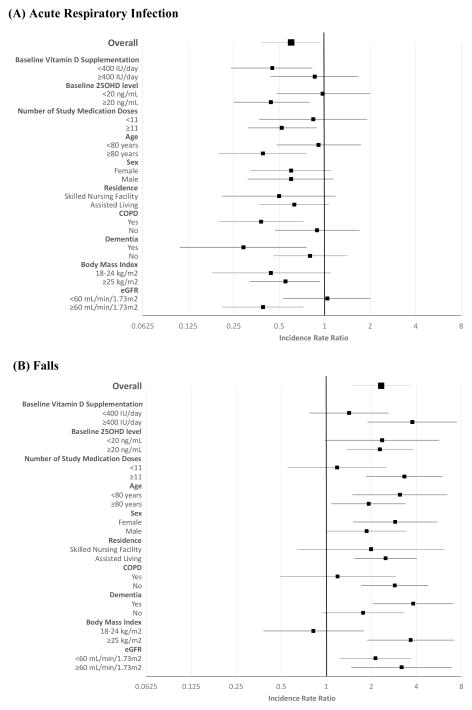
Pre-specified subgroup analyses of the primary ARI outcome is displayed in Figure 3, along with post-hoc subgroup analyses for the secondary fall outcome. The following subgroups had a significantly lower incidence of ARI among those assigned to high dose vs standard dose: baseline vitamin D supplementation <400 IU, baseline 25OHD level ≥20 ng/mL, ≥11 study medication doses received, age ≥80 years, type of LTC residence, dementia, and eGFR≥60 mL/min/1.73m2. The following subgroups had a particularly high observed incidence of falls in the high dose vs. standard dose group: baseline vitamin D supplementation ≥400 IU, ≥11 study medication doses received, age <80 years, dementia, and BMI ≥25 kg/m2.
Figure 3. Subgroup analyses of acute respiratory infection and fall outcomes.
(A) Acute Respiratory Infection
(B) Falls
Abbreviations: 25OHD, 25-hydroxyvitamin D; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtrate rate
DISCUSSION
In this double-blinded, phase II randomized control trial, older LTC residents receiving monthly high dose vitamin D supplementation had a 40% lower incidence of ARI during the 12 month follow-up period, compared to those receiving standard dose vitamin D. While there were no observed safety concerns for hypercalcemia, hypervitaminosis D, kidney stones, hospitalizations, death, or fractures, there was a markedly higher incidence of falls in participants assigned to the high dose vitamin D group, driven by participants with multiple falls.
To our knowledge, this is the first trial to evaluate high dose vitamin D supplementation for prevention of ARI in older LTC residents. Secondary analyses of two randomized controlled trials suggest that 800 IU per day vitamin D supplementation had modest benefit in preventing ARI in older adults.18,19 Tran et al reported that 60,000 IU monthly vitamin D reduced antibiotic use in community dwelling adults aged ≥70 years.20 However, these were post-hoc analyses of trials designed for other purposes. In a recent randomized trial, Martineau et al found that 96,000 IU vitamin D every 2 months to residential care facility residents in the U.K. did not influence the incidence of ARI.21 However, these participants were younger and healthier than our population of older LTC residents and received half the dose used in our trial.
Although not a primary focus of this trial, falls are also an important cause of morbidity in older adults. There is growing interest regarding the role of vitamin D in fall prevention, relating to muscle function and balance. The American Geriatrics Society consensus statement recommends 1,000–4,000 IU daily of vitamin D supplementation for fall prevention in older adults.22 Indeed, recruitment into our trial was challenging because of clinician use of high dose vitamin D for usual care of many older LTC residents. However, we found that participants in the high dose group had a 2.3-fold higher incidence of falls. The mechanism of this finding requires further investigation, including the hypothesis that high dose vitamin D leads to increased mobility resulting in greater exposure to falls. Two prior trials of high, intermittent doses of vitamin D (500,000 IU annually and 60,000 IU monthly) in older community dwelling adults have also reported a higher fall rates in the intervention group.23,24 Although higher rates of fractures have not been reported in our trial nor these 2 prior trials, increased falls from these 3 trials call into question the potential safety of high, intermittent doses of vitamin D for older adults. We used this dosing paradigm to reduce the need to deliver daily study doses to these LTC residents. There remains equipoise for daily high dose vitamin D supplementation and fall prevention in older adults, which is the focus of an ongoing phase III trial (NCT02166333).
Several other secondary and subgroup analyses could have important clinical implications, meriting further investigation. The incidence of skin and soft tissue infections was lower in the high dose group and is an underexplored infectious outcome in vitamin D trials. High dose vitamin D may be particularly effective in ARI prevention for patients with COPD, which has important clinical implications due to ARI-associated disease exacerbation. Several recent clinical trials support the potential for high dose vitamin D to improve COPD outcomes, particularly those with severe disease and severe vitamin D deficiency.25–27
The results of this trial should be interpreted in the context of several limitations. The sample size was modest and did not reach goal recruitment; this impacts the trial power and precision. However, outcome data did not influence the decision to end the trial, limiting the potential for bias and type 1 error. Modest differences in some baseline characteristics may have influenced results, although subgroup analyses suggest that this was unlikely. We selected intermittent bolus dosing largely for feasibility, and the regimen is not physiologic relative to daily dosing. We did not measure or adjust for baseline fall history, and thus the higher rate of total falls in the high dose group may have been driven by imbalance in the number of frequent fallers in this group. However, as others have also observed higher falls with high, intermittent doses of vitamin D, we recommend equivalent daily doses be tested in future trials for ARI prevention in this population. Due to ethical concerns to ensure at least standard amounts of vitamin D for the control group in this vulnerable population, there was no true placebo group. This may have reduced the effect size for some outcomes of interest. In addition, some missed doses of study medication (due to logistical issues/hospitalizations) may have also led to regression to the mean. The observed rise in 25OHD levels was not as robust as anticipated, which may have mitigated potential benefits (as well as risks) of high dose supplementation. Indeed, completers (those receiving ≥11 out of 12 possible doses of study medication) appeared to have a more robust signal for ARI prevention and higher fall incidence.
In summary, monthly high dose vitamin D supplementation reduced the incidence of ARI but increased falls, without an increase in fractures, in older LTC residents. If our results are confirmed by a larger trial, high dose vitamin D, ideally using daily dosing to minimize fall risk, has the potential for substantial public health benefit through ARI prevention for the large and growing population of LTC residents.
Supplementary Material
Supplementary Table S1. Summary of adverse events by system organ class in high dose vs. standard dose vitamin D groups
Supplementary Figure S1. Time for first acute respiratory infection in high dose vs standard dose vitamin D groups
Supplementary Figure S2. Time for first fall in high dose vs standard dose vitamin D groups
Acknowledgments
We thank the long-term care facilities, residents, and families who participated in this trial, and the Data Safety and Monitoring Board members for their careful review and oversight of this trial.
Funding Sources: This trial was supported by the Beeson Career Development Award (NIH/NIA grant K23AG040708), NIH/NCATS Colorado CTSA Grant UL1TR001082, and the American Geriatrics Society Jahnigen Career Development Scholars Award. Dr. Blatchford and Dr. Schwartz were supported by the Eastern Colorado VA Geriatric Research Education and Clinical Center (GRECC). Contents are the authors’ sole responsibility and do not necessarily represent official NIH or VA views.
Footnotes
Conflict of Interest Disclosures:
| Elements of Financial/Personal Conflicts | AAG | PB | KB | LZ | SAL | JIW | RSS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | X | X | X | X | X | X | X | |||||||||
| Grants/Funds | X | X | X | X | X | X | X | |||||||||
| Honoraria | X | X | X | X | X | X | X | |||||||||
| Speaker Forum | X | X | X | X | X | X | X | |||||||||
| Consultant | X | X | X | X | X | X | X | |||||||||
| Stocks | X | X | X | X | X | X | X | |||||||||
| Royalties | X | X | X | X | X | X | X | |||||||||
| Expert Testimony | X | X | X | X | X | X | X | |||||||||
| Board Member | X | X | X | X | X | X | X | |||||||||
| Patents | X | X | X | X | X | X | X | |||||||||
| Personal Relationship | X | X | X | X | X | X | X | |||||||||
Author Contributions
Dr. Ginde had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Ginde, Schwartz.
Acquisition of data: Ginde, Breese, Zarrabi.
Statistical analysis: Blatchford.
Interpretation of data: Ginde, Blatchford, Breese, Zarrabi, Linnebur, Wallace, Schwartz.
Drafting of the manuscript: Ginde.
Critical revision of the manuscript for important intellectual content: Ginde, Blatchford, Breese, Zarrabi, Linnebur, Wallace, Schwartz.
Sponsor’s Role: The sponsors had no role in the design, methods, subject recruitment, data collection, analysis, interpretation, or presentation of the study. Contents are the authors’ sole responsibility and do not necessarily represent official NIH or VA views.
References
- 1.Fried TR, Gillick MR, Lipsitz LA. Short-term functional outcomes of long-term care residents with pneumonia treated with and without hospital transfer. J Am Geriatr Soc. 1997;45:302–306. doi: 10.1111/j.1532-5415.1997.tb00944.x. [DOI] [PubMed] [Google Scholar]
- 2.Hasley PB, Brancati FL, Rogers J, et al. Measuring functional change in community-acquired pneumonia. A preliminary study using the Sickness Impact Profile. Med Care. 1993;31:649–657. doi: 10.1097/00005650-199307000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Muder RR. Pneumonia in residents of long-term care facilities: epidemiology, etiology, management, and prevention. Am J Med. 1998;105:319–330. doi: 10.1016/s0002-9343(98)00262-9. [DOI] [PubMed] [Google Scholar]
- 4.Loeb M, McGeer A, McArthur M, et al. Risk factors for pneumonia and other lower respiratory tract infections in elderly residents of long-term care facilities. Arch Intern Med. 1999;159:2058–2064. doi: 10.1001/archinte.159.17.2058. [DOI] [PubMed] [Google Scholar]
- 5.Drinka PJ, Goodwin JS. Prevalence and consequences of vitamin deficiency in the nursing home: a critical review. J Am Geriatr Soc. 1991;39:1008–1017. doi: 10.1111/j.1532-5415.1991.tb04050.x. [DOI] [PubMed] [Google Scholar]
- 6.Lipski PS, Torrance A, Kelly PJ, et al. A study of nutritional deficits of long-stay geriatric patients. Age Ageing. 1993;22:244–255. doi: 10.1093/ageing/22.4.244. [DOI] [PubMed] [Google Scholar]
- 7.Chandra RK. Effect of vitamin and trace-element supplementation on immune responses and infection in elderly subjects. Lancet. 1992;340:1124–1127. doi: 10.1016/0140-6736(92)93151-c. [DOI] [PubMed] [Google Scholar]
- 8.High KP. Nutritional strategies to boost immunity and prevent infection in elderly individuals. Clin Infect Dis. 2001;33:1892–1900. doi: 10.1086/324509. [DOI] [PubMed] [Google Scholar]
- 9.Fortes C, Forastiere F, Agabiti N, et al. The effect of zinc and vitamin A supplementation on immune response in an older population. J Am Geriatr Soc. 1998;46:19–26. doi: 10.1111/j.1532-5415.1998.tb01008.x. [DOI] [PubMed] [Google Scholar]
- 10.Girodon F, Galan P, Monget AL, et al. Impact of trace elements and vitamin supplementation on immunity and infections in institutionalized elderly patients: a randomized controlled trial. MIN. VIT. AOX. geriatric network. Arch Intern Med. 1999;159:748–754. doi: 10.1001/archinte.159.7.748. [DOI] [PubMed] [Google Scholar]
- 11.Liu BA, McGeer A, McArthur MA, et al. Effect of multivitamin and mineral supplementation on episodes of infection in nursing home residents: a randomized, placebo-controlled study. J Am Geriatr Soc. 2007;55:35–42. doi: 10.1111/j.1532-5415.2006.01033.x. [DOI] [PubMed] [Google Scholar]
- 12.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 13.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 14.Ginde AA, Mansbach JM, Camargo CA., Jr Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2009;169:384–390. doi: 10.1001/archinternmed.2008.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jolliffe DA, Griffiths CJ, Martineau AR. Vitamin D in the prevention of acute respiratory infection: systematic review of clinical studies. J Steroid Biochem Mol Biol. 2013;136:321–9. doi: 10.1016/j.jsbmb.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 16.Bergman P, Lindh AU, Bjorkhem-Bergman L, et al. Vitamin D and respiratory tract infections: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2013;8:e65835. doi: 10.1371/journal.pone.0065835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGeer A, Campbell B, Emori TG, et al. Definitions of infection for surveillance in long-term care facilities. Am J Infect Control. 1991;19:1–7. doi: 10.1016/0196-6553(91)90154-5. [DOI] [PubMed] [Google Scholar]
- 18.Avenell A, Cook JA, Maclennan GS, Macpherson GC. Vitamin D supplementation to prevent infections: a sub-study of a randomised placebo-controlled trial in older people (RECORD trial, ISRCTN 51647438) Age Ageing. 2007;36:574–577. doi: 10.1093/ageing/afm091. [DOI] [PubMed] [Google Scholar]
- 19.Aloia JF, Li-Ng M. Re: epidemic influenza and vitamin D. Epidemiol Infect. 2007;135:1095–6. doi: 10.1017/S0950268807008308. author reply 7–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tran B, Armstrong BK, Ebeling PR, et al. Effect of vitamin D supplementation on antibiotic use: a randomized controlled trial. Am J Clin Nutr. 2014;99:156–161. doi: 10.3945/ajcn.113.063271. [DOI] [PubMed] [Google Scholar]
- 21.Martineau AR, Hanifa Y, Witt KD, et al. Double-blind randomised controlled trial of vitamin D3 supplementation for the prevention of acute respiratory infection in older adults and their carers (ViDiFlu) Thorax. 2015;70:953–960. doi: 10.1136/thoraxjnl-2015-206996. [DOI] [PubMed] [Google Scholar]
- 22.American Geriatrics Society Workgroup on Vitamin D Supplementation for Older Adults. Recommendations abstracted from the American Geriatrics Society consensus statement on vitamin D for prevention of falls and their consequences. J Am Geriatr Soc. 2014;62:147–152. doi: 10.1111/jgs.12631. [DOI] [PubMed] [Google Scholar]
- 23.Sanders KM, Stuart AL, Williamson EJ, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303:1815–1822. doi: 10.1001/jama.2010.594. [DOI] [PubMed] [Google Scholar]
- 24.Bischoff-Ferrari HA, Dawson-Hughes B, Orav EJ, et al. Monthly high-dose vitamin D treatment for the prevention of functional decline: a randomized clinical trial. JAMA Intern Med. 2016;176:175–183. doi: 10.1001/jamainternmed.2015.7148. [DOI] [PubMed] [Google Scholar]
- 25.Zendedel A, Gholami M, Anbari K, et al. Effects of vitamin D intake on FEV1 and COPD exacerbation: a randomized clinical trial study. Glob J Health Sci. 2015;7:243–238. doi: 10.5539/gjhs.v7n4p243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martineau AR, James WY, Hooper RL, et al. Vitamin D3 supplementation in patients with chronic obstructive pulmonary disease (ViDiCO): a multicentre, double-blind, randomised controlled trial. Lancet Respir Med. 2015;3:120–130. doi: 10.1016/S2213-2600(14)70255-3. [DOI] [PubMed] [Google Scholar]
- 27.Lehouck A, Mathieu C, Carremans C, et al. High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: a randomised trial. Ann Intern Med. 2012;156:105–114. doi: 10.7326/0003-4819-156-2-201201170-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Summary of adverse events by system organ class in high dose vs. standard dose vitamin D groups
Supplementary Figure S1. Time for first acute respiratory infection in high dose vs standard dose vitamin D groups
Supplementary Figure S2. Time for first fall in high dose vs standard dose vitamin D groups