Abstract
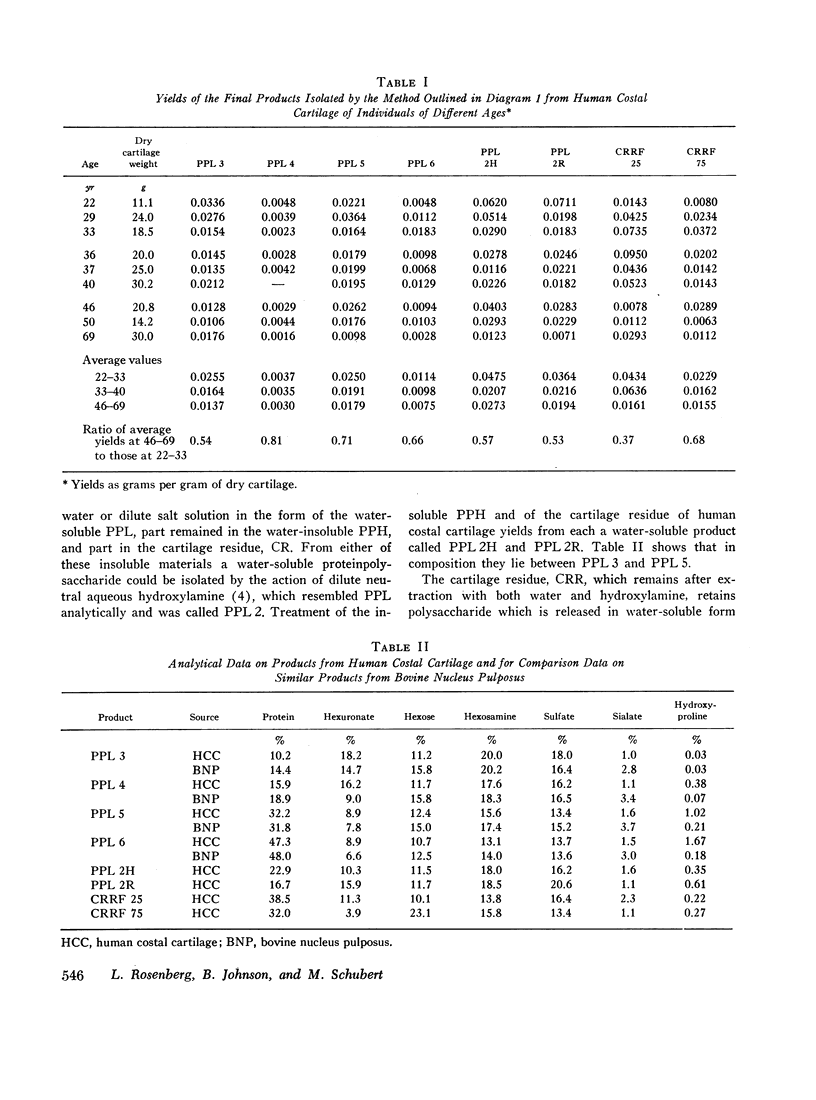
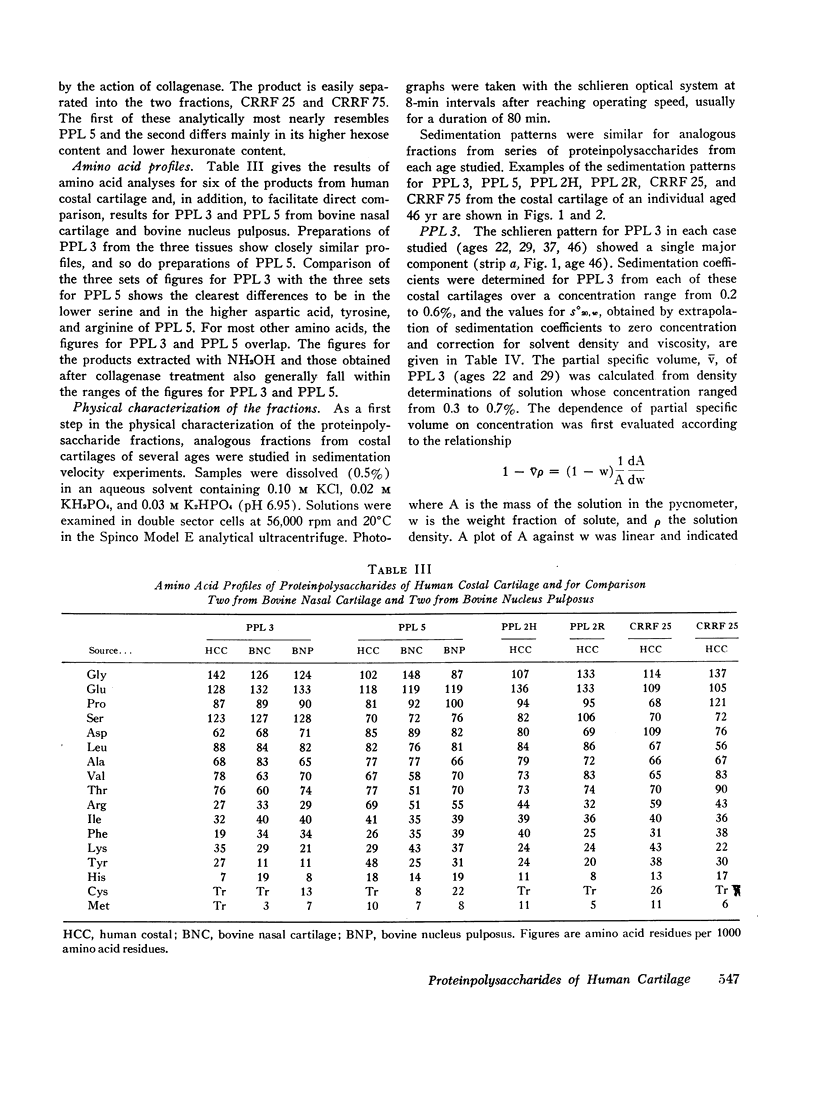
Water-soluble proteinpolysaccharides, called PPL, can be extracted from bovine nucleus pulposus in yields of 45%, and from bovine nasal cartilage in yields of 37% of the dry tissue weight. From human costal cartilage only 7% can be extracted. The method used to separate PPL from each of the first two tissues into four distinct fractions separates the PPL of human costal cartilage into four fractions called PPL 3, PPL 4, PPL 5, and PPL 6, which show an increase in protein content, a decrease in chondroitin sulfate content, a nearly constant keratan sulfate content, and an increase in ease of sedimentability and molecular weight. From each of the three tissues mentioned. PPL 3 has a similar amino acid profile and so does PPL 5, but PPL 5 differs from PPL 3 in having a lower content of serine and higher contents of aspartic acid, tyrosine, and arginine. A more extensive effort to characterize these products has been made by analytical ultracentrifugation, and this has led to a further fractionation of PPL 5.
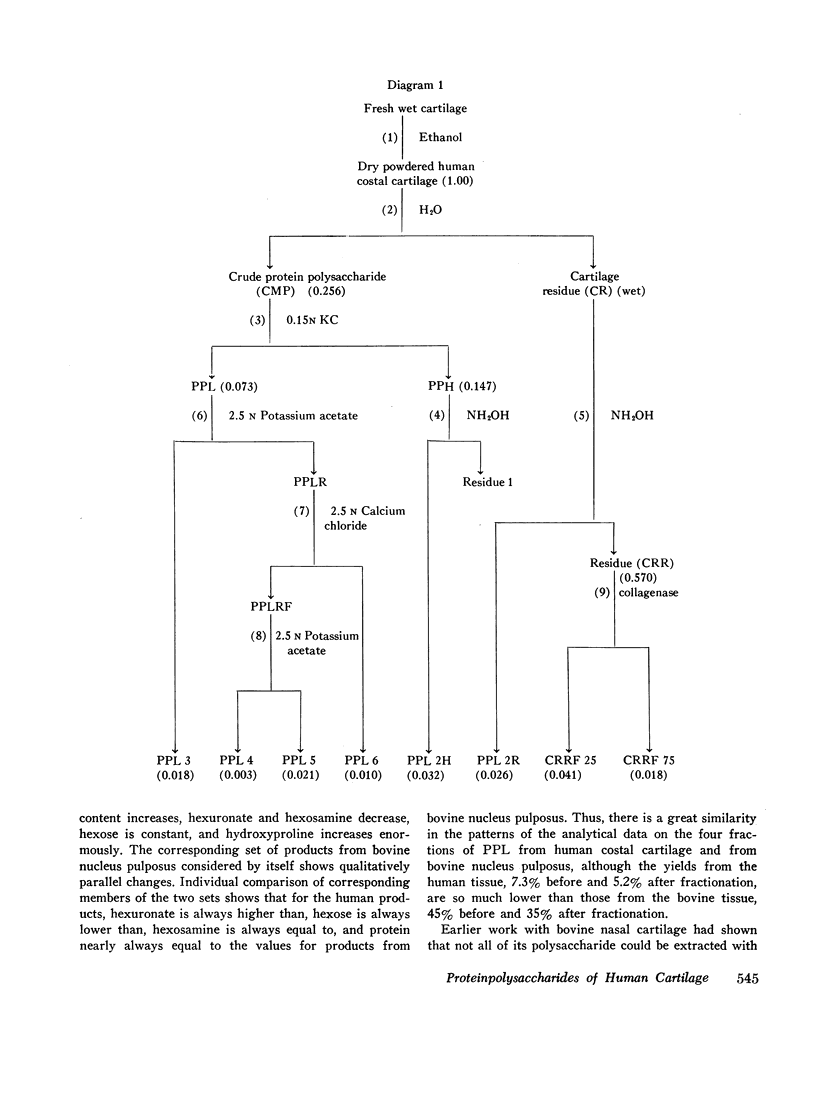
Treatment of the cartilage residue or the water-insoluble protein polysaccharide called PPH, with neutral NH2OH solution releases water-soluble protein polysaccharides which in composition resemble PPL 4. The water-insoluble residue left after NH2OH treatment, when treated with collagenase, yields two soluble products, one resembling PPL 5 in composition, the other with a much lower chondroitin sulfate and much higher keratan sulfate content. The possibility is suggested that in human costal cartilage, binding of some forms of PPL to collagen may occur.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Di Salvo J., Schubert M. Specific interaction of some cartilage proteinpolysaccharides with freshly precipitating calcium phosphate. J Biol Chem. 1967 Feb 25;242(4):705–710. [PubMed] [Google Scholar]
- GREEN J. P., ROBINSON J. D., Jr Cerebroside sulfate (sulfatide A) in some organs of the rat and in a mast cell tumor. J Biol Chem. 1960 Jun;235:1621–1624. [PubMed] [Google Scholar]
- KAPLAN D., MEYER K. Ageing of human cartilage. Nature. 1959 May 2;183(4670):1267–1268. doi: 10.1038/1831267a0. [DOI] [PubMed] [Google Scholar]
- Mathews M. B., Glagov S. Acid mucopolysaccharide patterns in aging human cartilage. J Clin Invest. 1966 Jul;45(7):1103–1111. doi: 10.1172/JCI105416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews M. B. The molecular evolution of cartilage. Clin Orthop Relat Res. 1966 Sep-Oct;48:267–283. [PubMed] [Google Scholar]
- Muir H., Jacobs S. Protein-polysaccharides of pig laryngeal cartilage. Biochem J. 1967 May;103(2):367–374. doi: 10.1042/bj1030367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAL S., SCHUBERT M. THE ACTION OF HYDROXYLAMINE ON THE PROTEINPOLYSACCHARIDES OF CARTILAGE. J Biol Chem. 1965 Aug;240:3245–3248. [PubMed] [Google Scholar]
- Pal S., Doganges P. T., Schubert M. The separation of new forms of the proteinpolysaccharides of bovine nasal cartilage. J Biol Chem. 1966 Sep 25;241(18):4261–4266. [PubMed] [Google Scholar]
- Partridge S. M. Chondroitin sulfate-protein of bovine cartilage. Fed Proc. 1966 May-Jun;25(3):994–996. [PubMed] [Google Scholar]
- Rosenberg L., Johnson B., Schubert M. Proteinpolysaccharides from human articular and costal cartilage. J Clin Invest. 1965 Oct;44(10):1647–1656. doi: 10.1172/JCI105271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg L., Schubert M. The proteinpolysaccharides of bovine nucleus pulposus. J Biol Chem. 1967 Oct 25;242(20):4691–4701. [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- WOESSNER J. F., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961 May;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]