Abstract
OBJECTIVES
To develop and validate the Dental Activities Test (DAT), a clinical tool for measuring dentally-related function in cognitively-impaired older adults.
DESIGN
Cross-sectional study design.
SETTING
Three assisted living (AL) residences in North Carolina.
PARTICIPANTS
90 AL residents ranging from normal to impaired cognition who were 50 years of age or older; not blind, deaf, or severely physically disabled; and English speaking.
MEASUREMENTS
Items for the DAT were developed based on focus group discussions, a literature review, and clinical relevance. Cronbach’s alpha, interrater reliability, and test-retest reliability were examined, and construct validity was assessed in relation to correlations with cognitive and functional assessments. Correlations between the DAT and oral health measures, were also analyzed to evaluate the concurrent validity of the DAT.
RESULTS
The DAT has excellent internal consistency reliability (Cronbach's alpha = 0.90), test-retest reliability (r=0.84) and inter-rater reliability (r=0.90). In terms of construct validity, higher scores on the DAT were significantly associated with better cognitive function, along with better physical and instrumental activities of daily living function. Finally, the DAT was significantly associated with oral hygiene and gingival health.
CONCLUSION
The DAT is a reliable and valid instrument to measure dentally-related function in older adults with cognitive impairment.
Keywords: Cognitive impairment, Functional assessment, Oral self-care function, Oral health
INTRODUCTION
Cognitive impairment, which affects more than one-third of Americans aged 71 and older,1, 2 is a major determinant of functional loss.3, 4 For many persons with cognitive impairment (PWCI), these losses also affect dentally-related function (DRF). For example, impaired prospective memory3 can disrupt daily oral and denture hygiene routines. Impaired executive function3 compromises oral self-care activities such as brushing and flossing. Anosognosia, language impairment and altered pain interpretation can compromise help seeking for pain and other oral health needs5. Difficulties consenting for treatment and following clinical and homecare instructions can affect clinical care3, 4, 6. Apraxia7 can compromise prosthetic treatment outcomes. Taken together, these impairments can lead to worse oral hygiene and a higher incidence of dental carries, periodontal disease, tooth loss, oral soft tissue pathology, and denture related problems compared to persons without impairment8–10, resulting in lower quality of life, malnutrition, increased insulin resistance, recurrent respiratory infections, delirium and other life-threatening conditions11–14.
Given the relationship between cognitive impairment and oral health, it is essential that DRF assessment be a standard part of geriatric dental care. Unfortunately, no objective, standardized approach is available. Existing cognitive (e.g., the Mini Mental State Examination [MMSE]15 and the Montreal Cognitive Assessment [MoCA]16) and functional (e.g., the Katz Index of Independence in Activities of Daily Living [ADL]17 and the Lawton Instrumental Activities of Daily Living [IADL] Scale18) assessment tools are not specific to DRF, nor are they designed for use in dental environments. As a result, dental professionals tend to rely on idiosyncratic, informal impressions rather than evidence-based approaches,19 raising concerns of suboptimal clinical outcomes and increased risk of avoidable complications.
In response, we have developed and validated the Dental Activities Test (DAT), an objective and reliable instrument for quickly assessing DRF among PWCI.
MATERIALS AND METHODS
Design Phase
The DAT was grounded in our work on the mediation effect of oral self-care function on the association between cognitive impairment and dental caries among dementia patients20 and a survey of the need for such a tool among the members of the Special Care Dentistry Association.19 Development was further informed by clinical observations and an extensive literature review, which identified five DRF domains essential to maintain oral health in PWCIs. These include the capacity to: (1) manage dentally-related medications (e.g., use analgesics), (2) comprehend and follow instructions (e.g., implement post-surgical instructions), (3) perform oral hygiene (e.g., brush teeth), (4) perceive and react to oral health problems (e.g., verbalize complaints), and (5) manage dentures. Examples of oral health related activities (OHRAs) were also identified for each domain (Online supplementary Table S1). Next, cognitive (e.g., executive function, prospective memory, language, praxis) and other factors (e.g., vision, eye-hand coordination, manual dexterity) that can contribute to these DRF domains were identified, and 17 candidate DAT items that originated from the OHRAs commonly performed in clinics or at home by PWCIs were also developed. Additional literature review and focus group discussion with geriatric dentists, dental hygienists, neurologists, geriatricians, occupation therapists and neuropsychologists were used to review and revise the DRF domains and candidate items. Two domains (oral hygiene and denture management) had sufficient overlap to combine into a single domain titled oral care capacity. Some candidate items were also eliminated. The prototype DAT included 9 items designed to measure the factors underlying four DRF domains. Items with similar underlying cognitive/physical factors were grouped together, not necessary reflecting the sequence of a particular OHRA.
Evaluation Phase
1. Expert review, cognitive testing and instrument revision
The prototype DAT was presented to eight dental experts including geriatric dentists, general dentists, and dental hygienists for feedback on clinical relevance, completeness and validity of domains, and anticipated barriers of use in dental/non-dental settings. Revisions were made to make the DAT suitable for use by non-dental professionals (e.g., dental jargon was removed).
We then pilot tested the DAT with five cognitively and functionally intact assisted living (AL) residents aged 65 and older. In addition to completing the DAT, participants were asked to interpret the instructions in their own words. Based on these results, the DAT items and instructions were re-worded to avoid confusions and reduce complexity.
2. Final instrument
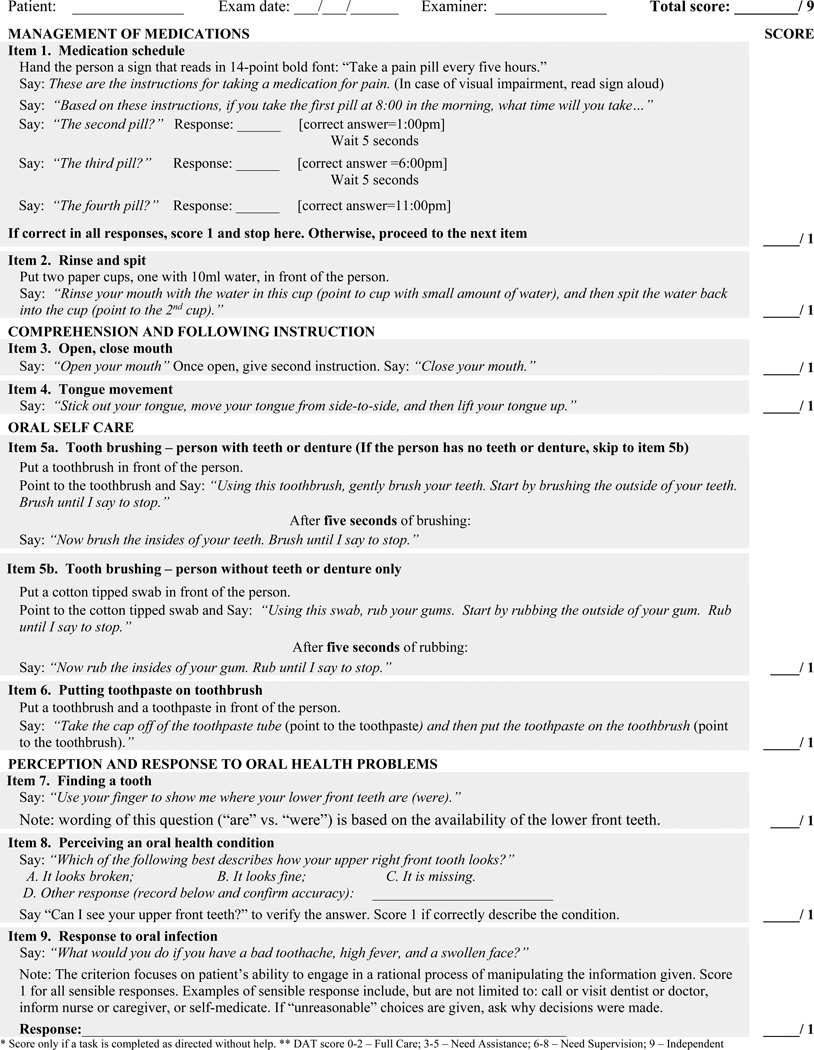
The final DAT is a 9-item assessment (Figure 1). One point is assigned for each activity completed exactly as directed and without help. The summative score (ranging from 0–9) reflects overall function in performing OHRAs, with a higher score indicating higher DRF.
Figure 1.
The Dental Activities Test
3. Psychometric testing
Measures
To assess construct validity, the DAT was compared to scores on standardized and widely used assessments of cognition (the Saint Louis University Mental Status [SLUMS]21 and the Minimum Data Set Cognition Scale [MDS-COGS]22) and function (the MDS Activities of Daily Living [MDS-ADL] scale23 and the IADL18). Concurrent validity was evaluated via the association between the DAT and residents’ oral hygiene status [Debris Index (DI) of the Oral Hygiene Index24, Gingival Index (GI)25 and Denture Plaque Index (DPI)26].
Sample
Study participants included 90 older adults with normal to impaired cognition, recruited from three AL communities in North Carolina. Sample size was determined based on measurement development literature27 that recommends at least 5–10 subjects per test item. Eligibility included: 50 years of age or older; not blind, deaf, or with a severe physical disability (e.g., hemiplegia); and English speaking. Individuals with an oral health condition that required antibiotic prophylaxis prior to dental treatment and/or an immediate dental referral were excluded. All procedures were approved by the University of North Carolina Institutional Review Board and the University of Iowa Institutional Review Board.
Data collection
Oral assessments were completed by a geriatric dentist (XC) and dental hygienist. Within one week, a research staff who had no dental background and was blind to the oral exam results visited the resident to complete the DAT and the SLUMS and interviewed staff to obtain information for the MDS-COGS, MDS-ADL and IADL.
Inter-rater Reliability Assessment
Forty-three subjects completed the DAT and SLUMS twice within three days by two different research staff, but at the same general time of day. These subjects were evenly divided among the three research sites and had a range of cognitive status.
Test-retest Reliability Assessment
Two weeks after the initial test, 43 residents different those involved in inter-rater reliability testing were re-administered the DAT. The same examiner who completed the first assessment completed the second assessment.
Throughout these assessments, the research and dental teams did not communicate about the real or perceived oral, cognitive or functional status of any residents.
Statistical Analysis
Means and frequencies were generated for descriptive statistics. Pearson correlations were calculated for inter-rater reliability, test-retest reliability, and construct validity associations. Cronbach’s alpha was used to assess internal consistency reliability, and analysis of variance was used to determine whether DAT scores differentiated residents on cognitive and functional measures. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated to identify a single item for screening.
RESULTS
Characteristics of the Study Subjects
Subjects mean age was 83.71 years old (SD=8.88, rang=53–102, Online supplementary Table S2). Seventy-nine percent were female, 68% were white and 30% were black. The mean MDS-COGS was 3.56 (SD=3.40, rang=0–10), with 37.4% cognitively intact or mildly impairment, 25.3% moderately impaired, 25.3% severely impaired, and 12.1% very severely impaired. The mean SLUMS was 8.21 (SD=7.81, rang=0–27), with 88.9% of the subjects showing dementia. Mean MDS-ADL and IADL scores were 11.4 (SD=9.6, range=0–32) and 4.8 (SD=3.0, range=0–12), respectively.
Reliability
The DAT has showed excellent internal consistency (α=0.90). Except for item-1 (understanding medication schedule), all items correlated well with the total scale. However, removal of this item increased alpha only by 0.008, and because it seems to address a component of DRF not clearly evident in the other items, it was retained in the final measure.
The DAT also showed excellent interrater (r=0.90) and test-retest reliability (r=0.84).
Construct and Concurrent Validity
Because OHRAs are associated with, but not exactly the same as, basic ADLs and IADLs, the DAT should significantly but less strongly correlate with these functional assessments. As expected, individuals who performed better on the DAT had lower MDS-ADL scores (which indicate better function; r= −0.52) and higher IADL scores (r=0.47; see Table 1). Stronger correlations were seen with the cognitive measures, where the correlation coefficients ranged from −0.78 with the MDS-COGS (lower scores are better) and 0.74 for the SLUMS, indicating that individuals with better cognitive function also performed better in the DAT exam.
Table 1.
Correlations among the Dental Activities Test (DAT) and cognitive, functional, and oral hygiene measures
| Measure | Pearson Correlation Coefficient |
P value | |
|---|---|---|---|
| Functional measures |
MDS-ADL*§ | −.52 | <.001 |
| IADL§ | .47 | <.001 | |
| Cognitive measures |
MDS-COGS*§ | −.78 | <.001 |
| SLUMS§ | 0.74 | <.001 | |
| Oral hygiene measures |
Debris Index* | −.32 | 0.01 |
| Gingival Index* | −.36 | <.01 | |
| Denture Plaque Index* |
−.19 | .37 |
Lower scores are favorable
MDS-ADL – Minimum Data Set Activities of Daily Living; IADL – Instrumental Activities of Daily Living, MDS-COGS – Minimum Data Set Cognition Scale; SLUMS – St Louis University Mental Status Exam;
DAT score was significantly but moderately correlated with the DI (r= −0.32, P=0.01) and GI scores (r=−0.36, P<0.01), indicating that individuals with better DAT performance had better oral hygiene and less severe gingival inflammation. However, the correlation between the DAT score and the DPI score was not significant (P=0.37).
DRF categories
The mean DAT score overall was 5.9 (SD 3.0; range 0–9). To understand the connection between DAT scores and differing DRF levels, we examined the distribution (mean, standard deviation and 95% confidence interval) of DAT scores within the levels of cognition and function. We then proposed four DRF level categories: those scoring 0–2 (full care), 3–5 (needs assistance), 6–8 (needs supervision), and 9 (independent, Table 2). Post hoc analysis indicated that cognitive (MDS-COGS and SLUMS) and functional (MDS-ADL and IADL) scores all significantly differed according to these categories (P<.001, Table 2). Significant differences with regard to cognitive and functional measures (especially the MDS-COGS and SLUMS) also were observed in most pairwise comparisons between the DRF categories (data not shown).
Table 2.
Categories differentiating levels of dentally-related function as measured by the Dental Activities Test (DAT)
| DRF level (Score) | ||||||
|---|---|---|---|---|---|---|
| Independent (9) |
Needs supervision (6–8) |
Needs assistance (3–5) |
Full care (0–2) |
Total | P value | |
| MDS-COGS* (Mean, SD) |
0.87 (1.41) | 2.12 (2.29) | 5.47 (2.76) | 8.00 (2.59) | 3.60 (3.40) |
<.001 |
| SLUMS (Mean, SD) |
18.80 (5.92) | 10.22 (5.79) | 1.53 (1.02) | 0.60 (1.18) | 8.21 (7.81) |
<.001 |
| IADL (Mean, SD) |
6.40 (3.54) | 5.39 (2.90) | 4.37 (2.41) | 1.93 (1.39) | 4.77 (3.04) |
<.001 |
| MDS-ADL* (Mean, SD) |
7.60 (9.05) | 8.07 (7.32) | 15.21 (10.05) | 19.93 (8.57) | 11.48 (9.58) |
<.001 |
Lower scores are favorable
MDS-ADL – Minimum Data Set Activities of Daily Living; IADL – Instrumental Activities of Daily Living, MDS-COGS – Minimum Data Set Cognition Scale. Score 0–1 represents cognitively intact – mild cognitive impairment; 2–4 represents mild-moderate impairment; 5–8 represents moderate-severe impairment and 9–10 represents severe-very severe impairment; SLUMS – St Louis University Mental Status Exam. Score 1–19 dementia; 20–24 mild cognitive impairment; 25–30 normal.
Identifying a Pre-Screen Item
To allow quick identification of patients who might benefit from the DAT, we examined items and item combinations to find one item for use as a pre-screen. The item relating to understanding a medication schedule (Item 1) demonstrated 97% sensitivity and 100% specificity, meaning failure in this item identified 97% of those whose full score indicated a need for supervision, assistance, or full care. It also demonstrated an excellent PPV (1.00) and NPV (0.88).
Administration time
The DAT took 4–15 minutes (mean=6, SD = 2). Eighty-one percent of subjects required 7 minutes or less. PWCIs took longer than those without, but the difference was not significant. The screening item took 30–40 seconds to complete for cognitively intact subjects and 1–2 minutes for those with impairment.
DISCUSSION
PWCIs can vary in their DRF in ways that do not necessarily parallel their cognitive status. Those with mild cognitive impairment can show significant decline in oral hygiene, indicating impaired oral self-care ability,28 while those with mild dementia do not.29 Reasons for this can include “overlearning” of oral self-care through lifelong practice. Geriatric dentists therefore need an easy-to-us tool for specifically evaluating DRF for PWCIs.
The DAT showed excellent internal consistency, interrater reliability, and test-retest reliability, as well as excellent construct and concurrent validity. Correlations with cognitive measures were strong, suggesting that the DAT performs well in assessing the cognitive deficits contributing to functional loss among PWCIs. Significant but less strong correlations with functional measures demonstrate that the oral health related dysfunction captured by the DAT is related to, but different than, general functional impairment. Additionally, the DAT is moderately associated with the DI and GI, but not with the DPI. This may be due to the small sample size of denture wearers (N=25) and other factors (e.g., denture designs, denture care skills and frequency, and level of caregiver support), but should be explored in future studies.
The excellent psychometric properties of the DAT enables dental professionals to reliably stage patients’ DRF and design functionally-tailored interventions to achieve desired oral health outcome. For mildly-impaired patients who are still able to learn new skills, home care interventions should target both patients and their caregivers, helping patients regain/maintain oral care function while teaching caregivers how to effectively supervise/assist patients in oral care. For moderately-impaired patients, oral care intervention should target more on caregivers to improve their oral caregiving skills and ability to work with PWCIs with behavior symptoms. Medication instructions should also be directed to caregivers. In-office fluoride/chlorhexidine varnish treatment can be considered to reduce the risk of mishandling of these medications. Due to an impaired ability to adapt to new dentures, it may be best to design treatment plans involving minimal changes to the existing dentures of these patients (e.g., relining dentures rather than remaking them when possible). For severely-impaired patients, especially for those with a short life expectancy, stage-appropriate palliative dental care should be considered.30 Besides oral caregiving skills, it is also important to teach caregivers how to recognize signs, symptoms, and behavior change indicating dental pain/infection for nonverbal patients.
When using the DAT, it is important to note three issues. First, the DAT was designed to measure global DRF via a summative score. Based on direct observation of a particular item (e.g., Rinse and Spit), one may obtain insights regarding a related OHRA (e.g., use of chlorhexidine oral rinse), but should not judge a particular DRF domain (e.g., medication management) based on the patient’s performance on a subset of items. Second, due to the complex intercorrelations among the cognitive and functional determinants of DRF function, it is difficult to specify the underlying cause(s) for any particular item. Finally, Item 1, understanding a medication schedule, can serve as a triage, which can be completed in 30–40 seconds in individuals with normal cognition. Failure suggests a high likelihood of impaired DRF and the full assessment is recommended. This highly-reliable, valid and easy-to-use pre-screen addresses dentists’ concerns regarding the use of a functional assessment in dental practices19.
In addition to the pre-screen item, several aspects of the DAT increase its attractiveness for clinicians in daily practice. The incorporation of OHRAs that are highly relevant to oral health and/or clinical care allows dental professionals with little training in cognitive and functional assessment to easily understand and administer the tool. The instructions were written in a way that allied dental professionals and individuals without a healthcare background can also easily administer the DAT with minimal training. Finally, all the materials required for assessment (e.g., paper cups, toothbrush and toothpaste) are readily available in dental offices, increasing the feasibility of this instrument to be used in daily practices.
Neverthless, several limitations exist in the present study. First, the DAT is a performance-based assessment that provides an objective, quantifiable measure of a patients’ capacity, not skill, in performing OHRAs. When interpreting DAT scores, one should bear in mind that a high score does not necessarily imply good quality oral care, especially in patients with lower oral health literacy, poor oral hygiene skills, and/or inadequate caregiver support. Therefore, DAT assessment must be considered in relation to a clinical oral assessment. Although direct observation of these activities may clarify discrepancies between self- or proxy-reports and what a person can actually do, the DAT may fail to capture the typical performance of this individual in his/her home where the familiar environment may serve as a cue. Further, the DAT cannot distinguish unmotivated from incapable persons. Finally, the sensitivity of this instrument in detecting and predicting functional loss over time has yet to be established.
In summary, the DAT appears to be a reliable, easy-to-use and objective tool to measure DRF, providing useful information for clinicians when designing functionally-tailored oral health interventions for individuals with cognitive impairment.
Supplementary Material
Test items corresponding to the dentally-related functional domains that are essential to maintain oral health in cognitively-impaired patients
Characteristics of study subjects (N=90)
Acknowledgments
The authors thank Drs. James D. Beck, Stephen K. Shuman and Daniel I. Kaufer and the research staff in the Cecil G. Sheps Center for Health Services Research at the University of North Carolina for their support on this project. We also thank the AL residents and staff who participate in the Collaborative Studies of Long-Term Care, for their time and effort to better understand resident needs and promote better care.
Xi Chen was partially supported by K23DE022470 from the National Institute of Dental and Craniofacial Research.
Sponsor’s Role: None.
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions: Chen, X: study concept and instrument development. Chen, X, Zimmerman, S., Potter G.G., Sloane, P.D. and Cohen, L.W.: study design and instrument revision. Chen, X. and Cohen, L.W.: data collection. Chen, X. and Reed, D.: data analysis and interpretation. Chen, X, Zimmerman, S., Potter G.G., Sloane, P.D., Cohen, L.W. and Reed, D.: preparation of manuscript.
Contributor Information
Xi Chen, Department of Preventive and Community Dentistry, University of Iowa, Iowa City, Iowa.
Sheryl Zimmerman, Cecil G. Sheps Center for Health Services Research and the School of Social Work, University of North Carolina, Chapel Hill, North Carolina.
Guy G Potter, Department of Psychiatry and Behavioral Sciences, Duke University, Durham, North Carolina.
Philip D Sloane, Cecil G. Sheps Center for Health Services Research and the Department of Family Medicine, University of North Carolina, Chapel Hill, North Carolina.
Lauren W Cohen, Duke Clinical Research Institute, Duke University School of Medicine, Durham, North Carolina.
David Reed, Cecil G. Sheps Center for Health Services Research, University of North Carolina, Chapel Hill, North Carolina.
REFERENCE
- 1.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148(6):427–434. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29(1–2):125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcotte T, Grant I. Neuropsychology of everyday functioning. 1st. Guilford Press; 2009. [Google Scholar]
- 4.Groome D. Introduction to Cognitive Psychology : Processes and disorders. 3rd. London, GBR: Psychology Press; 2013. [Google Scholar]
- 5.Hadjistavropoulos T, Herr K, Prkachin KM, et al. Pain assessment in elderly adults with dementia. Lancet Neurol. 2014;13(12):1216–1227. doi: 10.1016/S1474-4422(14)70103-6. [DOI] [PubMed] [Google Scholar]
- 6.Marson D, Harrell L. Executive dysfunction and loss of capacity to consent to medical treatment in patients with Alzheimer's disease. Semin Clin Neuropsychiatry. 1999;4(1):41–49. doi: 10.1053/SCNP00400041. [DOI] [PubMed] [Google Scholar]
- 7.Zadikoff C, Lang AE. Apraxia in movement disorders. Brain. 2005;128(Pt 7):1480–1497. doi: 10.1093/brain/awh560. [DOI] [PubMed] [Google Scholar]
- 8.Chalmers JM, Carter KD, Spencer AJ. Oral diseases and conditions in community-living older adults with and without dementia. Spec Care Dentist. 2003;23(1):7–17. doi: 10.1111/j.1754-4505.2003.tb00283.x. [DOI] [PubMed] [Google Scholar]
- 9.Chen X, Clark JJ, Naorungroj S. Oral health in nursing home residents with different cognitive statuses. Gerodontology. 2013;30(1):49–60. doi: 10.1111/j.1741-2358.2012.00644.x. [DOI] [PubMed] [Google Scholar]
- 10.Wu B, Fillenbaum GG, Plassman BL, Guo L. Association Between Oral Health and Cognitive Status: A Systematic Review. J Am Geriatr Soc. 2016;64(4):739–751. doi: 10.1111/jgs.14036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kandelman D, Petersen PE, Ueda H. Oral health, general health, and quality of life in older people. Spec Care Dentist. 2008;28(6):224–236. doi: 10.1111/j.1754-4505.2008.00045.x. [DOI] [PubMed] [Google Scholar]
- 12.Azarpazhooh A, Leake JL. Systematic review of the association between respiratory diseases and oral health. J Periodontol. 2006;77(9):1465–1482. doi: 10.1902/jop.2006.060010. [DOI] [PubMed] [Google Scholar]
- 13.Sadamori S, Hayashi S, Hamada T. The relationships between oral status, physical and mental health, nutritional status and diet type in elderly Japanese women with dementia. Gerodontology. 2008;25(4):205–209. doi: 10.1111/j.1741-2358.2008.00224.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee KH, Wu B, Plassman BL. Cognitive function and oral health-related quality of life in older adults. J Am Geriatr Soc. 2013;61(9):1602–1607. doi: 10.1111/jgs.12402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 16.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 17.Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist. 1970;10(1):20–30. doi: 10.1093/geront/10.1_part_1.20. [DOI] [PubMed] [Google Scholar]
- 18.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- 19.Chen X, Clark JJ. Assessment of dentally related functional competency for older adults with cognitive impairment--a survey for special-care dental professionals. Spec Care Dentist. 2013;33(2):48–55. doi: 10.1111/scd.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen X, Clark JJ, Chen H, Naorungroj S. Cognitive impairment, oral self-care function and dental caries severity in community-dwelling older adults. Gerodontology. 2015;32(1):53–61. doi: 10.1111/ger.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tariq SH, Tumosa N, Chibnall JT, Perry MH, 3rd, Morley JE. Comparison of the Saint Louis University mental status examination and the mini-mental state examination for detecting dementia and mild neurocognitive disorder--a pilot study. Am J Geriatr Psychiatry. 2006;14(11):900–910. doi: 10.1097/01.JGP.0000221510.33817.86. [DOI] [PubMed] [Google Scholar]
- 22.Hartmaier SL, Sloane PD, Guess HA, Koch GG. The MDS Cognition Scale: a valid instrument for identifying and staging nursing home residents with dementia using the minimum data set. J Am Geriatr Soc. 1994;42(11):1173–1179. doi: 10.1111/j.1532-5415.1994.tb06984.x. [DOI] [PubMed] [Google Scholar]
- 23.Morris JN, Fries BE, Morris SA. Scaling ADLs within the MDS. J Gerontol A Biol Sci Med Sci. 1999;54(11):M546–M553. doi: 10.1093/gerona/54.11.m546. [DOI] [PubMed] [Google Scholar]
- 24.Greene JC, Vermillion JR. a method for classifying oral hygiene status. J Am Dent Assoc. 1960;61(2):172–179. [Google Scholar]
- 25.Loe H. The Gingival Index, the Plaque Index and the Retention Index Systems. J Periodontol. 1967;38(6 Suppl):610–616. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 26.Augsburger RH, Elahi JM. Evaluation of seven proprietary denture cleansers. J Prosthet Dent. 1982;47(4):356–359. doi: 10.1016/s0022-3913(82)80079-6. [DOI] [PubMed] [Google Scholar]
- 27.Boomsma A, Hoyle R, Panter A. The structural equation modeling research report. In: Hoyle R, editor. Handbook of Structural Equation Modeling. 1st. New York: Guilford Press; 2012. [Google Scholar]
- 28.Lee KH, Plassman BL, Pan W, Wu B. Mediation Effect of Oral Hygiene on the Relationship Between Cognitive Function and Oral Health in Older Adults. J Gerontol Nurs. 2016;42(5):30–37. doi: 10.3928/00989134-20151218-03. [DOI] [PubMed] [Google Scholar]
- 29.Associaiton AP. Diagnostic and statistical manual of mental disorders (DSM-V) [Accessed 01/05 2016]; " http://dsm.psychiatryonline.org/doi/full/10.1176/appi.books.9780890425596.dsm17#CIHJBAIG". [Google Scholar]
- 30.Chen X, Kistler CE. Oral health care for older adults with serious illness: when and how? J Am Geriatr Soc. 2015;63(2):375–378. doi: 10.1111/jgs.13240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Test items corresponding to the dentally-related functional domains that are essential to maintain oral health in cognitively-impaired patients
Characteristics of study subjects (N=90)