Abstract
Lipoarabinomannan (LAM) is a lipoglycan found in abundant quantities in the cell envelope of all mycobacteria. The non-reducing arabinan termini of LAM display species-specific structural micro-heterogeneity that impacts the biological activity of the entire molecule. Mycobacterium tuberculosis, for instance, produces mannoside caps made of one to three α-(1→2)-Manp-linked residues that may be further substituted with an α-(1→4)-linked methylthio-d-xylose (MTX) residue. While the biological functions and catalytic steps leading to the formation of the mannoside caps of M. tuberculosis LAM have been well established, the biosynthetic origin and biological relevance of the MTX motif remain elusive. We here report on the discovery of a five-gene cluster dedicated to the biosynthesis of the MTX capping motif of M. tuberculosis LAM, and on the functional characterization of two glycosyltransferases, MtxS and MtxT, responsible, respectively, for the production of decaprenyl-phospho-MTX (DP-MTX) and the transfer of MTX from DP-MTX to the mannoside caps of LAM. Collectively, our NMR spectroscopic and mass spectrometric analyses of mtxS and mtxT overexpressors and knock-out mutants support a biosynthetic model wherein the conversion of 5´-methylthioadenosine, which is an ubiquitous byproduct of spermidine biosynthesis, into 5´-methylthioribose-1-phosphate precedes the formation of a 5´-methylthioribose nucleotide sugar, followed by the epimerization at C-3 of the ribose residue, and the transfer of MTX from the nucleotide sugar to decaprenyl-phosphate yielding the substrate for transfer onto LAM. The conservation of the MTX biosynthetic genes in a number of Actinomycetes suggests that this discrete glycosyl substituent may be more widespread in prokaryotes than originally thought.
Keywords: Mycobacterium, tuberculosis, lipoarabinomannan, methylthioxylose
INTRODUCTION
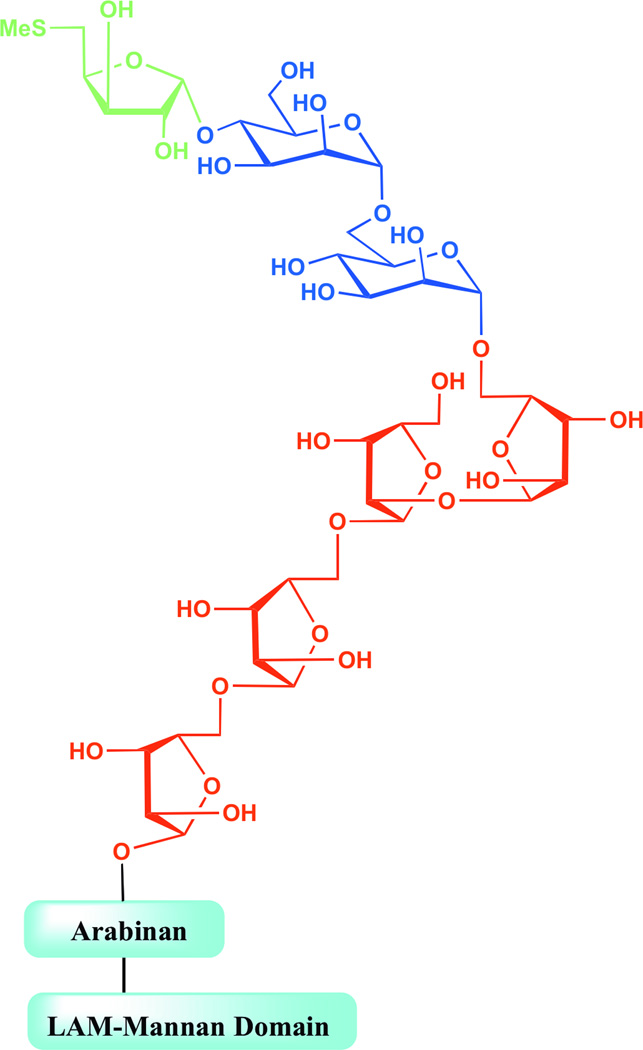
The mycobacterial cell envelope is characterized by its high content of mannosylated molecules. The mannosyl-phosphatidyl-myo-inositol-based glycolipids (PIMs) and metabolically related lipoglycans comprising lipomannan (LM) and lipoarabinomannan (LAM), in particular, are found in abundant quantities in the inner and outer membranes of all mycobacterial species (1–2). The most common forms of LM and LAM share a linear α-(1→6)-linked mannan backbone made up on average of 20–25 mannopyranose (Manp) residues occasionally substituted at C-2 by single Manp units (3). The major LAM glycoforms contain about 110 glycosyl residues (approximately 60 Araf and 50 Manp units) and consist of a single d-arabinan chain attached to the α-(1→6) d-mannan backbone (4). The arabinan polymer contains about 60 Araf units and consists of a linear α-(1→5)-linked Araf backbone punctuated with branched hexaarabinofuranosides (Ara6) and linear tetraarabinofuranosides (Ara4) (5). Succinate or lactate residues may substitute the C-2 position of some of the α-(3→5)-Araf interior residues of the arabinan (6–7). Importantly, the non-reducing arabinan termini of LAM display considerable species-specific structural micro-heterogeneity that is key to the biological activity of the entire molecule (8–11). M. tuberculosis and other pathogenic slow-growing mycobacteria (M. bovis, M. leprae, M. avium, M. xenopi, M. marinum and M. kansasii), for instance, produce mannoside caps made of one to three α-(1→2)-Manp-linked residues giving rise to mannosylated LAM (referred to as ManLAM). Intriguingly, the mannoside caps of ManLAM of all M. tuberculosis isolates analyzed to date may be further substituted with an α-(1→4)-linked methylthio-d-xylose (MTX) residue [Fig. 1] (12–16). The same MTX motif apparently substitutes the ManLAM of M. avium and M. kansasii although, in the latter species, its attachment is to the mannan backbone rather than to the non-reducing termini of the arabinan domain (8,15,17). MTX is a discrete motif that occurs at the level of one molecule per entire molecule of ManLAM in M. tuberculosis (or one molecule for every five to six mannose caps) (12,18). It is unusual in that it is one of the few reports of a xylo-configured sugar outside the plant kingdom and the first report of a thiosugar in a bacterial polysaccharide (16). The biosynthetic origin of this motif is unknown although models have been proposed (12,14,16).
Figure 1. Representation of the non-reducing arabinan termini of M. tuberculosis ManLAM.
The conservation of the MTX motif of ManLAM in M. tuberculosis and other pathogenic mycobacteria and its exposure on the cell surface suggest that it may play a beneficial role to the bacterium in host-pathogen interactions. Accordingly, in vitro studies indicate that disaccharide mimetics of the MTX capping motif of ManLAM downregulate cytokine production by activated human macrophages in culture (15) and that the MTX motif of purified ManLAM may account for the anti-oxidative properties of the lipoglycan (18–19). To what extent these properties impact the interactions of intact M. tuberculosis bacilli with phagocytic cells and the immunopathogenesis of tuberculosis (TB) remains to be determined.
With the goals of elucidating the biosynthetic pathway leading to the formation of the MTX motif of ManLAM and of generating isogenic MTX-deficient M. tuberculosis mutants that subsequently could be used to investigate the biological relevance of this motif in TB infection, a combination of genetic and (bio)chemical approaches was here used to identify the enzymes responsible for the synthesis and transfer of the MTX motif to the t-Manp residue of the mannoside caps of ManLAM.
RESULTS AND DISCUSSION
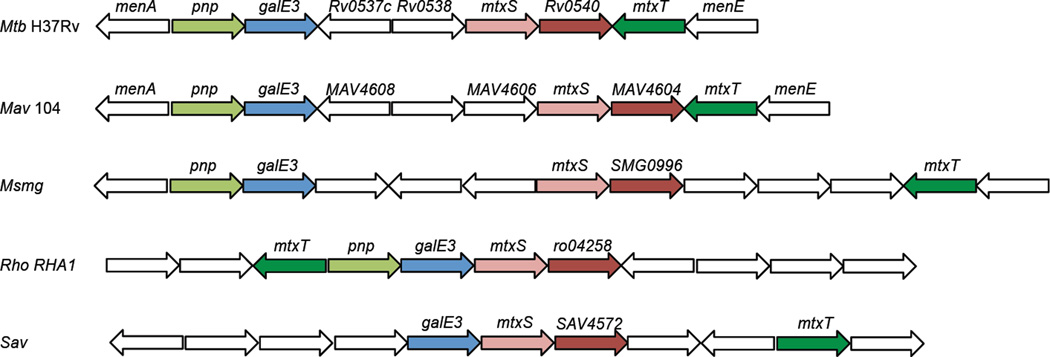
Identification of a gene cluster potentially involved in the biosynthesis of the MTX substituent of ManLAM in M. tuberculosis
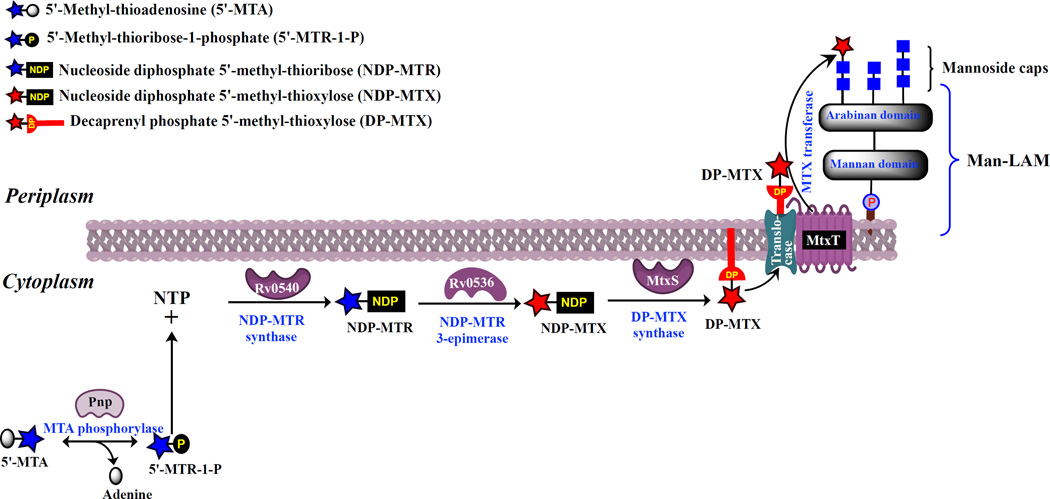
It has been postulated that the MTX motif was likely to be derived biosynthetically from 5´-methylthioadenosine (MTA), which is a ubiquitous byproduct of spermidine biosynthesis (12,15–16). MTA is toxic to the cells and is therefore rapidly converted to adenine and 5´-methylthioribose-1-phosphate, which may be used in the purine and methionine salvage pathways, respectively. Whereas this conversion is catalyzed by an MTA phosphorylase (MTAP, E.C 2.4.2.28) in Eukarya, bacteria rely on the coupled action of an MTA nucleosidase (MTAN, E.C 3.2.2.16) and a kinase. Mycobacterium smegmatis and M. tuberculosis are unusual in apparently being endowed with both MTAN and MTAP enzymes (20–21). Intriguingly, Rv0535 (pnp) which was enzymatically established to encode an MTA phosphorylase in M. tuberculosis (21), lies in the genomes of the MTX-producing species M. tuberculosis and M. avium in close vicinity to ORFs potentially encoding an NAD-dependent nucleotide sugar epimerase (Rv0536), two glycosyltransferases (Rv0539 and Rv0541c) and a protein with structural similarity to a wide range of enzymes that transfer nucleotides onto phosphosugars (Rv0540) [Fig. 2]. Rv0539 (hereafter referred to as MtxS; 210 amino acid residues) is predicted to be an inverting GT-2 glycosyltransferase and therefore shares significant sequence similarities with polyprenyl-monophosphosugar synthases from M. tuberculosis, including the polyprenyl-monophosphomannose synthase Ppm1 (Rv2051c; 33% identity and 49% similarity on a 214 amino acid overlap) and the polyprenyl-monophospho-N-acetylgalactosaminyl synthase PpgS (Rv3631; 33% identity and 47% similarity on a 115 amino acid overlap). Rv0541c (hereafter referred to as MtxT) is predicted to be a GT-C superfamily glycosyltransferase dependent on decaprenyl-phosphate-linked sugar donors rather than nucleotide-sugar donors (22). pnp and Rv0536 are likely to be co-transcribed as would be mtxS and Rv0540. This cluster of genes was not found in M. leprae, a species known to be devoid of the MTX motif on ManLAM (23). However, the five genes are conserved in the genomes of M. kansasii, M. smegmatis, many other slow- and fast-growing Mycobacterium species (M. avium complex species, M. vanbaalenii, M. marinum, M. ulcerans, etc.), as well as a number of other Actinomycetes, particularly Rhodococcus and Streptomyces [Fig. 2]. The conservation, syntenic arrangement and sequence similarities of these ORFs to genes involved in the detoxification of MTA and the further processing of sugar residues was suggestive of their participation in the formation of the MTX capping motif of ManLAM. Based on what is known of the biogenesis of polysaccharides in mycobacteria in general (3), and that of the mannoside caps of ManLAM and galactosamine substituent of arabinogalactan in particular (24–26), it is indeed reasonable to assume that the formation of the MTX motif of ManLAM is topologically split across the plasma membrane and proceeds through a three-step mechanism as shown in the proposed biosynthetic model presented in Figure 3. The initial cytoplasmic enzymatic reactions would lead to the formation of a 5´-methylthioribose (MTR) nucleotide sugar followed by that of decaprenyl-phosphoryl-MTX (DP-MTX). DP-MTX would then be translocated from the cytosolic to the periplasmic face of the plasma membrane by a dedicated translocase, and the MTX motif finally transferred from DP-MTX to the t-Manp residue of the mannoside caps of ManLAM by a lipid-linked sugar-dependent glycosytransferase (3,22). Although the epimerization at C-3 of the ribose residue leading to the xylo-configured sugar donor may occur at different stages of the pathway, i.e., directly on MTA as has been reported in the nudibranch mollusk Doris verrucosa (27), at the level of 5´-methylthioribose-1-phosphate or at that of the nucleotide- or decaprenyl-phosphate-linked sugar donors, the sequence similarity that Rv0536 shares with nucleotide sugar epimerases suggests that the reactions leading to DP-MTX from MTA follow the sequential order presented in Fig. 3.
Figure 2. Representation of the MTX gene cluster in M. tuberculosis and other Actinomycetes.
Mtb H37Rv, Mycobacterium tuberculosis strain H37Rv; Mav 104, Mycobacterium avium strain 104; Msmg, Mycobacterium smegmatis mc2155; Rho RHA1, Rhodococcus sp. RHA1; Sav, Streptomyces avermitilis MA-4680. Similarly colored genes are orthologs. White genes are thought to be unrelated to the MTX biosynthetic pathway. Genes are not drawn to scale. Pnp, MTA phosphorylase; GalE3, putative NAD-dependent 5´-methylthioribose-nucleotide-3-epimerase; MtxS, decaprenyl-phosphoryl-MTX synthase; Rv0540 (and orthologs, MAV4604, SMG0996, ro04258, SAV4572), nucleotide-5´-methylthioribose synthase; MtxT, MTX-transferase. The pnp gene of S. avermitilis (SAV3679) is found in a different region of the genome. Rv0537c and MAV4608 display sequence similarities with the 5´-methylthioribose-1-phosphate isomerase from Bacillus licheniformis and are likely to be involved in the methionine salvage pathway. Rv0538 encodes a conserved membrane protein; orthologs of this gene are lacking in the MTX clusters of M. avium, M. smegmatis, S. avermitilis and Rhodococcus RHA1.
Figure 3. Proposed biosynthetic pathway for the formation of the MTX motif of ManLAM in M. tuberculosis.
The proposed catalytic steps leading to the synthesis of the MTX motif and its transfer onto the mannoside caps of M. tuberculosis ManLAM are represented. The identity of the translocase involved in the translocation of DP-MTX from the cytoplasmic to the periplasmic side of the inner membrane and the precise nature of the nucleotide-MTX donor used by MtxS in the formation of DP-MTX are not known.
The genetic inactivation of mtxS and mtxT abolishes the synthesis of the MTX motif of ManLAM in M. tuberculosis
To investigate the putative involvement of the glycosyltransferases MtxS and MtxT in the formation of the MTX motif of ManLAM, the genes encoding these enzymes were disrupted by allelic replacement in M. tuberculosis H37Rv [Fig. 4]. The two mutants displayed wild-type (WT) morphotypes and grew similarly to their M. tuberculosis H37Rv parent strain on agar plates (data not shown). Genetic complementation was achieved by transforming the mtxT knock-out mutant with pMVGH1mtxT, and the mtxS mutant with both pMVGH1mtxS and pMVGH1[mtxS-Rv0540] since the operon-like structure of mtxS-Rv0540 raised concerns of a polar effect of the inactivation of mtxS on the expression of Rv0540.
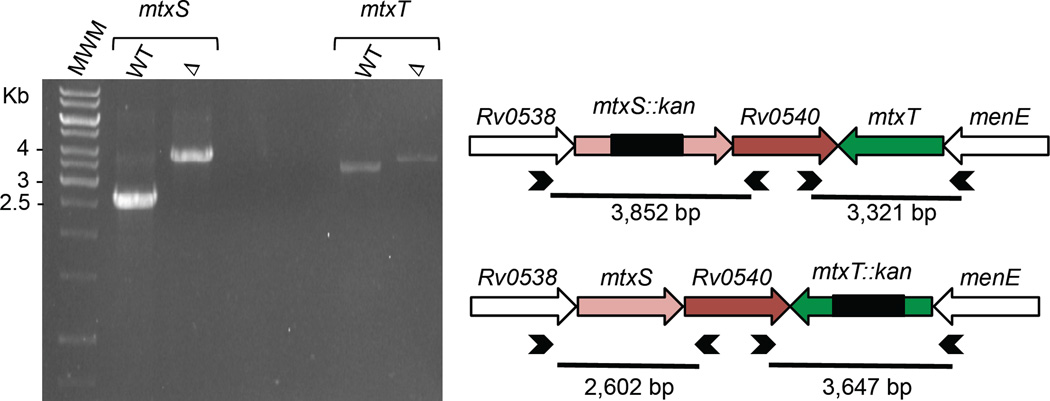
Figure 4. Allelic replacement at the mtxS and mtxT glycosyltransferase loci of M. tuberculosis H37Rv.
Allelic replacement at the mtxS and mtxT loci of M. tuberculosis H37Rv was confirmed by PCR using sets of primers (represented as arrowheads) located outside the allelic exchange substrates. The WT 2,602 bp mtxS fragment is replaced by a 3,852-bp fragment in the mtxS knock-out mutant due to the insertion of a 1.2 kb-kanamycin resistance cassette at the unique NcoI restriction site of mtxS. The WT 3,321 bp mtxT fragment is replaced by a 3,647-bp fragment in the mtxT knock-out mutant due to a 1.2 kb-kanamycin resistance cassette replacing 924-bp of the mtxT gene flanked between two BlpI restriction sites in mtxT.
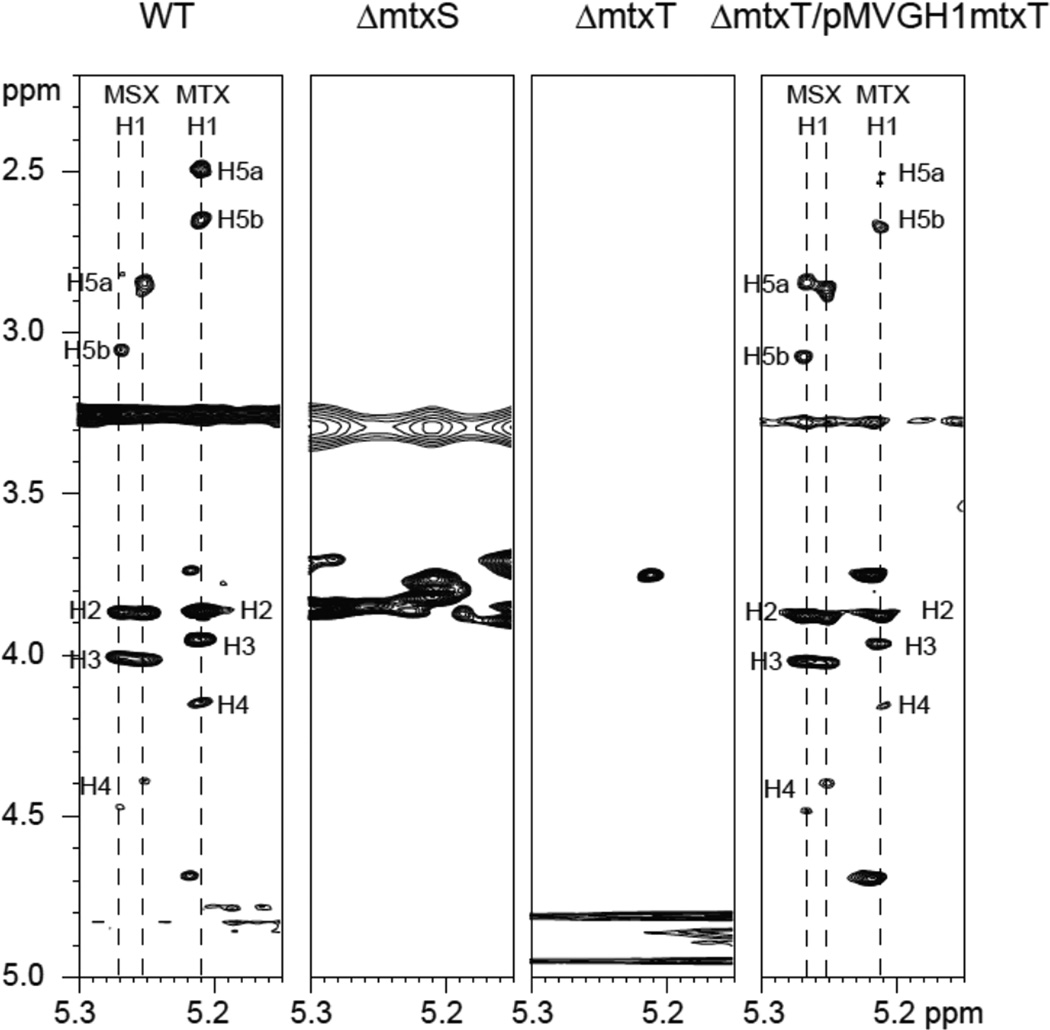
ManLAM was prepared from the WT, mutant and complemented mutant strains and analyzed for the presence of MTX motif by NMR spectroscopy (12–13). H-1 signals corresponding to 5´-methylthiopentose at δ 5.21 and corresponding oxidation products (5´-methylsulfoxypentose) at δ 5.25 and δ 5.27 were observed in the anomeric region of the 1-D 1H NMR spectrum of the WT strain. The corresponding complete spin systems were further identified in the TOCSY spectrum [Fig. 5]. Indicative of the involvement of MtxS and MtxT in the synthesis of the MTX motif of ManLAM, these correlations were not observed in the mtxS and mtxT knock-out mutants [Fig. 5]. They were, however, restored in the complemented mtxT mutant [Fig. 5]. Perhaps due to the insufficient level of expression of mtxS and mtxS-Rv0540 from the pMVGH1 plasmid in M. tuberculosis, attempts to complement H37RvΔmtxS with either one of these plasmids have thus far failed to restore the synthesis of the MTX motif of ManLAM to detectable levels in this mutant [Supplementary Figure 1].
Figure 5. Expanded region of the 2D 1H-1H TOCSY spectrum in DMSO-d6 at 315 K of ManLAM purified from M. tuberculosis H37Rv WT, the mtxS and mtxT knock-out mutants and the complemented mtxT mutant strain.
Coupling systems corresponding to 5´-methylsulfoxypentose and 5´-methylthiopentose (labeled as MSX and MTX, respectively) are presented. Numerals correspond to the proton number of the 5´-methylthiopentose and the 5´-methylsulfoxypentose units.
Effect of inactivating mtxT on the DP-MTX content of M. tuberculosis cells
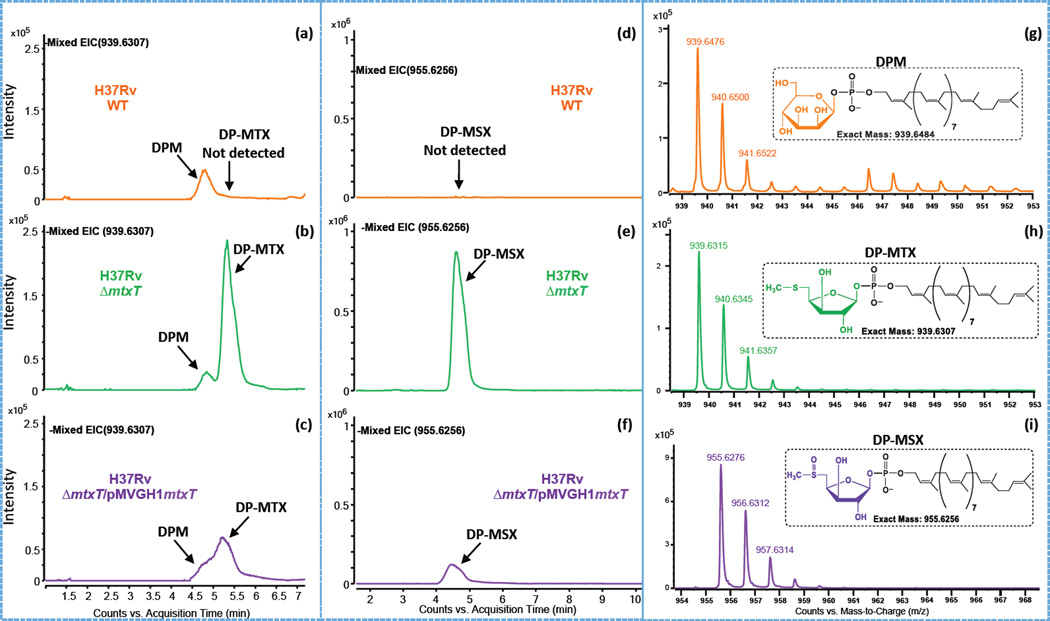
To further investigate the reason for the absence of MTX motif on the ManLAM of the M. tuberculosis mtxS and mtxT mutants, we next sought to determine whether these two genes were involved in the synthesis or utilization of DP-MTX, the likely sugar donor used in the periplasmic transfer of MTX onto the mannoside caps of ManLAM. To this end, an LC/MS method allowing for the detection of DP-MTX in intact mycobacterial cells was first developed and applied to the analysis of the M. tuberculosis H37Rv WT, mutant and complemented mutant strains. Figures 6a–i show the extracted ion chromatograms and mass spectra for the [M-H]− ion of various decaprenyl-phosphoryl-sugars. The analysis was complicated by the fact that DP-MTX and decaprenyl-phosphoryl-mannose (DPM) have almost the same molecular weight (m/z 939.6307 for DP-MTX and m/z 939.6484 for DPM). Thus, both species appear on the extracted ion chromatographs. DP-MTX and DPM, however, separate by HPLC [Fig. 6b] and their difference in mass can be seen on the mass spectra [Fig. 6g and 6h]. The presence of a deprotonated ion typifying DP-MTX could not be detected in the M. tuberculosis WT parent strain [Fig. 6a], the mtxS knock-out mutant or the mtxS knock-out complemented with mtxS-Rv0540 [Supplementary Figure 2]. In sharp contrast, the mtxT knock-out mutant showed a clear accumulation of DP-MTX [Fig. 6b] and an oxidized form of DP-MTX, decaprenyl-phosphoryl-methylsulfoxyxylose (referred to as DP-MSX), with a deprotonated ion at m/z 955.6256 [Fig. 6e]. The build-up of DP-MTX and DP-MSX in H37RvΔmtxT was partially reverted upon complementation with pMVGH1mtxT [Fig. 6c and 6f]. In line with the biosynthetic model proposed in Figure 3, the results of these analyses thus support the hypothesis that DP-MTX serves as a substrate for MtxT in the transfer of MTX to ManLAM.
Figure 6. Negative ion LC/MS of decaprenyl-phosphoryl-sugars from total lipids extracts of M. tuberculosis H37Rv WT, the mtxT knock-out mutant and the complemented mtxT mutant strain.
Panels (a–c) are the extracted ion chromatograms (EICs) of m/z 939.6307 showing the presence of DPM (actual [M-H]− at mass m/z 939.6484; see text for details) and the presence or absence of DP-MTX in the different M. tuberculosis strains. The detection of DP-MSX at m/z 955.6256 as [M-H]− ions are shown in the EICs presented in panels d–f. The insets in the mass spectra shown in panels (g–i) show the possible structures and the exact mass of the [M-H]− ions of the decaprenyl-phosphoryl sugars detected.
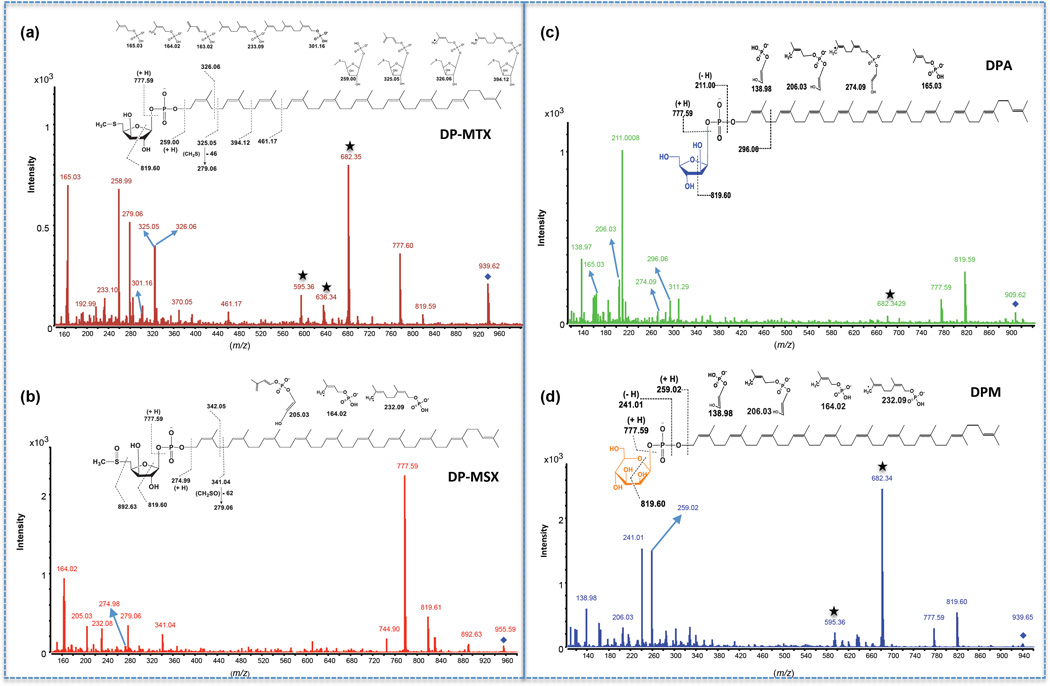
To confirm the structures of DP-MTX and DP-MSX, their corresponding precursor ions at m/z 939.6307 and m/z 955.6256 were selected and analyzed by LC-MS/MS in the negative mode with a collision energy of 65 V. Figures 7a and 7b display the mass spectra of fragment ions obtained for DP-MTX and DP-MSX and, for comparative purposes, Fig. 7c and 7d display the fragmentation patterns of decaprenyl-phosphoryl-arabinose (DPA) and DPM, respectively. All decaprenyl-phosphoryl-sugar spectra present characteristic fragment ions at m/z 777.59 and 819.60 (28–29). The fragment ion at m/z 777.59 arises from the cleavage of the glycosidic bond as shown in Fig. 7a–d. Similarly, the fragment ion at m/z 819.60 corresponds to the cross-ring cleavage of their glycosyl residues between the ring oxygen and C-1 and between C-2 and C-3 as shown in Fig. 7a–d. The finding of these two ions in DP-MTX and DP-MSX [Fig. 7a–b] is thus consistent with these products being decaprenyl-phosphoryl-sugars. The resulting decaprenyl-phosphate ion at m/z 777.59 (DP-MTX) further fragments and yields a sequential loss of isoprene units with m/z 165.03, 233.10, 301.16. In a similar fashion, two fragment ions separated by 68 mass units containing isoprene phosphate at m/z 164.02 and 232.08 were present on the DP-MSX spectrum. Most importantly, the cleavage between the oxygen of the phosphate group and the polyisoprene unit (with a hydrogen transfer) shows the molecular weight of the glycosyl residue, as seen at m/z 258.99 for DP-MTX and at m/z 274.99 for DP-MSX. Fragments containing the glycosyl residue for DP-MTX are further seen at m/z 325.05, 325.06, 394.12 and 461.18 [Fig. 7a], and at m/z 341.04 and 342.05 for DP-MSX [Fig. 7b]. Additionally, ions typifying the loss of the sulfur substituent are seen for both DP-MTX and DP-MSX (diagnostic ions at m/z 279.06) [Fig. 7a–b]. The MS/MS results are therefore consistent with the proposed structures.
Figure 7. LC-MS/MS analysis of decaprenyl-phosphoryl sugars from total lipids extracts of the M. tuberculosis H37Rv mtxT knock-out mutant.
MS/MS spectra and proposed fragmentation patterns of DP-MTX (a), DP-MSX (b), DPA (c) and DPM (d). The fragmented precursor [M-H]− ions are identified with a diamond. The details of the cleavages are discussed in the text. Ions labeled with stars come from the fragmentation of a contaminant ion with a mass close to the m/z value of 939.6307. For DP-MTX and DPM, this ion is at m/z 939.4671.
In further support of the identity of DP-MTX and DP-MSX with formula C56H93O7PS and C56H93O8PS, respectively, is the isotopic distribution of the [M-H]− ion observed in the ESI-TOF mass spectra. Compounds containing sulfur exhibit a characteristic isotopic distribution with a greater relative intensity at the M+2 ion than compounds without S, due to the relatively high natural abundance of 34S (4.2 %) (30). The modeled isotopic distribution for C56H92O7PS ([M-H]−) predicts the M+2 ion at m/z 941.6353 at a relative intensity of 25.2 %, whereas the modeled isotopic distribution of DPM, with formula C56H92O9P ([M-H]−) predicts the relative intensity of the M+2 ion at 20.7 %. The difference of 4.5 % is explained by the combined number of molecules containing 34S instead of 32S, plus minor contributions from 18O, 2H and 13C2. Similarly, the predicted relative intensity of the M+2 ion for DP-MSX ([M-H]−) at m/z 957.6302 is 25.5 %. The good fit of the formula C56H92O7PS for DP-MTX ([M-H]−) and C56H92O8PS for DP-MSX ([M-H]−) with expected and observed isotopic distributions is illustrated with the overlaid modeled and observed data in the mass spectra [Supplementary Figure 3].
Effect of overexpressing mtxS on the DP-MTX content of M. smegmatis
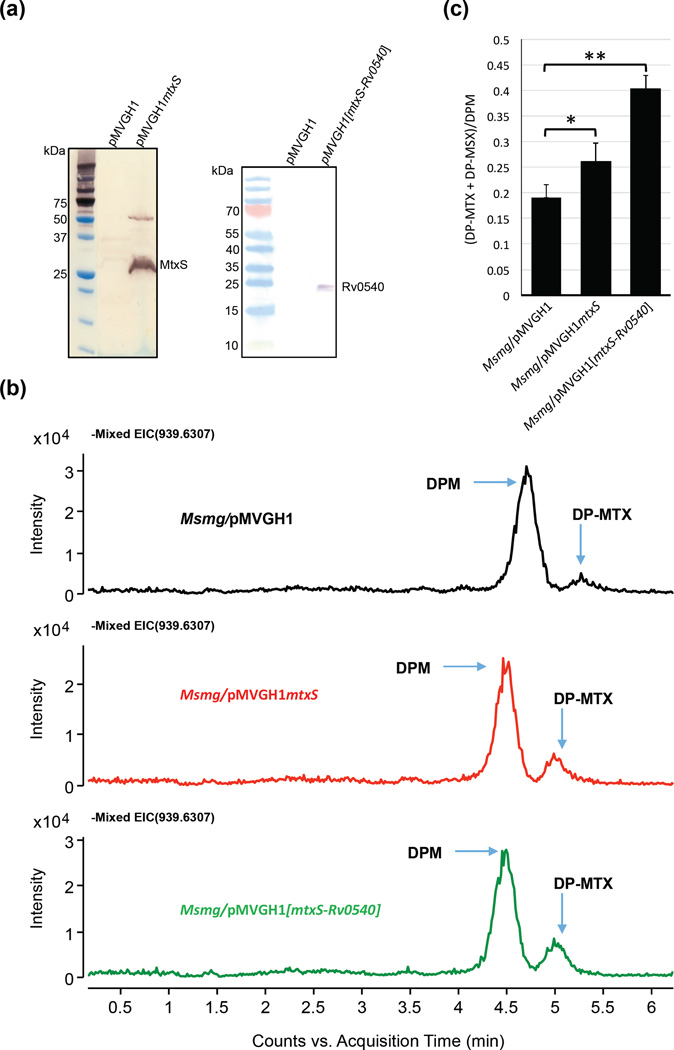
The absence of detectable amounts of DP-MTX in M. tuberculosis H37Rv WT making a direct comparison of the DP-MTX content of the WT and mtxS knock-out uninformative [Supplementary Figure 2], we set out to overexpress mtxS to gain further insights into the function of this gene. M. smegmatis rather than M. tuberculosis was used as a model organism in these experiments because greater levels of expression of mtxS were achieved in this species [Fig. 8a] and the presence of DP-MTX in WT M. smegmatis cells [Fig. 8b] indicated that the four genes required for the synthesis of DP-MTX from MTA [Fig. 2 and Fig. 3] are functional in this species. Overexpression of the M. tuberculosis mtxS gene from pMVGH1mtxS in M. smegmatis consistently resulted in a 1.4-fold increase in the production of DP-MTX in the cells [Fig. 8b–c]. This effect was even more pronounced in an M. smegmatis recombinant strain co-expressing mtxS and the putative 5´-methylthioribose-1-phosphate nucleotidyl transferase gene (Rv0540) from pMVGH1[mtxS-Rv0540] indicating that the formation of NDP-MTR may be a rate-limiting step in the pathway. The amount of DP-MTX and DP-MSX detected in the membranes of this overexpressor was about 2.1-fold that measured in the membranes of the M. smegmatis control strain, Msmg/pMVGH1 [Fig. 8b–c]. Collectively, the build-up of DP-MTX in M. smegmatis strains overexpressing mtxS, the sequence resemblance that MtxS shares with other polyprenyl-monophosphosugar synthases from M. tuberculosis, and the fact that disrupting mtxS abolishes the formation of the MTX motif of ManLAM strongly support MtxS as the DP-MTX synthase of the pathway.
Figure 8. Effect of overexpressing mtxS and mtxS-Rv0540 on DP-MTX synthesis in M. smegmatis.
(a) Immunoblot analysis of MtxS and Rv0540 produced in M. smegmatis. M. smegmatis protein extracts prepared from mtxS and mtxS-Rv0540 overexpressing strains and a control strain carrying an empty pMVGH1 plasmid were separated by SDS-PAGE, blotted onto a nitrocellulose membrane, and the recombinant proteins (harboring an C-terminal His-tag) were detected using a monoclonal anti-polyhistidine monoclonal antibody (mouse Ig2a isotype; Sigma) as the first antibody and an anti-mouse IgG-alkaline phosphatase-conjugated antibody as the secondary antibody. Immune complexes were detected by monitoring alkaline phosphatase activity using NBT/BCIP (Thermo Scientific). The expected size of the recombinant Rv0540 protein is about 22.9 kDa; that of the recombinant MtxS protein is about 22.4 kDa; this protein consistently migrates with an apparent higher molecular weight, possibly due to its association with the membrane. A possible dimer of MtxS is also seen around 50 kDa.
(b) Negative ion LC/MS of DPM and DP-MTX from total lipids extracts of the M. smegmatis control strain (Msmg/pMVGH1) and the mtxS and mtxS-Rv0540 overexpressors. Decaprenyl-phosphoryl sugars were analyzed as described in Fig. 6.
(c) Relative amounts of DP-MTX and its sulfoxide form, DP-MSX, in the membranes of the M. smegmatis control strain, Msmg/pMVGH1, the mtxS overexpressor and the mtxS-Rv0540 overexpressor. The amounts of DP-MTX and DP-MSX relative to DPM were determined and the averages and standard deviations of two independent membrane preparations for each strain are shown. Statistical comparison (Student’s t-test) between control and overexpressing strains: ** p < 0.010; * p < 0.05.
CONCLUSIONS
Altogether, our genetic and biochemical data validate the hypothesis that the MTX motif of M. tuberculosis ManLAM arises from a byproduct of spermidine biosynthesis and indicate that the formation and transfer of this motif proceeds through the overall biosynthetic scheme depicted in Fig. 3. The processes involved in the covalent modification of ManLAM with this unique thiosugar bear obvious resemblance to that responsible for the formation of the galactosaminyl substituent of arabinogalactan wherein a polyprenyl-phospho-N-acetyl galactosamine synthase encoded by PpgS (homologous to MtxS) generates the lipid-linked sugar donor used by Rv3779 (homologous to MtxT) for transfer onto arabinogalactan on the periplasmic side of the plasma membrane (26). Despite these significant advances in our understanding of the origin of the MTX motif, a number of outstanding questions about its synthesis and transfer to ManLAM remain. Among them is the identity of the translocase involved in the translocation of DP-MTX from the cytoplasmic to the periplasmic side of the inner membrane and the precise nature of the nucleotide-MTX donor used by MtxS in the formation of DP-MTX, whether UDP-MTX, GDP-MTX or otherwise.
The conservation of the core five genes of the pathway (pnp, Rv0536, mtxS, Rv0540 and mtxT) in slow- and fast-growing Mycobacterium species, including M. smegmatis mc2155 in which no MTX motif has yet been reported, as well as a number of other Actinomycetes suggests that the MTX motif, may be more widespread than previously appreciated. Whether the MTX motif may substitute other molecules than LM- and LAM-like polysaccharides, including glycoproteins and secondary metabolites, remains to be determined. This finding also brings into question the putative biological significance of this motif. The important impact that discrete glycosyl substituents have on the biological activities of bacterial lipopolysaccharides and teichoic acids, and the interactions of bacterial pathogens with their host is well documented (31–33). The restricted distribution of the MTX motif of ManLAM to pathogenic Mycobacterium species, its conservation in all M. tuberculosis isolates analyzed to date and exposure on the cell surface all point to an important role in immunopathogenesis. The MTX-deficient M. tuberculosis mutants generated in the course of this work open the way to host-pathogen interaction studies aimed at testing this hypothesis.
METHODS
Bacterial strains and growth conditions
M. tuberculosis H37Rv ATCC 25618 was grown at 37°C in Glycerol-Alanine-Salts (GAS) medium, Middlebrook 7H9 medium supplemented with 10% oleic acid-albumin-dextrose-catalase (OADC, BD Sciences) and 0.05% Tween 80, or Middlebrook 7H11 agar supplemented with 10% OADC. M. smegmatis mc2155 was grown at 37°C in Luria Bertani (LB) medium (Difco). Kanamycin (Kan) and hygromycin (Hyg) were added to final concentrations of 25 µg mL−1 and 50 µg mL−1, respectively.
Generation of M. tuberculosis knock-out and complemented knock-out mutants
The mtxS gene and flanking regions were PCR-amplified from M. tuberculosis H37Rv genomic DNA using primers Rv0539.1 (5’-gatacttctagatcgctcggcgccgttgagcc-3’) and Rv0539.2 (5’-gatacttctagagatccggctgccatctgctgc-3’) and a disrupted allele, mtxS::kan, was obtained by inserting the Tn903 kanamycin resistance cassette at the NcoI restriction site of mtxS. mtxS::kan was then cloned in the XbaI-cut pPR27-xylE, yielding pPR27mtxSKX. The mtxT gene and flanking regions were PCR-amplified using primers Rv0541c.1 (5’-gatacttctagagcagctggctgttggctgtgc-3’) and Rv0541c.2 (5’-gatacttctagagctggcgtgtcgtcgaactcg-3’) and a disrupted allele, mtxT::kan, was obtained by replacing 924 bp of the mtxT ORF flanked between two BlpI restriction sites with the kanamycin resistance gene from Tn903. mtxT::kan was then cloned in the XbaI-cut pPR27-xylE, yielding pPR27mtxTKX. The Ts/sacB method was used to achieve allelic replacement at the Rv0539 (herein renamed mtxS) and Rv0541c (herein renamed mtxT) loci of M. tuberculosis H37Rv (34). Allelic replacement at the mtxS and mtxT chromosomal loci was verified by PCR using upstream and downstream primers located outside the allelic exchange substrates.
For complementation studies and expression in M. smegmatis, the entire coding sequences of mtxS and mtxT were PCR-amplified from M. tuberculosis H37Rv genomic DNA and cloned into the replicative expression plasmid pMVGH1 (35), yielding pMVGH1mtxS and pMVGH1mtxT, respectively. pMVGH1[mtxS-Rv0540] was generated by amplifying the mtxS and Rv0540 genes together and cloning the resulting PCR fragment in the same expression plasmid. These constructs allow for the constitutive expression of C-terminally hexahistidine-tagged MtxS, MtxT or Rv0540 proteins under control of the hsp60 promoter. Primer sequences for these constructs are available upon request.
Lipid and lipoglycan extraction
Total lipid and lipoglycan extractions and LAM purification from mycobacterial cells followed earlier procedures (25).
Analytical procedures
LC-MS analyses were conducted on an Agilent 1200 binary pump liquid chromatograph (Agilent technologies/ Palo Alto, CA), using a 2.1 inner diameter × 150 mm, 3.5 µm XBridge reverse phase C18 column (Waters) heated to 45 °C as described (36). Decaprenyl phosphoryl-sugars were detected using an Agilent 6220A time-of-flight (TOF) mass spectrometer equipped with an electrospray ionization/atmospheric pressure chemical ionization (ESI/APCI), and a multimode source operated in the negative ion mode. Mass spectra were acquired at a rate of 1.02 spectra/second from m/z 250 to 3,200 Da and the data collected analyzed using the Mass Hunter software (Agilent).
Modeled isotopic distributions for DP-MTX and DP-MSX were calculated, overlaid with observed data, and visualized with the Isotope Distribution Calculator (Agilent MassHunter Workstation Data Analysis Core Version 4.0.479.0).
LC-MS/MS analyses were performed on an Agilent binary pump G4220A LC using a 2.1 × 100 mm, 2.6 µm Agilent Poroshell 120 EC-C18 column heated to 40 °C. Five µL of total lipid extracts were injected with an autosampler and separated at a flow rate of 0.3 mL min−1 as described (36). Decaprenyl phosphoryl-sugars were detected using an Agilent 6530 QTOF mass spectrometer equipped with electrospray ionization and a Jet Stream source operated in the negative ion mode. Precursor ions m/z 909.6379 (DPA), m/z 939.6307 (DP-MTX), m/z 939.6484 (DPM) and m/z 955.6256 (DP-MSX) were selected and fragmented using a collision energy setting of 65 V. Mass spectra were acquired at a rate of 1.0 spectrum/second from m/z 100 to 1,700 Da and the data collected analyzed using the Mass Hunter software (Agilent).
NMR spectra were recorded at 315 K on a Bruker Avance 600 MHz spectrometer equipped with a cryogenic probe TCi (Bruker Biospin, Germany). Purified ManLAM samples were exchanged in D2O (D, 99.97 % from Euriso-top, Saint-Aubin, France), with intermediate lyophilization, and then dissolved in 0.5 mL DMSO (D, 99.96 % from Euriso-top, Saint-Aubin, France). Samples were analyzed in a 200 × 5 mm UL-5 NMR tube (Euriso-Top). Proton chemical shifts are expressed in part per million (ppm) and referenced relative to internal DMSO at 2.52 ppm. All 2D NMR data sets were recorded without sample spinning. 1H–1H correlation spectra were acquired in the echo/antiecho-TPPI gradient selection mode (512 data points) using the “mlevetgp” sequence from the Topspin v2.1 software (Bruker Biospin).
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Allergy and Infectious Diseases/National Institutes of Health grant AI064798 (to M. Jackson), and the Alberta Glycomics Centre. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The IPBS NMR equipment was financed by the French Research Ministry, CNRS, Université Paul Sabatier, the Région Midi- Pyrénées and the European structural funds. We thank D. Dick, T. Sours and B. Cranmer (Colorado State University) for their help with LC/MS and MS/MS analyses.
Footnotes
Author Contributions
S. k. A., M. Joe, M. G., M. J., M. R. M., J. N. and T. L. L. conceived the study. M. J., M. R. M., J. N. and T. L. L. coordinated the study. S. k. A., M. J., M. R. M., C. M. B. and M. G. wrote the paper. S. k. A., L. S. and H. P. constructed and analyzed recombinant mycobacterial strains. S. k. A., S. Z., C. M. B., M. R. M. and M. G. analyzed the cell envelope composition of the recombinant strains and performed the NMR experiments. All authors reviewed the results and approved the final version of the manuscript.
Supporting Information Available - This material is available free of charge via the Internet.
REFERENCES
- 1.Ortalo-Magné A, Lemassu A, Lanéelle MA, Bardou F, Silve G, Gounon P, Marchal G, Daffé M. Identification of the surface-exposed lipids on the cell envelopes of Mycobacterium tuberculosis and other mycobacterial species. J. Bacteriol. 1996;178:456–461. doi: 10.1128/jb.178.2.456-461.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pitarque S, Larrouy-Maumus G, Payré B, Jackson M, Puzo G, Nigou J. The immunomodulatory lipoglycans, lipoarabinomannan and lipomannan, are exposed at the mycobacterial cell surface. Tuberculosis. 2008;88:560–565. doi: 10.1016/j.tube.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angala SK, Belardinelli JM, Huc-Claustre E, Wheat WH, Jackson M. The cell envelope glycoconjugates of Mycobacterium tuberculosis. Crit. Rev. Biochem. Mol. Biol. 2014;49:361–399. doi: 10.3109/10409238.2014.925420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaur D, Angala SK, Wu SW, Khoo KH, Chatterjee D, Brennan PJ, Jackson M, McNeil MR. A single arabinan chain is attached to the phosphatidylinositol mannosyl core of the major immunomodulatory mycobacterial cell envelope glycoconjugate, lipoarabinomannan. J. Biol. Chem. 2014;289:30249–30256. doi: 10.1074/jbc.M114.599415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi L, Berg S, Lee A, Spencer JS, Zhang J, Vissa V, McNeil MR, Khoo K-H, Chatterjee C. The carboxy terminus of EmbC from Mycobacterium smegmatis mediates chain length extension of the arabinan in lipoarabinomannan. J. Biol. Chem. 2006;281:19512–19526. doi: 10.1074/jbc.M513846200. [DOI] [PubMed] [Google Scholar]
- 6.Hunter SW, Gaylord H, Brennan PJ. Structure and antigenicity of the phosphorylated lipopolysaccharide antigens from the leprosy and tubercle bacilli. J. Biol. Chem. 1986;261:12345–12351. [PubMed] [Google Scholar]
- 7.Delmas C, Gilleron M, Brando T, Vercellone A, Gheorghiu M, Rivière M, Puzo G. Comparative structural study of the mannosylated-lipoarabinomannans from Mycobacterium bovis BCG vaccine strains: characterization and localization of succinates. Glycobiology. 1997;7:811–817. doi: 10.1093/glycob/7.6.811. [DOI] [PubMed] [Google Scholar]
- 8.Gilleron M, Jackson M, Nigou J, Puzo G. Structure, activities and biosynthesis of the Phosphatidyl-myo-Inositol-based lipoglycans. In: Daffé M, Reyrat J-M, editors. The Mycobacterial Cell Envelope. Washington, DC: ASM Press; 2008. pp. 75–105. [Google Scholar]
- 9.Torrelles JB, Schlesinger LS. Diversity in Mycobacterium tuberculosis mannosylated cell wall determinants impacts adaptation to the host. Tuberculosis (Edinb) 2010;90:84–93. doi: 10.1016/j.tube.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mishra AK, Driessen NN, Appelmelk BJ, Besra GS. Lipoarabinomannan and related glycoconjugates: structure, biogenesis and role in Mycobacterium tuberculosis physiology and host-pathogen interaction. FEMS Microbiol. Rev. 2011;35:1126–1157. doi: 10.1111/j.1574-6976.2011.00276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neyrolles O, Guilhot C. Recent advances in deciphering the contribution of Mycobacterium tuberculosis lipids to pathogenesis. Tuberculosis (Edinb) 2011;91:187–195. doi: 10.1016/j.tube.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Treumann A, Xidong F, McDonnell L, Derrick PJ, Ashcroft AE, Chatterjee D, Homans SW. 5-methylthiopentose: a new substituent on liporabinomannan in Mycobacterium tuberculosis. J. Mol. Biol. 2002;316:89–100. doi: 10.1006/jmbi.2001.5317. [DOI] [PubMed] [Google Scholar]
- 13.Ludwiczak P, Gilleron M, Bordat Y, Martin C, Gicquel B, Puzo G. Mycobacterium tuberculosis phoP mutant: lipoarabinomannan molecular structure. Microbiology. 2002;148:3029–3037. doi: 10.1099/00221287-148-10-3029. [DOI] [PubMed] [Google Scholar]
- 14.Turnbull WB, Shimizu KH, Chatterjee D, Homans SW, Treumann A. Identification of the 5-methylthiopentose substituent in Mycobacterium tuberculosis lipoarabinomannan. Angew. Chem. Int. Ed. 2004;43:3918–3922. doi: 10.1002/anie.200454119. [DOI] [PubMed] [Google Scholar]
- 15.Joe M, Sun D, Taha H, Completo GC, Croudace JE, Lammas DA, Besra GS, Lowary TL. The 5-deoxy-5-methylthio-xylofuranose residue in mycobacterial lipoarabinomannan. Absolute stereochemistry, linkage position, conformation, and immunomodulatory activity. J. Am. Chem. Soc. 2006;128:5059–5072. doi: 10.1021/ja057373q. [DOI] [PubMed] [Google Scholar]
- 16.Turnbull WB, Stalford SA. Methylthioxylose--a jewel in the mycobacterial crown? Org. Biomol. Chem. 2012;10:5698–5706. doi: 10.1039/c2ob25630d. [DOI] [PubMed] [Google Scholar]
- 17.Guérardel Y, Maes E, Briken V, Chirat F, Leroy Y, Locht C, Strecker G, Kremer L. Lipomannan and lipoarabinomannan from a clinical isolate of Mycobacterium kansasii: Novel structural features and apoptosis-inducing properties. J. Biol. Chem. 2003;278:36637–36651. doi: 10.1074/jbc.M305427200. [DOI] [PubMed] [Google Scholar]
- 18.Stalford SA, Fascione MA, Sasindran SJ, Chatterjee D, Dhandayuthapani S, Turnbull WB. A natural carbohydrate substrate for Mycobacterium tuberculosis methionine sulfoxide reductase A. Chem. Commun. (Camb) 2009:110–112. doi: 10.1039/b817483k. [DOI] [PubMed] [Google Scholar]
- 19.Chan J, Fan XD, Hunter SW, Brennan PJ, Bloom BR. Lipoarabinomannan, a possible virulence factor involved in persistence of Mycobacterium tuberculosis within macrophages. Infect. Immun. 1991;59:1755–1761. doi: 10.1128/iai.59.5.1755-1761.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buckoreelall K, Wilson L, Parker WB. Identification and characterization of two adenosine phosphorylase activities in Mycobacterium smegmatis. J. Bacteriol. 2011;193:5668–5674. doi: 10.1128/JB.05394-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buckoreelall K, Sun Y, Hobrath JV, Wilson L, Parker WB. Identification of Rv0535 as methylthioadenosine phosphorylase from Mycobacterium tuberculosis. Tuberculosis (Edinb) 2012;92:139–147. doi: 10.1016/j.tube.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berg S, Kaur D, Jackson M, Brennan PJ. The glycosyltransferases of Mycobacterium tuberculosis- roles in the synthesis of arabinogalactan, lipoarabinomannan, and other glycoconjugates. Glycobiology. 2007;17:35R–56R. doi: 10.1093/glycob/cwm010. [DOI] [PubMed] [Google Scholar]
- 23.Torrelles JB, Khoo KH, Sieling PA, Modlin RL, Zhang N, Marques AM, Treumann A, Rithner CD, Brennan PJ, Chatterjee D. Truncated structural variants of lipoarabinomannan in Mycobacterium leprae and an ethambutol-resistant strain of Mycobacterium tuberculosis. J. Biol. Chem. 2004;279:41227–41239. doi: 10.1074/jbc.M405180200. [DOI] [PubMed] [Google Scholar]
- 24.Dinadayala P, Kaur D, Berg S, Amin AG, Vissa VD, Chatterjee D, Brennan PJ, Crick DC. Genetic basis for the synthesis of the immunomodulatory mannose caps of lipoarabinomannan in Mycobacterium tuberculosis. J. Biol. Chem. 2006;281:20027–20035. doi: 10.1074/jbc.M603395200. [DOI] [PubMed] [Google Scholar]
- 25.Kaur D, Obregón-Henao A, Pham H, Chatterjee D, Brennan PJ, Jackson M. Lipoarabinomannan of Mycobacterium; mannose capping by a multifunctional terminal mannosyltransferase. Proc. Natl. Acad. Sci. USA. 2008;105:17973–17977. doi: 10.1073/pnas.0807761105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Škovierová H, Larrouy-Maumus G, Pham H, Belanova M, Barilone N, Dasgupta A, Mikušová K, Gicquel B, Gilleron M, Brennan PJ, Puzo G, Nigou J, Jackson M. Biosynthetic origin of the galactosamine substituent of Arabinogalactan in Mycobacterium tuberculosis. J. Biol. Chem. 2010;285:41348–41355. doi: 10.1074/jbc.M110.188110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Porcelli M, Cacciapuoti G, Cimino G, Gavagnin M, Sodano G, Zappia V. Biosynthesis and metabolism of 9-[5'-deoxy-5'-(methylthio)-beta-D-xylofuranosyl]adenine, a novel natural analogue of methylthioadenosine. Biochem. J. 1989;263:635–640. doi: 10.1042/bj2630635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolucka BA, McNeil MR, de Hoffmann E, Chojnacki T, Brennan PJ. Recognition of the lipid intermediate for arbinogalactan/arabinomannan biosynthesis and its relation to the mode of action of ethambutol on mycobacteria. J. Biol. Chem. 1994;269:23328–23335. [PubMed] [Google Scholar]
- 29.Wolucka BA, de Hoffmann E. Isolation and characterization of the major form of polyprenyl-phospho-mannose from Mycobacterium smegmatis. Glycobiology. 1998;8:955–962. doi: 10.1093/glycob/8.10.955. [DOI] [PubMed] [Google Scholar]
- 30.Budzikiewicz H, Grigsby RD. Mass spectrometry and isotopes: a century of research and discussion. Mass Spectrom. Rev. 2006;25:146–157. doi: 10.1002/mas.20061. [DOI] [PubMed] [Google Scholar]
- 31.Swoboda JG, Campbell J, Meredith TC, Walker S. Wall teichoic acid function, biosynthesis, and inhibition. ChemBioChem. 2010;11:35–45. doi: 10.1002/cbic.200900557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Needham BD, Trent MS. Fortifying the barrier: the impact of lipid A remodelling on bacterial pathogenesis. Nat. Rev. Microbiol. 2013;11:467–481. doi: 10.1038/nrmicro3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wheat WH, Dhouib R, Angala SK, Larrouy-Maumus G, Dobos K, Nigou J, Spencer JS, Jackson M. The presence of a galactosamine substituent on the arabinogalactan of Mycobacterium tuberculosis abrogates full maturation of human peripheral blood monocyte-derived dendritic cells and increases secretion of IL-10. Tuberculosis (Edinb) 2015;95:476–489. doi: 10.1016/j.tube.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jackson M, Camacho LR, Gicquel B, Guilhot C. Gene replacement and transposon delivery using the negative selection marker sacB. In: Parish T, Stocker NG, editors. Mycobacterium tuberculosis protocols. Totowa N J: Humana Press; 2001. pp. 59–75. [DOI] [PubMed] [Google Scholar]
- 35.Grzegorzewicz AE, Pham H, Gundi VAKB, Scherman MS, North EJ, Hess T, Jones V, Gruppo V, Born SEM, Korduláková J, Chavadi SS, Morisseau C, Lenaerts AJ, Lee RE, McNeil MR, Jackson M. Inhibition of mycolic acid transport across the Mycobacterium tuberculosis plasma membrane. Nat. Chem. Biol. 2012;8:334–341. doi: 10.1038/nchembio.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sartain MJ, Dick DL, Rithner CD, Crick DC, Belisle JT. Lipidomic analyses of Mycobacterium tuberculosis based on accurate mass measurements and the novel Mtb LipidDB. J. Lipid Res. 2011;52:861–872. doi: 10.1194/jlr.M010363. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.