Abstract
Gemini analogs of calcitriol, characterized by the extension of the C21-methyl group of calcitriol with a second chain, act as agonists of the vitamin D receptor (VDR). This second side chain of gemini is accommodated in a new cavity inside the VDR created by the structural rearrangement of the protein core. The resulting conformational change preserves the active state of the receptor and bestows gemini compounds with biological activities that exceed those of calcitriol. Of particular interest are gemini’s anti-cancer properties, and in this study we demonstrate anti-proliferative and tumor-reducing abilities of BXL0124 and BXL0097, differing only by the presence or absence, respectively, of the methylene group on the A ring. BXL0124 acts as a more potent VDR agonist than its 19-nor counterpart by activating VDR-mediated transcription at lower concentrations. In a similar manner, BXL0124 is more active than BXL0097 in growth inhibition of breast cancer cells and reduction of tumor volume. Structural comparisons of BXL0097 and BXL0124, as their VDR complexes, explain the elevated activity of the latter.
Keywords: vitamin D, VDR agonist, vitamin D analog, gemini analog, breast cancer
Graphical Abstract
Introduction
Breast cancer is a complex progressive disease with multiple subtypes and varying clinical outcomes. Ductal carcinoma in situ (DCIS) is an early, non-malignant lesion of the breast and recognized as a precursor of invasive breast cancer. A cancerous stem-cell-like population has the capacity to drive malignant progression to invasive ductal carcinoma (IDC), manifest by transgression of cancerous growth through the ductal lining and initiating metastasis and resistance to conventional therapies (Morrison et al. 2008). Calcitriol (1α,25-dihydroxy vitamin D3 or 1α,25(OH)2D3) and its analogs, particularly gemini compounds, can act as inhibitors of breast cancer progression by retarding or preventing the transition of DCIS to IDC (Wahler et al. 2014). Indeed, gemini reveal multifunctional activities that include inhibition of different types of breast carcinogenesis without displaying hypercalcemic toxicity and with potencies that are 10 to 100 fold higher than those of calcitriol (Lee et al. 2008; Pazos et al. 2014). Initial synthesis procedure of gemini analogs was described in (Norman et al. 2000). A novel synthetic methodology for gemini compounds was recently described (Pazos et al. 2016). Among the gemini analogs investigated in breast cancer models, BXL0124 (Figure 1) has been shown to be a potent agent for the prevention of different types of human breast cancer; it modifies cancer stem cell subpopulations into less stem-like differentiated cells, it inhibits mammosphere formation and suppresses mammary tumor growth in animals (Lee et al. 2006; So et al. 2011; Lee et al. 2010; Wahler et al. 2014; So et al. 2015).
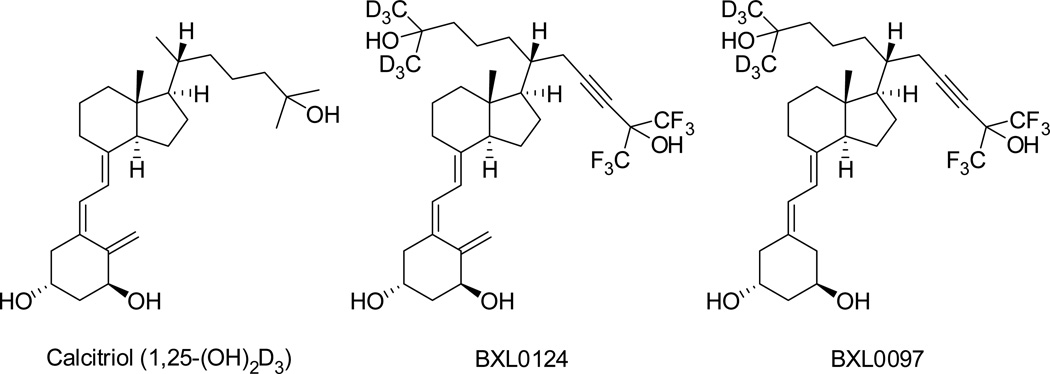
Figure 1.
Chemical structures of calcitriol and gemini analogs, BXL0124 and BXL0097.
Similarly to calcitriol, gemini compounds act in conjunction with the vitamin D nuclear receptor (VDR). We previously reported the crystal structures of the VDR ligand-binding domain (LBD) in complex with the parental gemini bearing two identical side chains and several of its derivatives (Ciesielski, Rochel, and Moras 2007; Huet et al. 2011; Maehr et al. 2013). A common feature of gemini compounds is their ability to induce a conformational change of the protein core by creating a new cavity that accommodates the second, saturated side chain obtained by C21 elongation. Most significantly, this structural rearrangement preserves the active state of the agonist-bound LBD.
The present study gains insights into the structure-activity relationships of BXL0124 and BXL0097 (Figure 1). Both compounds are closely related to cholecalciferol wherein the side chain of cholecalciferol was modified by a 23-triple bond and the six hydrogen atoms at positions 26 and 27 were replaced with fluorine atoms, and the position 21 was extended with a (3-hydroxy-3-trideuteromethyl-4,4,4-trideuterobutyl) group, thus maintaining the 20(R) configuration of cholecalciferol (Maehr et al. 2009). These chemical alterations were selected to prevent or retard biological degradation initiated by 24-hydroxylation thus extending the half-life of the compound. In contrast to BXL0097 which has a 19-nor A-ring, BXL0124 maintains the methylene group at position 19 of calcitriol. The 19-nor analogs have the advantage of enhanced chemical stability due to the absence of the triene function. Interestingly, binding of 19-nor-calcitriol to the VDR has been shown to be only 30% of that of calcitriol while the effects on HL60 differentiation were similar and calcemic effects were reduced (Bouillon et al. 1993; Perlman et al. 1990). Although numerous 19-nor-1,25(OH)2D3 analogs have been synthesized and their crystal structures solved [reviewed in (Belorusova and Rochel 2014)], there is no structural comparison of agonists differing only by the presence or absence of the C19 methylene group.
In this study we examine and compare the biological activities of BXL0124 and BXL0097. We show that both ligands are more potent in VDR-mediated transcriptional activation and in inhibition of breast cancer cell proliferation than the calcitriol, with BXL0124 displaying slightly higher activity than BXL0097. Importantly, BXL0124 is more effective in the suppression of mammary tumor growth than BXL0097. We further report the crystal structure of the zebrafish zVDR LBD in complex with BXL0124 explaining elevated potency of this compound.
Material and Methods
Compounds
Calcitriol was purchased from Sigma, and all gemini compounds (>95% purity) (Figure 1) were prepared as described (Maehr et al. 2009). All compounds were used in ethanol solutions.
Crystallization and structure determination
cDNA encoding zVDR LBD (156–453 AA) was cloned into the pET28b vector to generate N-terminal His-tag fusion protein. Purification was carried out as previously described, including metal affinity chromatography and gel filtration (Ciesielski, Rochel, and Moras 2007). The protein was concentrated using Amicon ultra-30 (Millipore) to 3–7 mg/ml and incubated with a two-fold excess of ligand and a three-fold excess of the coactivator SRC-2 peptide (686-KHKILHRLLQDSS-698). Crystals were obtained in a solution containing 50 mM Bis–Tris pH 6.5, 1.6 M lithium sulfate and 50 mM magnesium sulfate. Protein crystals were mounted in a fiber loop and flash-cooled under a nitrogen flux after cryo-protection with 20% glycerol. Data collection from a single frozen crystal was performed at 100 K on the ID30 beamline at ESRF (France). The raw data were processed and scaled with the HKL2000 program suite (Otwinowski and Minor 1997). The crystals belong to the space group P6522, with one LBD complex per asymmetric unit. The structure was solved and refined using BUSTER (version 2.11.2. Cambridge, United Kingdom: Global Phasing Ltd (Bricogne et al. 2016)), Phenix (Adams et al. 2010) and iterative model building using COOT (Emsley and Cowtan 2004). Crystallographic refinement statistics are presented in Supplementary Table 1. All structural figures were prepared using PyMOL (www.pymol.org).
Cell culture
The MCF10DCIS.com line was provided by Dr. Fred Miller at the Barbara Ann Karmanos Cancer Institute (Detroit, MI). MCF10DCIS.com cells were maintained in DMEM/F12 medium supplemented with 5% horse serum, 1% penicillin/streptomycin, and 1% HEPES solution at 37°C and 5% CO2. The cells were passed every 3 to 4 days. MCF-7 cells were acquired from ATCC. MCF-7 cells were maintained in DMEM/F12 supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin at 37°C and 5% CO2. 17-β Estradiol supplementation for MCF-7 cells was purchased from Sigma Aldrich (St. Louis, MO) and dissolved in ethanol. HEK293 EBNA cells were maintained in DMEM medium supplemented with 5% fetal bovine serum, 40 µg/mL gentamycin and 1 mg/mL geneticin G418 at 37°C and 5% CO2.
Transient Transfection and Luciferase Reporter Gene Assays
HEK293 EBNA cells plated into 24-well plates at 105 cells per well were cotransfected with 150 ng of the expression plasmid pSG5-hVDR, 150 ng of the reporter plasmid pLuc-MCS (Stratagene, La Jolla, USA) containing the proximal promoter region (-414 to -64) of the human CYP24A1 gene, 3 ng of the pRL plasmid (Promega, Madison, USA) containing the Renilla luciferase gene (transfection and cell viability control), and 697 ng of the carrier plasmid pBluescript (Stratagene). Transfection was performed with jetPEI (PolyPlus Transfection, Illkrich, France) according to the manufacturer’s instructions. Six hours after transfection, test-compounds were added. Cells were harvested after eighteen hours of incubation with ligands. The amounts of reporter gene product (firefly luciferase) and constitutively expressed Renilla luciferase produced in the cells were measured using Dual-Luciferase® Reporter Assay System (Promega) on a luminometer plate reader LB 96P (Berthold Technologies). Luminescence of firefly luciferase values were normalized to the Renilla luciferase activity. Luciferase activities were expressed as relative units of light intensity.
Cell proliferation assay
The assay protocol of cell proliferation is reported previously (Lee et al. 2008). For cell proliferation assay, MCF10DCIS cells were incubated with compound-containing DMEM/F12 medium supplemented with 5% horse serum medium for three days. MCF-7 cells were incubated with phenol-red free RPMI medium supplemented with 5% charcoal stripped fetal bovine serum and 17β-estradiol (100 pM) for three days. One microCi of [3H]-thymidine was added to each well three hours before the harvest. Incorporation of [3H]-thymidine into the cells was analyzed with a liquid scintillation spectrometer (Beckman Coulter).
Xenograft tumor studies
The detailed protocol of xenograft tumor studies is reported previously (So et al. 2011). In brief, MCF10DCIS.com cells (one million cells per mouse) were injected in the mammary fat pad of severe combined immunodecifiency (SCID) mice. Vehicle control (0.1 mL) and gemini vitamin D compounds BXL0097 or BXL0124 (0.1 µg/kg body weight in 0.1 mL vehicle) were injected intraperitoneally daily from day three until the termination of the experiment. For in vivo animal experiments, compounds were diluted in cremophore/PBS (1:8, v/v). At autopsy, mammary tumors were measured and weighed. All animal studies were done in accordance with an institutionally approved protocol.
Results and Discussion
BXL0124 shows transcriptional activity higher than its 19nor counterpart
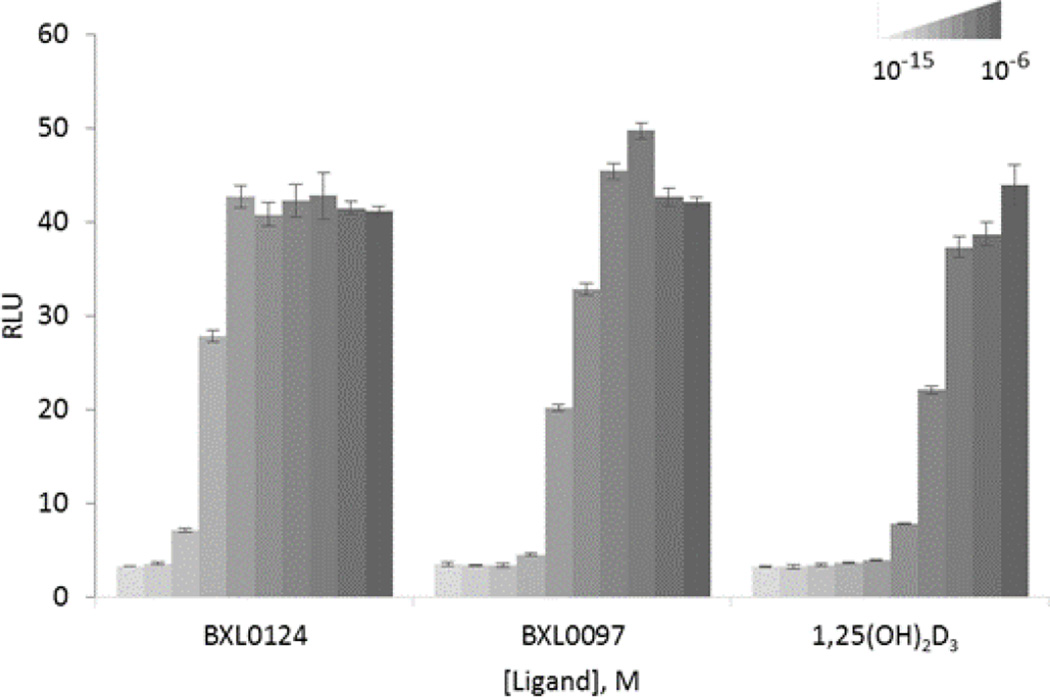
Transcriptional activities of BXL0097 and BXL0124 were evaluated and compared to the activity induced by calcitriol in HEK 293 EBNA cells transiently transfected with an expression vector encoding the full-length hVDR and a luciferase reporter plasmid encompassing the promoter region of the VDR target gene hCYP24A1. We found that VDR transcriptional activity is induced by the ligand BXL0124 at lower doses as compared to BXL0097 and calcitriol (Figure 2): this compound was able to activate transcription of the hCYP24A1 promoter at 10−12 M, which is one order of magnitude higher in comparison with BXL0097.
Figure 2.
Agonistic properties of BXL0124 and BXL0097 in VDR-mediated transcription. Transient transfection assays with expression vectors encoding full-length VDR protein were performed in HEK293 EBNA cells in order to evaluate transcriptional activity of the receptor on the hCYP24A1 promoter controlling luciferase reporter gene in response to increasing amounts of tested compounds or vehicle. Normalized amount of the expressed luciferase is presented in relative light units (RLU) intensity. Data are presented as mean ± standard error of the mean.
MCF10DCIS and MCF7 cell growth inhibition by BXL0124 and BXL0097
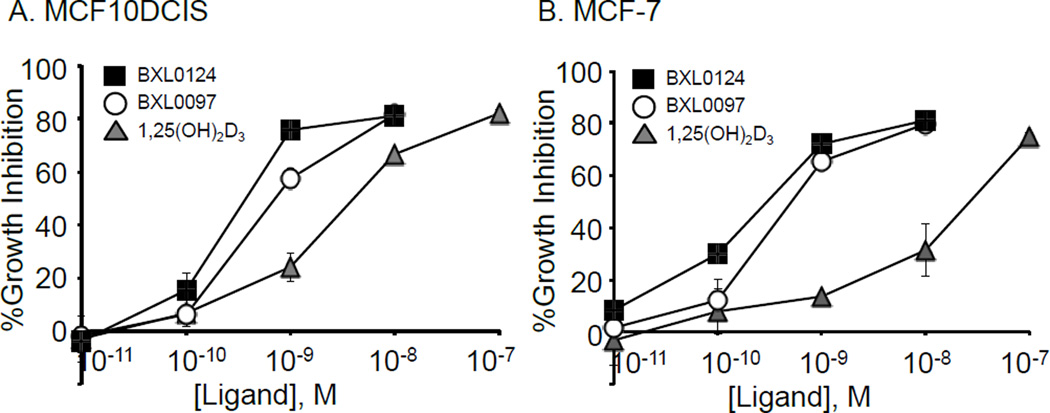
We determined the growth inhibitory effects of BXL0124 and BXL0097 on the cell proliferation of breast cancer cells, the ER-positive breast cancer cell line, MCF7 cells, and MCF10DCIS.com cells, a xenograft model of ER-negative mammary tumors that correspond to a basal-like breast tumor subtype. Both BXL0097 and BXL0124 are superior inhibitors of cell growth when compared to calcitriol, with BXL0124 being more active than BXL0097 (Figure 3). Both BXL0097 and BXL0124 showed subnanomolar ranges of IC50 values in the growth inhibition of the two breast cancer cell lines, which were 10-fold or better than that of 1,25(OH)2D3.
Figure 3.
Dose-dependent inhibition of cell proliferation by calcitriol (1,25(OH)2D3) (triangle) and gemini analogs, BXL0124 (square) and BXL0097 (circle) in (A) MCF10DCIS cells and (B) MCF-7 cells. The calculated IC50 for the inhibitory effect in MCF10DCIS of 1,25(OH)2D3, BXL0097 and BXL0124 are 2.65 nM, 0.56 nM and 0.23 nM, respectively and in MCF-7 24 nM, 0.36 nM and 0.19 nM, respectively.
BXL0124 inhibits MCF10DCIS human breast cancer xenograft tumor growth in SCID mice
Gemini analogs have been shown to prevent estrogen-receptor positive and negative mammary tumorigenesis without displaying hypercalcemic toxicity and to suppress mammary tumor growth in mice (Wahler et al. 2014; So et al. 2011). We now have compared the effects of BXL0124 and BXL0097 on MCF10DCIS human breast cancer xenograft tumor growth in SCID mice. Animals treated with BXL0124 are normocalcemic as previously shown for BXL0097 (Lee et al. 2008) and BXL0124 (Lee et al. 2010), and showed a significant reduction in tumor volume, with the effect larger than for the animals treated with BXL0097 (Table 1), suggesting that BXL0124 is more active in tumor reduction than its 19-nor analog.
Table 1.
Gemini compound BXL0124 inhibits MCF10DCIS human breast cancer xenograft tumor growth in SCID mice.
| Group | No. animals |
Average Body Weight at Day 3 (g) |
Average Body Weight at Autopsy (g) |
Final Tumor Volume at Autopsy (mm3)§ |
Final Tumor Weight (g)ठ|
Serum Calcium (mg/dl) |
|---|---|---|---|---|---|---|
| Control | 9 | 20.3 ± 1.7 | 21.9 ± 1.7 | 322.4 ± 214.4 | 0.29 ± 0.18 | 9.03 ± 0.45 |
| BXL0097 | 8 | 20.2 ± 1.3 | 22.4 ± 1.7 | 285.3 ± 202.7 | 0.33 ± 0.22 | ND |
| BXL0124 | 8 | 20.4 ± 1.3 | 21.4 ± 2.5 | 96.1 ± 81.0** | 0.11 ± 0.09** | 9.56 ± 0.65 |
MCF10DCIS.com cells (1 million cells per mouse) were injected into the mammary fat pad of female SCID mice. Three days after tumor cells injection, mice received daily i.p. injection of vehicle or Gemini vitamin D compounds (0.1 µg/kg body weight). Mice were sacrificed at day 34.
Data are expressed as mean ± S.D.
(p<0.01 by the Student’s t-test).
Average tumor burden in each mouse at autopsy. ND not determined.
Structural analysis of the BXL0124-VDR LBD complex
To characterize the molecular mechanisms underlying the enhanced activity of BXL0124, the crystal structure of zebrafish zVDR LBD in complex with BXL0124 and SRC-1 coactivator peptide was solved at 2.5 Å resolution. Data collection and refinement statistics are summarized in Supplementary Table 1. The complex preserves the general fold and canonical active agonist; the Cα atoms of the complex have a rmsd of 0.5 Å when compared to the zVDR LBD-calcitriol structure [PDB ID: 2HC4] and of 0.3 Å when compared to the zVDR LBD-BXL0097 [PDB ID: 3O1E].
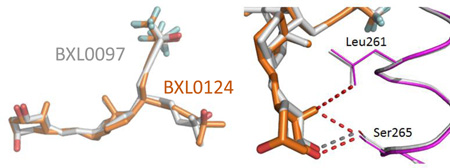
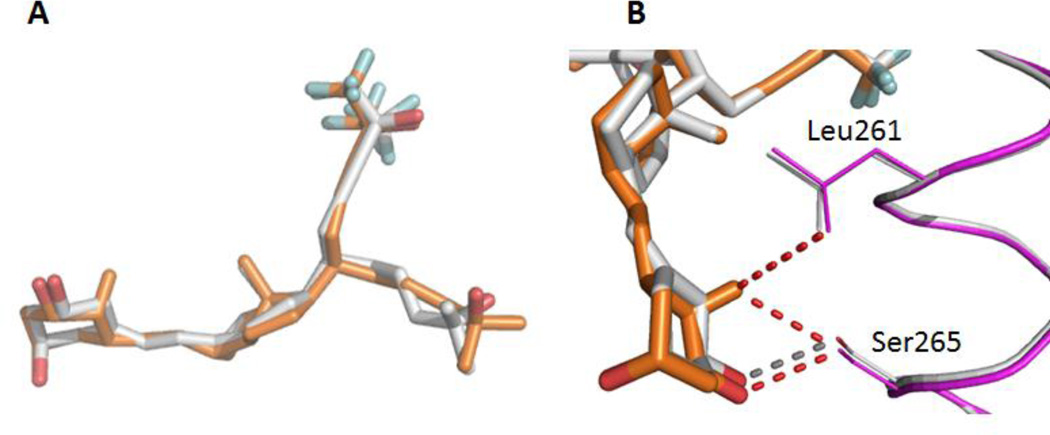
Positioning of BXL0124 ligand within the LBP is identical to that of BXL0097 (Figure 4A), although the trideuteromethyl containing side arm is more loosely positioned as seen from the weak electron density map (Supplementary Figure 1) of some atoms of this side chain. The side-chain containing the trifluoromethyl groups adopts the orientation of the natural chain of calcitriol and the trideuteromethyl-containing side chain occupies the additional pocket, created by the reorientation of the side chain at Leu337, similar to the zVDR LBD-gemini complexes (Ciesielski, Rochel, and Moras 2007; Huet et al. 2011). Thus, the previously observed propensity of the LBD to accommodate the unsaturated side chain containing the trifluoromethyl groups in the parental pocket, regardless of the gemini configuration at C20, is sustained.
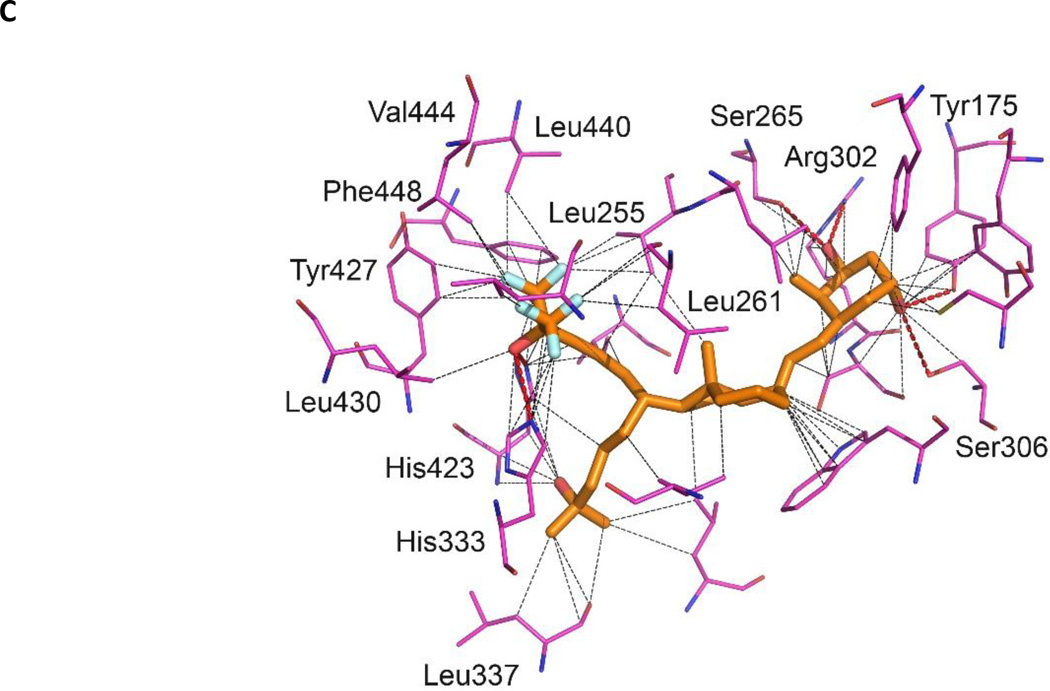
Figure 4.
Crystal structure of zVDR LBD-BXL0124 complex. (A) Overlay of BXL0124 (orange) and BXL0097 (grey) within VDR LBPs. (B) Enhanced visualization of the interactions formed by the C19 methylene group of BXL0124. (C) Details of interactions between LBP and BXL0124. Hydrophobic and van der Waals interactions (grey), and polar contacts (red) are displayed at 4.0 Ȧ cut-off (dotted lines). Amino acids with major contribution are named. Oxygen and fluorine atoms are shown in red and cyan, respectively.
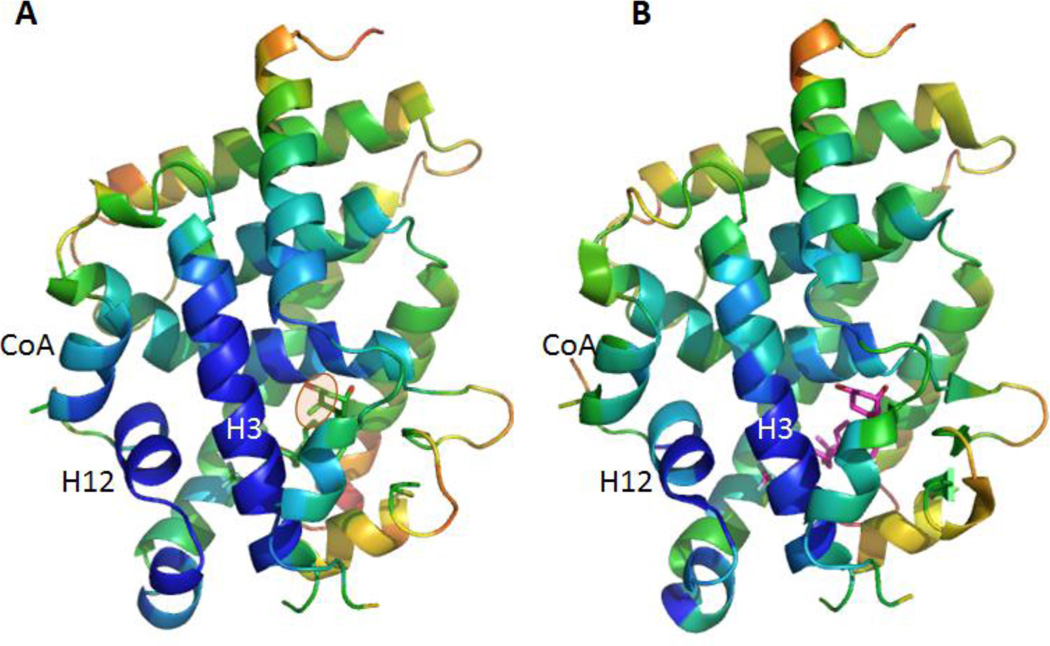
The hydroxyl groups of BXL0124 form similar hydrogen bonds as the zVDR LBD bound to BXL0097. More specifically, 1-OH interacts with Ser265 and Arg302, 3-OH with Tyr175 and Ser306 and the hydroxyl group of the trifluoromethyl-containing side chain with His333 or His423 (Figure 4C). Similarly to BXL0097, the trifluoromethyl groups form additional interactions explaining the increased biological activities of these gemini derivatives compared to the parental compound. These additional interactions involve Leu255 (H3), Val262 (H3), Tyr427 (H11), Leu430 (H11), Leu440 (H12), Val444 (H12) and Phe448 (H12). Although the two trideuteromethyl groups of BXL0124 adopt slightly different orientation than those of BXL0097, they engage similar contacts. The A-, seco B-, C-, and D-rings present conformations which are similar to those observed in the presence of the natural ligand. The main observed difference between BXL0124 and BXL0097 structures is the C19 methylene group which is present in BXL0124 and absent in BXL0097. The C19 atom in BXL0124 interacts with residues of helix H3, namely Leu261 (3.7 Å) and Ser265 (3.1 Å), and these interactions are not observed in the BXL0097 complex (Figure 4B). Analysis of the average temperature factors on the overall structure of the zVDR LBD in complex with BXL0124 and its comparison with the BXL0097 complex reveal stabilization of helix H3, but also additional stabilization of helix H12 and of the coactivator peptide in the presence of BXL0124 (Figure 5). While the additional contacts between fluorine atoms and hydrophobic residues of H3, H11 and H12 stabilize in a significant manner the agonist conformation of VDR and explain the superagonist potency of these compounds, the 19-methylene group on the A-ring provides additional stabilization of the agonist conformation of the complex, thus explaining the enhanced activity of this ligand.
Figure 5.
The C19 methylene group stabilizes the overall structure of the complex as shown by the B-factor plot on the overall structure of the VDR-BXL0124 complex (A) in comparison to VDR-BXL0097 (B). The secondary structural elements are colored by B-factors on a relative scale: highest B-factors, red; lowest, blue.
Conclusion
In this structure-function study, we have compared two related gemini compounds, BXL0124 bearing the 19-methylene group and its 19-nor counterpart BXL0097. We have shown that both analogs are potent superagonist ligands. Both compounds contain trifluoromethyl groups introduced at the terminus of one of the side chain, and these fluorine atoms are involved in the specific additional interactions stabilizing VDR H12 and contribute to the increased transactivation properties and pro-differentiating action in cancer cells. Moreover, BXL0124 is more active than BXL0097 in the different functional assays we performed. The methylene group at position 19 on the A-ring in BXL0124 forms additional interaction that stabilize helices H3 and H12 thus further stabilizing the agonist conformation of the complex.
Supplementary Material
Highlights.
-
○
Potent anticancer activity of BXL0124, a VDR agonist.
-
○
BXL0124 is more transcriptionally active than its 19nor counterpart.
-
○
The methylene group at position C-19 in BXL0124 stabilizes the VDR agonist conformation.
Acknowledgments
We thank Alastair McEwen (IGBMC) and the staff of ID30 (ESRF synchrotron) for their help in X-ray data collection. We thank the Agence Nationale de la Recherche (ANR-13-BSV8-0024-01), Alsace contre le Cancer, and French Infrastructure for Integrated Structural Biology (FRISBI) (ANR-10-INSB-05-01), and INSTRUCT as part of the European Strategy Forum on Research Infra-structures (ESFRI), for financial support. This work was supported in part by the National Institutes of Health National Cancer Institute R01 CA127645, the National Institute of Environmental Health Sciences Grant ES005022 and The Trustees Research Fellowship Program at Rutgers, The State University of New Jersey. AYB thanks FRM for a doctoral fellowship (FDT20140930978).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data deposition: The atomic coordinates, structure factors, and crystallography have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code: 5LGA)
Note: The atomic numbering system used refers to the conventional steroidal nomenclature.
References
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallographica Section D-Biological Crystallography. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belorusova AY, Rochel N. Modulators of vitamin D nuclear receptor: recent advances from structural studies. Curr Top Med Chem. 2014;14:2368–2377. doi: 10.2174/1568026615666141208095937. [DOI] [PubMed] [Google Scholar]
- Bouillon R, Sarandeses LA, Allewaert K, Zhao J, Mascarenas JL, Mourino A, Vrielynck S, de Clercq P, Vandewalle M. Biologic activity of dihydroxylated 19-nor-(pre)vitamin D3. J Bone Miner Res. 1993;8:1009–1015. doi: 10.1002/jbmr.5650080815. [DOI] [PubMed] [Google Scholar]
- Ciesielski F, Rochel N, Moras D. Adaptability of the Vitamin D nuclear receptor to the synthetic ligand Gemini: remodelling the LBP with one side chain rotation. J Steroid Biochem Mol Biol. 2007;103:235–242. doi: 10.1016/j.jsbmb.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Huet T, Maehr H, Lee HJ, Uskokovic MR, Suh N, Moras D, Rochel N. Structure-function study of gemini derivatives with two different side chains at C-20, Gemini-0072 and Gemini-0097. Medchemcomm. 2011;2:424–429. doi: 10.1039/C1MD00059D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Liu H, Goodman C, Ji Y, Maehr H, Uskokovic M, Notterman D, Reiss M, Suh N. Gene expression profiling changes induced by a novel Gemini Vitamin D derivative during the progression of breast cancer. Biochem Pharmacol. 2006;72:332–343. doi: 10.1016/j.bcp.2006.04.030. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Paul S, Atalla N, Thomas PE, Lin X, Yang I, Buckley B, Lu G, Zheng X, Lou YR, Conney AH, Maehr H, Adorini L, Uskokovic M, Suh N. Gemini vitamin D analogues inhibit estrogen receptor-positive and estrogen receptor-negative mammary tumorigenesis without hypercalcemic toxicity. Cancer Prev Res (Phila) 2008;1:476–484. doi: 10.1158/1940-6207.CAPR-08-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, So JY, DeCastro A, Smolarek A, Paul S, Maehr H, Uskokovic M, Suh N. Gemini vitamin D analog suppresses ErbB2-positive mammary tumor growth via inhibition of ErbB2/AKT/ERK signaling. J Steroid Biochem Mol Biol. 2010;121:408–412. doi: 10.1016/j.jsbmb.2010.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehr H, Lee HJ, Perry B, Suh N, Uskokovic MR. Calcitriol derivatives with two different side chains at C-20. V. Potent inhibitors of mammary carcinogenesis and inducers of leukemia differentiation. J Med Chem. 2009;52:5505–5519. doi: 10.1021/jm900780q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehr H, Rochel N, Lee HJ, Suh N, Uskokovic MR. Diastereotopic and deuterium effects in gemini. J Med Chem. 2013;56:3878–3888. doi: 10.1021/jm400032t. [DOI] [PubMed] [Google Scholar]
- Morrison BJ, Schmidt CW, Lakhani SR, Reynolds BA, Lopez JA. Breast cancer stem cells: implications for therapy of breast cancer. Breast Cancer Res. 2008;10:210. doi: 10.1186/bcr2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AW, Manchand PS, Uskokovic MR, Okamura WH, Takeuchi JA, Bishop JE, Hisatake JI, Koeffler HP, Peleg S. Characterization of a novel analogue of 1 alpha,25(OH)(2)-vitamin D-3 with two side chains: Interaction with its nuclear receptor and cellular actions. Journal of Medicinal Chemistry. 2000;43:2719–2730. doi: 10.1021/jm0000160. [DOI] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Macromolecular Crystallography, Pt A. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- Pazos G, Rivadulla ML, Perez-Garcia X, Gandara Z, Perez M. Gemini analogs of vitamin D. Curr Top Med Chem. 2014;14:2388–2397. doi: 10.2174/1568026615666141208100411. [DOI] [PubMed] [Google Scholar]
- Pazos G, Pérez M, Gándara Z, Gómez G, Fall Y. [3,3]-sigmatropic rearrangement mediated synthesis of chiral building blocks for the preparation of Gemini and its analogs. RCS Advances. 2016 in press. [Google Scholar]
- Perlman KL, Sicinski RR, Schnoes HK, Deluca HF. 1-Alpha, 25-Dihydroxy-19-nor-Vitamin-D3, a Novel Vitamin-D-Related Compound with Potential Therapeutic Activity. Tetrahedron Letters. 1990;31:1823–1824. [Google Scholar]
- So JY, Lee HJ, Smolarek AK, Paul S, Wang CX, Maehr H, Uskokovic M, Zheng X, Conney AH, Cai L, Liu F, Suh N. A novel Gemini vitamin D analog represses the expression of a stem cell marker CD44 in breast cancer. Mol Pharmacol. 2011;79:360–367. doi: 10.1124/mol.110.068403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So JY, Wahler J, Das Gupta S, Salerno DM, Maehr H, Uskokovic M, Suh N. HES1-mediated inhibition of Notch1 signaling by a Gemini vitamin D analog leads to decreased CD44(+)/CD24(−/low) tumor-initiating subpopulation in basal-like breast cancer. J Steroid Biochem Mol Biol. 2015;148:111–121. doi: 10.1016/j.jsbmb.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahler J, So JY, Kim YC, Liu F, Maehr H, Uskokovic M, Suh N. Inhibition of the transition of ductal carcinoma in situ to invasive ductal carcinoma by a Gemini vitamin D analog. Cancer Prev Res (Phila) 2014;7:617–626. doi: 10.1158/1940-6207.CAPR-13-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.