Abstract
The chemokine SDF-1α plays a critical role in mediating stem cell response to injury and disease and has specifically been shown to mobilize neural progenitor/stem cells (NPSCs) towards sites of neural injury. Current neural transplant paradigms within the brain suffer from low rates of retention and engraftment after injury. Therefore, increasing transplant sensitivity to injury-induced SDF-1α represents a method for increasing neural transplant efficacy. Previously, we have reported on a hyaluronic acid-laminin based hydrogel (HA-Lm gel) that increases NPSC expression of SDF-1α receptor, CXCR4, and subsequently, NPSC chemotactic migration towards a source of SDF-1α in vitro. The study presented here investigates the capacity of the HA-Lm gel to promote NPSC response to exogenous SDF-1α in vivo. We observed the HA-Lm gel to significantly increase NPSC transplant retention and migration in response to SDF-1α in a manner critically dependent on signaling via the SDF-1α-CXCR4 axis. This work lays the foundation for development of a more effective cell therapy for neural injury, but also has broader implications in the fields of tissue engineering and regenerative medicine given the essential roles of SDF-1α across injury and disease states.
Keywords: Tissue engineering, CXCL12, chemotaxis, neural progenitor cells, regenerative medicine, biomaterials
1. Introduction
The chemokine stromal cell-derived factor-1α (SDF-1α also known as CXCL12) has been implicated as a potent mediator of cellular mobilization in several physiological systems during developmental stages, as well as in maintenance of pluripotent stem cell niches and response to injury or disease. Through interaction with its primary receptor, CXCR4, SDF-1α directs primordial germ cell migration and influences the development of immune cells[1-3]. The SDF-1α-CXCR4 signaling axis is also essential in maintaining adult bone marrow and neural stem cell niches after development [4-7]. Beyond providing normal physiological cues, SDF-1α orchestrates inflammatory and thus endogenous reparative responses to injury or disease. Specifically, SDF-1α has been implicated in mobilizing beneficial stem cells in response to injury, such as the egress of marrow-derived stem cells to regions of injury or stress[8-11]; recruitment of mesenchymal stem cells to sites of myocardial infarction[12,13]; and recruitment of neural stem/progenitor cells (NPSCs) to sites of neural injury[14-17].
Viewing the SDF-1α-CXCR4 signaling axis through a therapeutic/intervention lens evokes the potential to exploit this endogenous cell recruitment mechanism for cell transplantation paradigms. Cell survival and retention in neural transplantation is particularly dismal with pre-clinical studies reporting survival and retention rates of 2-3% [18] [19]. Past research has explored the utility of overexpressing CXCR4 in mesenchymal stem cell transplants after stroke and traumatic brain injury resulting in improved transplant survival and retention, presumably through interaction with local, injury-induced increases in SDF-1α[14,15,20,21]. While effective, genetic engineering methods for increasing cell transplant sensitivity to SDF-1α are limited in their clinical relevance. As such, there is significant motivation for developing clinically translatable neural transplantation platforms to address transplant retention and survival. Observations of the endogenous NPSC response to SDF-1α and the roles of extracellular matrix components in mediating this response provides inspiration for alternative methods of increasing SDF-1α sensitivity in neural applications.
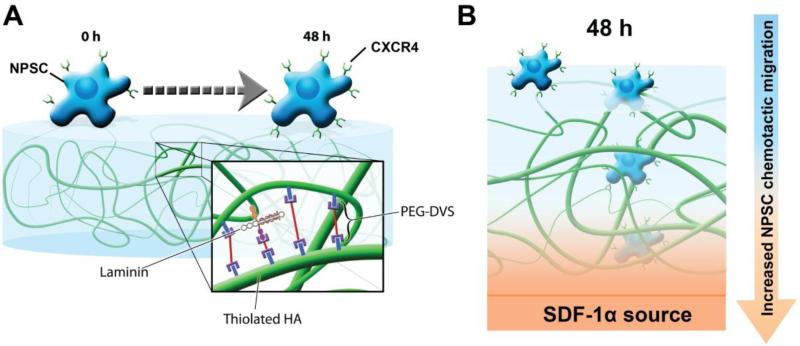
Endogenous NPSCs home to sites of neural injury in an SDF-1α-dependent manner, using the vasculature as a migratory pathway[16,22,23]. By doing so, NPSCs take advantage of the unique matrix composition of the vasculature, where crosstalk between the basement membrane protein laminin and SDF-1α has been shown to synergistically increase NPSC migration[24]. Moreover, the matrix composition of the neural stem cell niche itself may serve to prime endogenous NPSCs to respond to SDF-1α. Specifically, the niche is, in part, comprised of the glycosaminoglycan hyaluronic acid (HA)[25,26], which has been shown to increase CXCR4 expression in several stem cell types including NPSCs[27-30]. Therefore, we previously developed a hybrid tissue engineering hydrogel composed of HA and laminin-111 (HA-Lm) that serves to both increase CXCR4 expression in NPSCs (via HA interactions) and provide critical vascular basement membrane signaling to enhance NPSC migratory response to SDF-1α (via laminin interactions; Figure 1)[30]. This HA-Lm gel platform was shown to increase NPSC CXCR4 expression via CD44 signaling, where inhibition of NPSC CD44 abrogated the effect of the gel on CXCR4 expression [30].
Figure 1.
Schematic illustrating the effect of the HA-Lm gel on NPSCs. After 48 hours of culture on the HA-Lm gel, NSPCs (shown as blue cells) upregulate CXCR4 (shown as green cell surface receptor) (A). This correlates with increased NPSC chemotactic migration towards an SDF-1α source (shown in orange and labeled) that is dependent on both HA and laminin components of the gel (B)[30].
We previously demonstrated the ability of HA-Lm gels to increase NPSC migration in response to SDF-1α in vitro; however, signaling dynamics and complexity are exponentially amplified in vivo. As such, this study focuses on investigating the capacity of the HA-Lm gel to enhance NPSC transplant retention and migration in response to SDF-1α in an intact mouse brain model. We observed significant increases in NPSC retention and migration in response to SDF-1α when transplanted within the HA-Lm gel compared to vehicle. Moreover, increased NPSC migration was critically dependent on SDF-1α-CXCR4 signaling. The application of these findings directly impacts the neural transplantation field and may also extend to transplantation paradigms in numerous other physiological systems. As mentioned previously, HA has been shown to engage in crosstalk with SDF-1α in several stem cell populations, including mesenchymal and hematopoietic, which highlights the opportunity to develop this platform for other disease models. Moreover, a matrix-based hydrogel approach to increasing transplant sensitivity to SDF-1α escalates the clinical relevance of this therapy by circumventing the use of genetic engineering methods.
2. Results
2.1 HA-Lm gel significantly increases NPSC transplant retention within the brain
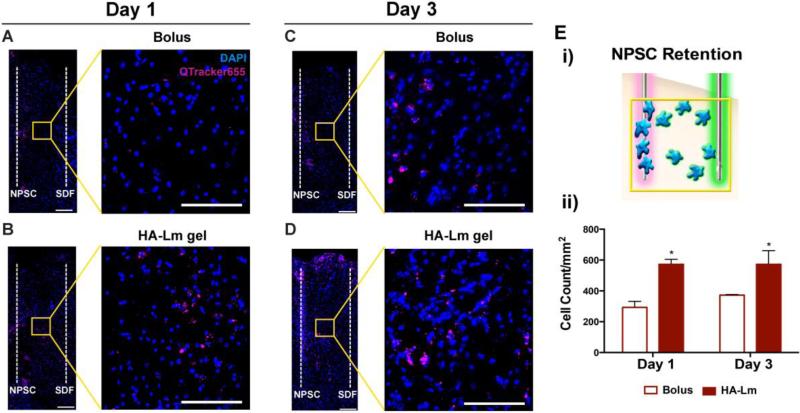
NPSCs used in this study were primary fetal-derived NPSCs harvested from the ganglionic eminences (medial and lateral) of E14.5 C57/BL 6 mice. Given their allogenic transplantation into C57/BL 6 mice, an immune response was not expected and immunosuppression was not necessary following transplantation into cortical tissue (Figure 2). The HA-Lm gel significantly increased average NPSC transplant retention at 1 and 3 days compared to bolus transplantation (p=0.0029, 0.0296 respectively, Figure 3). NPSC count per mm2 when transplanted bolus was 293.0±39.11 and 372.5±3.62 at days 1 and 3, respectively. When transplanted within the HA-Lm gel, NPSC count per mm2 increased to 572.6±32.60 and 547.2±63.52 at days 1 and 3, respectively. NPSC retention was not significantly different between day 1 and day 3 regardless of transplantation conditions.
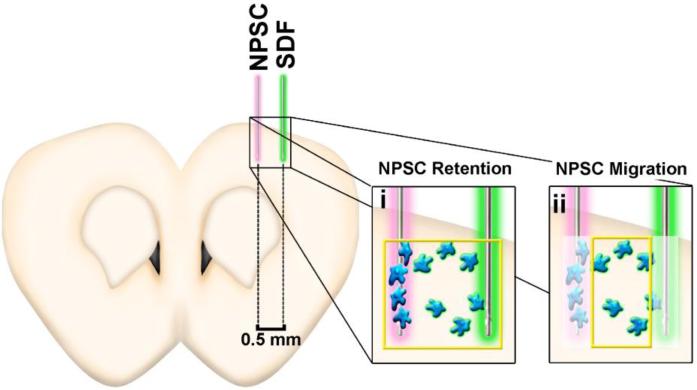
Figure 2.
Experimental overview and schematic illustrating NPSC retention and migration. SDF-1α was injected 0.5 mm lateral of the NPSC injection site and NPSC retention was calculated based on image sections including both needle tracks (i), where NPSC migration was calculated based on image sections excluding NPSCs that remained within the needle track (ii).
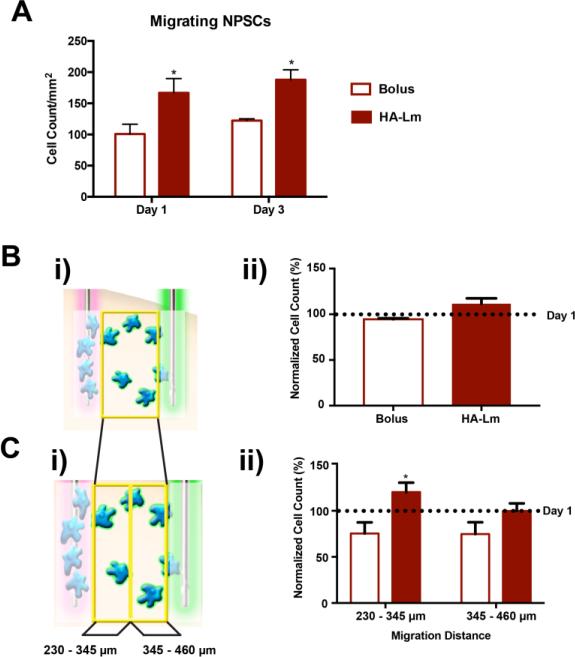
Figure 3.
NPSC retention and migration at days 1 and 3. Representative images demonstrate increased NPSC retention (overview) and migration (inset) in the HA-Lm gel at days 1 (B) and 3 (D) compared to bolus NPSC injections (A, C, respectively). NPSC retention was quantified as shown in the schematic (Ei). Average NPSC retention was significantly increased at 1 and 3 days when transplanted in the HA-Lm gel compared to bolus (Eii). Overview scale bar is 150 μm, inset scale bar is 100 μm. *p<0.05 compared to bolus of same time point.
2.2 HA-Lm gel significantly increases NPSC transplant migration in response to exogenous SDF-1α signaling
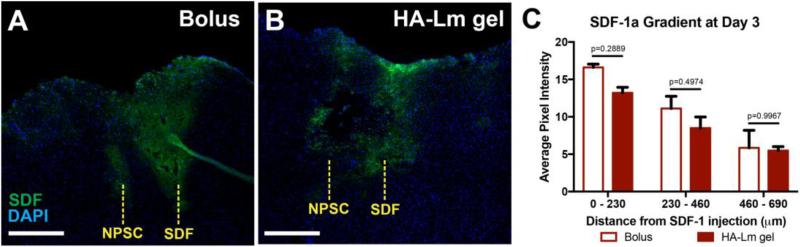
Exogenous SDF-1α gradients were effectively created in vivo using our novel experimental paradigm and maintained out to 3 days after injection (Figure 4). SDF-1α gradients were not significantly different between bolus NPSC and HA-Lm gel NPSC conditions (p≥0.2889, Figure 4C), demonstrating that NPSC transplants were exposed to similar SDF-1α concentration profiles across groups. NPSC migration out of the transplantation site towards the exogenous SDF-1α injection site was significantly increased at days 1 and 3 when transplanted within the HA-Lm gel compared to bolus (p= 0.0432, 0.0081, respectively, Figure 3,5A). When transplanted as bolus injection, migrating NPSC count per mm2 was 100.7±15.73 and 122.3±2.71 at days 1 and 3, respectively. When transplanted within the HA-Lm gel, migrating NPSC count per mm2 increased to 166.7±3.10 and 179.4±10.90 at days 1 and 3, respectively (Figure 5A). To differentiate between the effect of the HA-Lm gel on NPSC migration and potential effects of increased retention on migration, NPSC migration was normalized to NPSC retention. When normalized to average NPSC retention, the HA-Lm gel sustained increased NPSC migration at 3 days compared to day 1, where the bolus transplant conditions resulted in reduced NPSC migration at 3 days compared to day 1 (p=0.0967(ns), Figure 5B). Notably, the HA-Lm gel significantly increased sustained NPSC migration at a distance of 230 – 345 μm away from the injection site out to 3 days compared to bolus transplant condition (p=0.0467, Figure 5C). An increasing trend of sustained NPSC migration at a distance of 345 – 460 μm was observed when transplanted in the HA-Lm gel compared to bolus conditions; however it was not significantly different (p=0.1754, Figure 5C).
Figure 4.
SDF-1α gradient quantification. SDF-1α stained sections demonstrate the injection sites of bolus SDF-1α and NPSCs in bolus (A) and HA-Lm (B) transplant groups at 3 days after injection. SDF-1α gradients, reported as average pixel intensity of SDF-1α channel, were maintained out to 3 days after injection and were not significantly different between bolus and HA-Lm NPSC groups (p≥0.2889, C).
Figure 5.
Quantitative analysis of NSPC migration at days 1 and 3. The count of NPSCs migrating away from the injection site towards the exogenous SDF-1α was significantly increased in the HA-Lm gel compared to bolus injection at both 1 and 3 days (A). When normalized to NPSC retention, the HA-Lm gel increased sustained NPSC migration compared to bolus conditions (B,C). NPSCs transplanted bolus exhibited reduced numbers of migrating NPSCs at day 3 compared to day 1, while NPSC migration at day 3 was increased over that at day 1 when transplanted in the HA-Lm gel (not significantly different, B). Sustained NPSC migration at day 3 to a distance of 0.23 – 0.345 mm away from NPSC injection site was significantly increased when transplanted in the HA-Lm gel compared to bolus (C). *p<0.05 compared to bolus injection group of same time point.
2.3 SDF-1α signaling critically mediates NPSC transplant chemotactic migration, but not NPSC transplant retention
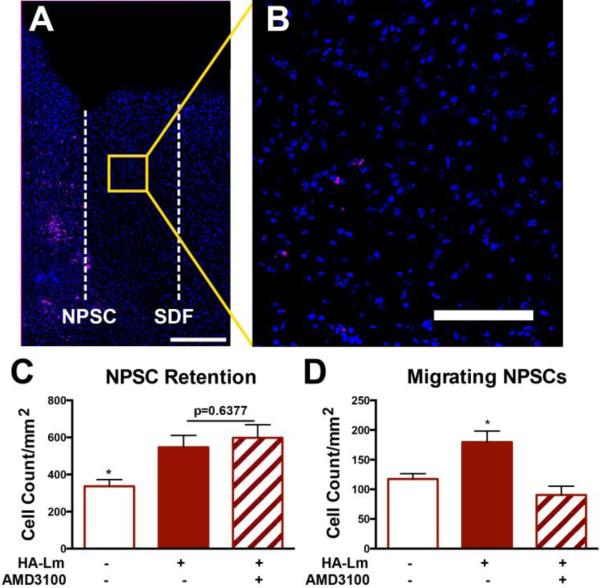
NPSCs were pre-incubated with the CXCR4 inhibitor, AMD3100, to control for SDF-1α-CXCR4 signaling effect on NPSC behavior following transplantation within the HA-Lm gel. Within an intact brain, injection of vehicle controls could initiate low levels of endogenous SDF-1α expression; therefore, inhibition of SDF-1α-CXCR4 interaction more effectively probes the effects of this signaling axis. NPSC pre-incubation with AMD3100 did not result in significantly different NPSC transplant retention compared to NSPCs transplanted in the HA-Lm gel without AMD3100 day 3 (p=0.6377, Figure 6). Both HA-Lm and HA-Lm with AMD3100 pre-incubation supported significantly higher average NSPC retention compared to bolus transplantation at day 3 (p=0.0296, 0.0336, respectively, Figure 6B). NPSC count per mm2 following pre-incubation with AMD3100 and transplantation within the HA-Lm gel was 597.8±70.25 compared to 547.2±63.52 without AMD3100 at day 3. This result indicates that NPSC interaction with SDF-1α does not play a significant role in increased NPSC retention within the HA-Lm gel, but rather that the gel itself is, in part, responsible for increased NPSC retention compared to bolus injection. However, increased NPSC migration was critically dependent on SDF-1α signaling as pre-incubation with AMD3100 significantly reduced NPSC transplant migration towards exogenous SDF-1α signaling compared to NPSC transplants without AMD3100 pre-incubation (p=.0127, Figure 6). Migrating NPSC count per mm2 was reduced to 90.50±10.50 when pre-incubated with AMD3100 compared to 179.4±10.90 without AMD3100.
Figure 6.
Pre-incubation with AMD3100 significantly reduced NPSC migration, but not NPSC retention at 3 days. Representative images illustrate high NPSC retention (A) and reduction in NPSC migration at day 3 (B). Average NPSC retention in the HA-Lm gel is not significantly different with the addition of AMD3100 pre-incubation at 3 days (p=0.6377, B). However, NPSC migration is significantly reduced when pre-incubated with AMD3100 compared to NPSCs transplanted within the HA-Lm gel without AMD3100 pre-incubation(D). *p<0.05 compared to all other groups. Overview scale bar is 250 μm, inset scale bar is 100 μm.
3. Discussion
Researchers have looked to increase neural transplant efficacy through several means in an effort to address the low rates of engraftment and survival that plague the field. One approach is to utilize the transplants as delivery devices where transplants are engineered to constitutively overexpress growth and/or trophic factors (i.e. fibroblast growth factor, brain-derived neurotrophic factor)[31-33]. While this approach has shown improvement in transplant engraftment and increased extracellular levels of their respective protein, it does not enable dynamic transplant interaction with and response to the host local microenvironment. Given the transient and progressive nature of pathophysiological signaling, the capacity of transplants to dynamically respond to the local microenvironment may prove beneficial.
One approach that provides transplants with the means to respond to the local microenvironment is enhancing transplant responsiveness/sensitivity to endogenous signaling factors, typically through genetic manipulation (i.e. transfection, transduction)[21,34]. Alternatively, transplants may be provided a scaffold environment as a tool to enable their retention and survival after injury. Transplant scaffolds may also serve to modulate transplant response to the microenvironment through biochemical priming of transplants to enhance responsiveness/sensitivity to the injury environment. Traditionally, the benefit of employing scaffolds has mainly focused on the capacity to create a permissive transplant environment that provides structural support and beneficial insoluble signaling to transplants[35]. Our approach focused on utilizing a scaffold as a means to enhance transplant responsiveness to local soluble signaling via biochemically priming transplants. In this way we aimed to provide transplants with a dual-purpose tool that combines the benefits of a permissive scaffolding environment with increased capacity to dynamically respond to local soluble signaling.
Specifically, enhancing neural progenitor/stem cell (NPSCs) transplant responsiveness to the chemokine SDF-1α may prove beneficial based on its critical role in mediating endogenous NPSC recruitment after neural injury[14,15,23]. Moreover, previous studies with CXCR4-overexpressing MSCs have also demonstrated enhanced engraftment in both stroke and brain injury paradigms [21,34]. Our data demonstrate that the HA-Lm gel enhances NPSC transplant chemotactic migration in response to exogenous SDF-1α in vivo, in part, by increasing the temporal window of NPSC transplant response to SDF-1α. NPSCs transplanted bolus did not sustain their migratory response to SDF-1α at 3 days, at which point less bolus NPSCs were migrating than at day 1 (Figure 5). However, NPSCs transplanted within the HA-Lm gel maintained an increased migratory response to SDF-1α out to 3 days, where 20% more NPSCs were migrating at day 3 than at day 1 (Figure 5).
Moreover, we found increased NPSC migration to be critically dependent on NPSC CXCR4 engagement as inhibition with AMD3100 significantly reduced migration. It is important to note that SDF-1α-CXCR4 engagement may initiate secondary signaling within the local microenvironment (i.e. VEGF production and inflammatory cytokine signaling) that, in turn, could have effects on NPSC transplant behavior. However, in our HA-Lm gel system, the critical role of SDF-1α-CXCR4 signaling in increasing NPSC transplant migration was evidenced by significant reduction in NPSC transplant migration when pre-incubated with AMD3100. The capacity of the HA-Lm gel to increase responsiveness of an NPSC population to soluble signaling without genetic engineering methods underlies its relevance to the clinic. However, data presented in this study were obtained in a population of fetal-derived NPSCs to demonstrate feasibility and design functionality. In translating this paradigm to the clinic, future work in this system will focus on augmenting the HA-Lm gel for more clinically relevant cell sources such as NPSCs derived from human induced pluripotent stem cells.
We have previously reported increased CXCR4 expression in NPSCs exposed to the HA-Lm gel in an HA-dependent manner[30], which may be responsible, in part, for increased NPSC migration towards exogenous SDF-1α in this study. However, this relationship may be bi-directional, where Avigdor et al. observed that exposure to SDF-1α rapidly induced cellular adhesion to HA in hematopoetic stem cells[28]. As such, the byproducts of the relationship between HA and SDF-1α-CXCR4 signaling in NPSCs are likely much more vast than only increased CXCR4 expression. While SDF-1α may enhance adhesion to HA, typically cellular adhesion and migration on HA alone is very limited, thus the laminin-111 component of the HA-Lm gel is critical for supporting/promoting NPSC migration in response to SDF-1α signaling[30]. Laminin also exhibits a unique crosstalk with SDF-1α in NPSCs where laminin and SDF-1α synergistically increases NPSC migration. This response was distinctly observed with laminin where other extracellular matrix (i.e. fibronectin, vitronectin) that readily engage in integrin-mediated NPSC migration do not exhibit the same relationship with SDF-1α [24]. As such, the mechanism of increased NPSC migration in the context of data presented here is likely a product of the interconnected relationships between HA, SDF-1α, and laminin.
Another parameter that may influence the effect of the HA-Lm gel on NPSC transplants is the phenotypic profile of the NPSC population. In this study, fetal-derived NPSCs were cultured as non-adherent neurospheres prior to transplantation, yielding a heterogeneous population of neural progenitor and stem cells. Subsets of NPSCs are known to express the HA receptor, CD44, enabling HA-mediated engagement with the HA-Lm gel and CD44-mediated overexpression of CXCR4, as previously demonstrated [36,37]. CD44-expressing NPSCs have been shown to typically differentiate down a glial lineage, which may serve to influence the phenotypic fate of NPSCs transplanted within the HA-Lm gel [37]. While the “ideal” phenotypic profile for NPSC transplants remains unclear, an enriched population of CD44-expressing NPSC may provide beneficial control over NPSC migration, as previous reports demonstrate endogenous migratory NPSCs to favor a glial lineage [38]. As such, future work will investigate the phenotypic profile of NPSCs that demonstrate increased migration within the HA-Lm gel.
Notably, inhibition of SDF-1α signaling did not significantly reduce NPSC retention within the HA-Lm gel. This result points to the benefit of providing a scaffolding material to NPSCs transplants on cell retention regardless of CXCR4 engagement. The study presented by Lisignoli et al. indicated that SDF-1α interactions increases cellular adhesion to HA [29]. Thus, one could postulate that inhibiting SDF-1α-NPSC interaction may reduce retention within the HA-Lm gel if SDF-1α-mediated binding to HA was a critical mechanism behind NPSC retention in the HA-Lm gel. However, we demonstrated that SDF-1α-CXCR4 engagement does not appear to play a critical role in increasing NPSC retention following transplantation within the HA-Lm gel. Identifying this relationship was only possible by negating SDF-1α signaling via AMD3100 since this study was performed in an intact mouse brain where the penetration of the needle itself represents a minor injury potentially initiating low levels of endogenous SDF-1α expression. The mechanisms of SDF-1α signal propagation may be such that a minor injury would still have a small effect on transplanted NPSCs. Therefore, inhibition of SDF-1α-CXCR4 signaling via AMD3100 provided a more robust mechanistic control compared to vehicle injections.
Regardless of transplantation paradigm, it is important to note that chemotactic response to SDF-1α follows a Gaussian distribution, where SDF-1α loses potency as a chemoattractive agent at high concentrations[39,40]. The SDF-1α concentration used for this study was based on previous work in vitro and the limited diffusivity of small molecules within the brain[41]. This concentration effectively generated an SDF-1α gradient for the duration of the experiment (Figure 4) and stimulated NPSC chemotactic migration, however, it is important to consider SDF-1α concentration as a function of the physiological tissue and injury/disease when looking towards pathophysiological applications of this work. For example, the full range of mechanisms of SDF-1α signal propagation remains unclear and the introduction of exogenous SDF-1α likely initiates the expression of endogenous SDF-1α within the surrounding tissue.
As such, one interesting effort to enhance endogenous repair has been through controlled release of SDF-1α by a degradable HA hydrogel. Purcell et al., have used an HA gel to deliver SDF-1α to regions of myocardial infarction, which resulted in increased recruitment of endogenous marrow-derived stem cells[27]. The effectiveness of this system after myocardial infarction highlights the application of HA, laminin, and SDF-1α systems beyond increasing NPSC transplant efficacy. Others have investigated these same components in a variety of permutations to elicit desired cellular response after injury/disease. For example, HA-based hydrogels containing collagen and laminin have been developed for spinal cord injury applications with promise in their capacity to support Schwann cells in vitro[42]. HA-based gels void of cells have been used to reconstruct the lesion cavity after traumatic brain injury; however their effect on the injury microenvironment remains to be thoroughly characterized[43,44].
SDF-1α signaling, specifically, plays important roles outside of a pathological context and, as such, our HA-Lm gel may also be useful in probing and/or enhancing the homeostatic roles for SDF-1α. For example, the neural stem cell niche in the subventricular zone is HA-rich and often described as a “vascular niche” due to its close proximity to vasculature[25,45,46]. Therefore, the HA-Lm gel may be optimized for use as an artificial niche with the goal of probing niche dynamics. A more thorough understanding of stem cell niche dynamics would inform therapies that aim to enhance the endogenous stem cell response to injury. While our work has been focused on the development of a neural transplantation platform, we believe the HA-Lm gel to be relevant to a broader scientific community given the diverse nature of SDF-1α signaling.
4. Experimental Methods
4.1 Materials for polymer synthesis
3,3 Dithiopropionic acid (DTPA), anhydrous methanol, anhydrous ethanol, hydrazine hydrate (HH), hexane, hyaluronic acid sodium salt (HA) from Streptococcus equi, N-3-dimethylaminopropyl-N′-ethylcarbodiimide hydrochloride (EDC), 5,5'-dithiobis-2-nitrobenzoic acid (Ellman's reagent) were purchased from Sigma Aldrich (St. Louis, MO, USA). Dithiothreitol (DTT) was purchased from Gold Biotechnology (St. Louis, MO, USA). Poly(ethylene glycol) divinyl sulfone (PEGDVS) 5 kDa was purchased from JenKem Technology USA (Allen, TX, USA).
4.2 HA-Lm Gel Formation
HA-Lm gel was formed as previously described[30]. Briefly, dithiopropionic dihydrazide (DTPH) was synthesized from DTPA and HH in a two-step reaction as previously described [47]. High molecular weight HA was functionalized with thiol groups (HA-S) through conjugation of the terminal hydrazides on DTPH to the carboxyl groups on HA using EDC chemistry and subsequent reduction of disulfide bonds using DTT as previously described [48]. HA-S was sterilized by ethylene oxide gas and stored at −20°C. HA free thiol concentration was 509 μM/g as determined by Ellman's test.
HA-Lm hydrogels were formed via Michael-type addition crosslinking with PEGDVS as previously reported [30,49]. Laminin was previously reported to have free thiol groups available for binding, presumably from its cysteine-rich β chain, enabling effective conjugation to PEGDVS within the gel. It was previously determined that the extent of vinyl sulfone consumption by laminin, as measured by NMR, was negligible in comparison to vinyl sulfone consumption by HA-S. HA-S was dissolved in pH 3 mitogenic growth factor-free culture media and titrated between pH 7 and pH 8 with 1 M NaOH using phenol red as a colorimetric indicator of pH. PEGDVS was dissolved in media at a concentration that yielded an equimolar ratio of thiol-reactive groups to thiols present on the HA-S and laminin. Laminin was conjugated to PEGDVS via Michael-type addition at 0.01 wt% and 0.10 wt% respectively in PBS for 15 minutes at room temperature. Cells were encapsulated within the HA-Lm gel at 3 × 104 cells/μL by suspending cells in the PEGDVS-Lm solution within 10 minutes of gel formation. Following laminin conjugation and cellular suspension, the HA-S solution was mixed with an equal volume of PEGDVS-Lm-cell solution and vortexed for 15 seconds prior to use. The final concentration of HA and laminin within the gel were 1.75 wt/v% and 0.01 wt/v%, respectively, based on previously reported optimization studies[30].
4.3 NPSC Isolation and Culture
Neural progenitor/stem cells (NPSCs) were isolated from the medial and lateral ganglionic eminences of E14.5 C57BL/6 mice based on previously published protocols[50] and in accordance with a protocol approved by the Institutional Animal Care and Use Committee at Arizona State University. Briefly, mice were anesthetized at 3% isoflurane, rapidly decapitated, and fetuses were extracted from both uterine horns. Fetal tissue was rinsed in cold Leibovitz medium (Life Technologies, Carlsbad, CA) at each stage of the ganglionic eminence dissection. The ganglionic eminences were rinsed with sterile, cold Leibovitz medium before mechanical dissociation in working NPSC medium (glucose (6 ng/mL, Acros Organics, Geel, Belgium), HEPES buffer (5mM, Sigma Aldrich, St. Louis, MO), progesterone (62.9 ng/mL, Sigma Aldrich), putrescine (9.6 μg/mL, Sigma Aldrich), heparin (1.83μg/mL , Sigma Aldrich), B27 growth supplement (1X, Life Technologies), epidermal growth factor (20 ng/mL, Sigma Aldrich), fibroblast growth factor (5 ng/mL, Sigma Aldrich), insulin (5 μg/mL, Sigma Aldrich), transferrin (5 μg/mL, Sigma Aldrich), and sodium selenite (5 ng/mL, Sigma Aldrich) in Dulbecco's Modified Eagle Medium (Life Technologies)) and plated at a density of 104 cells/mL in a humidified incubator at 37°C, 20% O2, and 5% CO2. NPSCs were cultured as non-adherent neurospheres in working NPSC medium, passaged by mechanical dissociation, and utilized for experiments between passages 3 through 6.
4.4 NPSC and SDF-1α dual injections into an intact brain
All transplantation studies were performed in accordance with the Arizona State University Institutional Animal Care and Use Committee. Immediately prior to transplantation, NPSCs were labeled with QTracker 655 according to the manufacturer protocol (Life Technologies). For all experiments, NPSCs were transplanted at a density of 3 × 104 cells/μL.
Adult male C57/BL6 mice (n=3 per group) were anesthetized, placed into a stereotaxic frame, and a 3 mm diameter craniotomy was performed using a biopsy punch centered 1.5 mm anterior of bregma and 1.5 mm lateral of midline, keeping dura intact during the craniotomy. NPSC suspension was transplanted bolus or within the HA-Lm gel (injection volume of 2 μL). To probe the dependence of NPSC behavior on SDF-1α, a third group included NPSCs pre-incubated with CXCR4 antagonist AMD3100 (5 μM, Santa Cruz Biotechnology, Santa Cruz, CA) for 45 minutes at 37 °C, prior to transplanting within the HA-Lm gel. Stereotaxic injections were 1.5 mm anterior of bregma, 1.5 mm lateral of midline at a depth of 0.8 mm. Immediately following NPSC transplantation, 2 μL of bolus exogenous mouse SDF-1α (100 μg/mL, PeproTech, Rocky Hill, NJ) was injected 0.5 mm away from the NPSCs (at 1.5 mm anterior to bregma, 2.0 mm lateral of midline) at a depth of 0.8 mm. Injection spacing was determined based on the smallest separable distance between needles that allowed for minimal interference with one another. Given the limited diffusion of small molecules within the brain[42], the smallest separable distance was preferable. Separate 5 μL Hamilton syringes were used for bolus NPSC and SDF-1α injections and a 25 μL Hamilton syringe was used for HA-Lm gel injections to accommodate the higher viscosity of the HA-Lm solution (Hamilton, Reno, NV). For all injections, the needle was stereotaxically placed before lowering 0.8 mm into the cortical tissue at a rate of 0.3 mm/min, held for 1 min, retracted to 0.6 mm, syringe contents injected at 0.5 μL/min and held again for 1 min before retracting at 0.3 mm/min. At 1 and 3 days post-injection, the mice were sacrificed by pericardial perfusion and post-fixed in 4% paraformaldehyde for histological analysis.
4.5 Immunohistochemistry
Following fixation, harvested brains were saturated with 30% sucrose, frozen and serially sectioned at 25 μm thickness. Sections were blocked with goat serum, permeabilized with Triton X-100 prior to staining for SDF-1α (rabbit anti-SDF-1α, Abcam, Cambridge, UK; AlexaFluor 488 goat anti-rabbit, Life Technologies), and visualized using fluorescence microscopy (DMI6000B, Leica). Sections used for cell counting were blocked but not permeabilized to maintain QTracker 655 retention within the transplanted NPSCs. Adjacent sections were used for cell counting and SDF-1α staining and all sections were counterstained with DAPI (Life Technologies). Images of the injection area were analyzed for SDF-1α gradient maintenance, NPSC retention within each brain section, and NPSC chemotactic migration away from their injection site and towards the exogenous SDF-1α injection site. For each brain section, the needle tracks and the area between injection sites were imaged with three 40X tile scan images of 0.23 mm width each to span the entire injection area as illustrated in Figure 2 (n=6 sections per animal; 3 animals per group). SDF-1α staining intensity of each tile scan was assessed by average pixel intensity normalized to area (0.23 mm × 1.5 mm) in ImageJ (NIH, Bethesda, MD). Cell retention was defined as the sum of labeled NPSC count across all three tile scans (Figure 2i), while migrating NPSCs were defined as the labeled NPSCs per section within the central section only (i.e. 0.23 – 0.36 mm away from transplant injection site, Figure 2ii). NPSC migration was also analyzed based on distance migrated into the central section (which began 0.23 mm away from the injection site and spanned a width of 0.23 mm) and normalized to average NPSC retention to control for potential variability in transplant number. Conditions for counting labeled NPSCs were pre-determined to minimize the incidence of false positives where a DAPI stained nuclei was required to be less than 10 μm away from two or more positive punctate QTracker 655 signals that were greater than 1 pixel in size. Moreover, if positive QTracker 655 signal within one cell was sharing a border with a second cell, then it was only counted as a single labeled cell. Examples of NPSCs that do and do not satisfy the conditions for being counted are shown in Supplementary Figure 1.
4.6 Statistical Analysis
Two-tailed t-tests were performed to compare bolus to HA-Lm encapsulated NPSCs in all studies where α=0.05. Error was reported as standard error of the mean and all statistical analysis was performed in Prism 6 (GraphPad Inc., La Jolla, CA). Post-hoc power analysis was performed to validate that experimental power was greater than 0.80 thus indicating that three animals per experimental group was adequately powered and did not warrant any further animals.
Supplementary Material
Highlights.
A hyaluronic-acid laminin (HA-Lm) hydrogel was investigated for its capacity to promote neural progenitor/stem cell (NPSC) transplant response to SDF-1α in vivo
The HA-Lm gel significantly increased NPSC transplant retention and migratory response to SDF-1α out to 3 days
Inhibition of SDF-1α-CXCR4 signaling significantly reduced NSPC migration, but not retention, when transplanted in the HA-Lm gel
Acknowledgements
The authors would like to acknowledge Emma Goddery for technical assistance, Amanda Witten for illustration assistance, and Jeff Kleim for surgical resources. This work was funded by the National Institutes of Health [NIH DP2 HD084067, (SES)] and the Barrow Neurological Foundation (RWS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 2.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 3.Doitsidou M, Reichman-Fried M, Stebler J, Koprunner M, Dorries J, Meyer D, et al. Guidance of primordial germ cell migration by the chemokine SDF-1. Cell. 2002;111:647–659. doi: 10.1016/s0092-8674(02)01135-2. [DOI] [PubMed] [Google Scholar]
- 4.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the Hematopoietic Stem Cell Pool by CXCL12-CXCR4 Chemokine Signaling in Bone Marrow Stromal Cell Niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharyya BJ, Banisadr G, Jung H, Ren D, Cronshaw DG, Zou Y, et al. The Chemokine Stromal Cell-Derived Factor-1 Regulates GABAergic Inputs to Neural Progenitors in the Postnatal Dentate Gyrus. J. Neurosci. 2008;28:6720–6730. doi: 10.1523/JNEUROSCI.1677-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goazigo AR-L, Van Steenwinckel J, Rostene W, Parsadaniantz SM. Current status of chemokines in the adult CNS. Prog. Neurobiol. 2013;104:67–92. doi: 10.1016/j.pneurobio.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Decimo I, Bifari F, Krampera M, Fumagalli G. Neural Stem Cell Niches in Health and Diseases. Curr. Pharm. Des. 2012;18:1755–1783. doi: 10.2174/138161212799859611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox A, Smythe J, Fisher N, Tyler MPH, McGrouther DA, Watt SM, et al. Mobilization of endothelial progenitor cells into the circulation in burned patients. Br. J. Surg. 2007;95:244–251. doi: 10.1002/bjs.5913. [DOI] [PubMed] [Google Scholar]
- 9.Kitaori T, Ito H, Schwarz EM, Tsutsumi R, Yoshitomi H, Oishi S, et al. Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum. 2009;60:813–823. doi: 10.1002/art.24330. [DOI] [PubMed] [Google Scholar]
- 10.Fiorina P, Pietramaggiori G, Scherer SS, Jurewicz M, Mathews JC, Vergani A, et al. The Mobilization and Effect of Endogenous Bone Marrow Progenitor Cells in Diabetic Wound Healing. Cell Transplant. 2010;19:1369–1381. doi: 10.3727/096368910X514288. [DOI] [PubMed] [Google Scholar]
- 11.Kucia M. Tissue-specific muscle, neural and liver stem/progenitor cells reside in the bone marrow, respond to an SDF-1 gradient and are mobilized into peripheral blood during stress and tissue injury. Blood Cell. Mol. Dis. 2004;32:52–57. doi: 10.1016/j.bcmd.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 12.Abbott JD. Stromal Cell-Derived Factor-1 Plays a Critical Role in Stem Cell Recruitment to the Heart After Myocardial Infarction but Is Not Sufficient to Induce Homing in the Absence of Injury. Circulation. 2004;110:3300–3305. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 13.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. The Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 14.Imitola J, Raddassi K, Park KI, Mueller F-J, Nieto M, Teng YD, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1α/CXC chemokine receptor 4 pathway. P. Natl. Acad. Sci. USA. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Itoh T, Satou T, Ishida H, Nishida S, Tsubaki M, Hashimoto S, et al. The relationship between SDF-1 α/CXCR4 and neural stem cells appearing in damaged area after traumatic brain injury in rats. Neurol. Res. 2009;31:90–102. doi: 10.1179/174313208X332995. [DOI] [PubMed] [Google Scholar]
- 16.Kojima T, Hirota Y, Ema M, Takahashi S, Miyoshi I, Okano H, et al. Subventricular Zone-Derived Neural Progenitor Cells Migrate Along a Blood Vessel Scaffold Toward the Post-Stroke Striatum. Stem Cells. 2010 doi: 10.1002/stem.306. N/A–N/A. [DOI] [PubMed] [Google Scholar]
- 17.Belmadani A. Chemokines Regulate the Migration of Neural Progenitors to Sites of Neuroinflammation. J. Neurosci. 2006;26:3182–3191. doi: 10.1523/JNEUROSCI.0156-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harting MT, Sloan LAE, Jimenez F, Baumgartner J, Cox CS. Subacute Neural Stem Cell Therapy for Traumatic Brain Injury. J. Surg. Res. 2009;153:188–194. doi: 10.1016/j.jss.2008.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tate CC, Shear DA, Tate MC, Archer DR, Stein DG, LaPlaca MC. Laminin and fibronectin scaffolds enhance neural stem cell transplantation into the injured brain. J. Tissue Eng. Regen. M. 2009;3:208–217. doi: 10.1002/term.154. [DOI] [PubMed] [Google Scholar]
- 20.Bang OY, Jin KS, Hwang MN, Kang HY, Kim BJ, Lee SJ, et al. The Effect of CXCR4 Overexpression on Mesenchymal Stem Cell Transplantation in Ischemic Stroke. Cell Med. 2012;4:65–76. doi: 10.3727/215517912X647172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Wang Y, Wang Z, Gutkind S, Wang Z, Wang F, et al. Engineered Mesenchymal Stem Cells with Enhanced Tropism and Paracrine Secretion of Cytokines and Growth Factors to Treat Traumatic Brain Injury. Stem Cells. 2014;33:456–467. doi: 10.1002/stem.1878. [DOI] [PubMed] [Google Scholar]
- 22.Bovetti S, Hsieh YC, Bovolin P, Perroteau I, Kazunori T, Puche AC. Blood Vessels Form a Scaffold for Neuroblast Migration in the Adult Olfactory Bulb. J. Neurosci. 2007;27:5976–5980. doi: 10.1523/JNEUROSCI.0678-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kokovay E, Goderie S, Wang Y, Lotz S, Lin G, Sun Y, et al. Adult SVZ Lineage Cells Home to and Leave the Vascular Niche via Differential Responses to SDF1/CXCR4 Signaling. Cell Stem Cell. 2010;7:163–173. doi: 10.1016/j.stem.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Addington CP, Pauken CM, Caplan MR, Stabenfeldt SE. The role of SDF-1α-ECM crosstalk in determining neural stem cell fate. Biomaterials. 2014;35:3263–3272. doi: 10.1016/j.biomaterials.2013.12.102. [DOI] [PubMed] [Google Scholar]
- 25.Preston M, Sherman LS. Neural Stem Cell Niches: Critical Roles for the Hyaluronan-Based Extracellular Matrix in Neural Stem Cell Proliferation and Differentiation. Front. Biosci. 2012;3:1165. doi: 10.2741/218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindwall C, Olsson M, Osman AM, Kuhn HG, Curtis MA. Selective expression of hyaluronan and receptor for hyaluronan mediated motility (Rhamm) in the adult mouse subventricular zone and rostral migratory stream and in ischemic cortex. Brain Res. 2013;1503:62–77. doi: 10.1016/j.brainres.2013.01.045. [DOI] [PubMed] [Google Scholar]
- 27.Purcell BP, Elser JA, Mu A, Margulies KB, Burdick JA. Synergistic effects of SDF-1α chemokine and hyaluronic acid release from degradable hydrogels on directing bone marrow derived cell homing to the myocardium. Biomaterials. 2012;33:7849–7857. doi: 10.1016/j.biomaterials.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Avigdor A. CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of human CD34+ stem/progenitor cells to bone marrow. Blood. 2004;103:2981–2989. doi: 10.1182/blood-2003-10-3611. [DOI] [PubMed] [Google Scholar]
- 29.Lisignoli G, Cristino S, Piacentini A, Cavallo C, Caplan AI, Facchini A. Hyaluronan-based polymer scaffold modulates the expression of inflammatory and degradative factors in mesenchymal stem cells: Involvement of Cd44 and Cd54. J. Cell. Physiol. 2006;207:364–373. doi: 10.1002/jcp.20572. [DOI] [PubMed] [Google Scholar]
- 30.Addington CP, Heffernan JM, Millar-Haskell CS, Tucker EW, Sirianni RW, Stabenfeldt SE. Enhancing neural stem cell response to SDF-1α gradients through hyaluronic acid-laminin hydrogels. Biomaterials. 2015;72:11–19. doi: 10.1016/j.biomaterials.2015.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dayer AG, Jenny B, Sauvain M-O, Potter G, Salmon P, Zgraggen E, et al. Expression of FGF-2 in neural progenitor cells enhances their potential for cellular brain repair in the rodent cortex. Brain. 2007;130:2962–2976. doi: 10.1093/brain/awm200. [DOI] [PubMed] [Google Scholar]
- 32.Ma H, Yu B, Kong L, Zhang Y, Shi Y. Neural stem cells over-expressing brain-derived neurotrophic factor (BDNF) stimulate synaptic protein expression and promote functional recovery following transplantation in rat model of traumatic brain injury. Neurochem. Res. 2012;37:69–83. doi: 10.1007/s11064-011-0584-1. [DOI] [PubMed] [Google Scholar]
- 33.Wang Z, Yao W, Deng Q, Zhang X, Zhang J. Protective Effects of BDNF Overexpression Bone Marrow Stromal Cell Transplantation in Rat Models of Traumatic Brain Injury. J. Mol. Neurosci. 2012;49:409–416. doi: 10.1007/s12031-012-9908-0. [DOI] [PubMed] [Google Scholar]
- 34.Yu X, Chen D, Zhang Y, Wu X, Huang Z, Zhou H, et al. Overexpression of CXCR4 in mesenchymal stem cells promotes migration, neuroprotection and angiogenesis in a rat model of stroke. J. Neurol. Sci. 2012;316:141–149. doi: 10.1016/j.jns.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Kim H, Cooke MJ, Shoichet MS. Creating permissivemicroenvironments for stem celltransplantation into the centralnervous system. Trends Biotechnol. 2011:1–9. [Google Scholar]
- 36.Naruse M, Shibasaki K, Yokoyama S, Kurachi M, Ishizaki Y. Dynamic changes of CD44 expression from progenitors to subpopulations of astrocytes and neurons in developing cerebellum. PLoS One. 2013;8:1–12. doi: 10.1371/journal.pone.0053109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y, Han S, Wu Y, Tuohy T, Xue H, Cai J, et al. CD44 expression identifies astrocyte-restricted precursor cells. Dev. Biol. 2004;276:31–46. doi: 10.1016/j.ydbio.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 38.Faiz M, Sachewsky N, Gascón S, Bang KWA, Morshead CM, Nagy A. Adult neural stem cells from the subventricular zone give rise to reactive astrocytes in the cortex after stroke. Cell Stem Cell. 2015;17:642–634. doi: 10.1016/j.stem.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Dutta D, Fauer C, Mulleneux HL, Stabenfeldt SE. Tunable controlled release of bioactive SDF-1α via specific protein interactions within fibrin/nanoparticle composites. J. Mater. Chem. B. 2015;3:7963–7973. doi: 10.1039/C5TB00935A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robin AM, Zhang ZG, Wang L, Zhang RL, Katakowski M, Zhang L, et al. Stromal cell-derived factor 1alpha mediates neural progenitor cell motility after focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2006;26:125–134. doi: 10.1038/sj.jcbfm.9600172. [DOI] [PubMed] [Google Scholar]
- 41.Sykova E, Nicholson C. Diffusion in Brain Extracellular Space. Physiol. Rev. 2008;88:1277–1340. doi: 10.1152/physrev.00027.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suri S, Schmidt CE. Cell-laden hydrogel constructs of hyaluronic acid, collagen, and laminin for neural tissue engineering. Tissue Eng. Part A. 2010;16:1703–1716. doi: 10.1089/ten.tea.2009.0381. [DOI] [PubMed] [Google Scholar]
- 43.Tian Y-Y, Tang C-J, Wang J-N, Feng Y, Chen X-W, Wang L, et al. Favorable effects of VEGF gene transfer on a rat model of Parkinson disease using adeno-associated viral vectors. Neurosci. Lett. 2007;421:239–244. doi: 10.1016/j.neulet.2007.05.033. [DOI] [PubMed] [Google Scholar]
- 44.Wei YT, Tian WM, Yu X, Cui FZ, Hou SP, Xu QY, et al. Hyaluronic acid hydrogels with IKVAV peptides for tissue repair and axonal regeneration in an injured rat brain. Biomed. Mater. 2007;2:S142–S146. doi: 10.1088/1748-6041/2/3/S11. [DOI] [PubMed] [Google Scholar]
- 45.Doetsch F. A niche for adult neural stem cells. Curr. Opin. Genet. Dev. 2003;13:543–550. doi: 10.1016/j.gde.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 46.Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, et al. A Specialized Vascular Niche for Adult Neural Stem Cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vercruysse KP, Marecak DM, Marecek JF, Prestwich GD. Synthesis and in vitro degradation of new polyvalent hydrazide cross-linked hydrogels of hyaluronic acid. Bioconjugate Chem. 1997;8:686–694. doi: 10.1021/bc9701095. [DOI] [PubMed] [Google Scholar]
- 48.Shu XZ, Liu Y, Palumbo F, Prestwich GD. Disulfide-crosslinked hyaluronan-gelatin hydrogel films: a covalent mimic of the extracellular matrix for in vitro cell growth. Biomaterials. 2003;24:3825–3834. doi: 10.1016/s0142-9612(03)00267-9. [DOI] [PubMed] [Google Scholar]
- 49.Heffernan JM, Overstreet DJ, Le LD, Vernon BL, Sirianni RW. Bioengineered Scaffolds for 3D Analysis of Glioblastoma Proliferation and Invasion. Ann. Biomed. Eng. 2014 doi: 10.1007/s10439-014-1223-1. [DOI] [PubMed] [Google Scholar]
- 50.Azari H, Sharififar S, Rahman M, Ansari S, Reynolds BA. Establishing Embryonic Mouse Neural Stem Cell Culture Using the Neurosphere Assay. JoVE. 2011:e2457–e2457. doi: 10.3791/2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.