Abstract
Objectives The nasoseptal flap (NSF) has become the workhorse for reconstruction in endoscopic endonasal skull-base surgery. The NSF, though useful in reconstruction, may lead to significant donor site morbidity. Published techniques to reduce the donor site morbidity, free mucosal grafts, and septal rotational flaps have shown to reduce crusting and remucosalization times. We present a novel technique utilizing posterior septal mucosa as a free mucosal graft for reconstruction of the anterior septal donor site. The septal mucosal graft is taken from the mucosa overlying the posterior septectomy site of the endonasal approach to skull base tumors.
Design Retrospective chart review.
Setting Single tertiary academic medical center.
Participants All patients who underwent endoscopic endonasal skull-base surgery between November 1, 2014 and August 30, 2015 with free mucosal graft reconstruction of the NSF donor site.
Main Outcome Measures Postoperative graft success.
Results Fifteen patients underwent septal reconstruction using a septal free mucosal graft. There was a 100% graft success rate with near complete remucosalization by 6 weeks postoperatively.
Conclusions The posterior septal free mucosal graft is a simple, reliable method for reconstructing the NSF donor site. The advantages of this technique include utilization of native septal mucosal tissue and middle turbinate preservation.
Keywords: skull base/surgery, skull base/pathology, nasal septum/surgery, nasal cavity/surgery, nasal mucosa/transplantation
Introduction
In the past two decades, endoscopic endonasal approaches for skull base tumors have advanced greatly. With advancement of these surgical techniques, the limits of their utility are being pushed continually; tumors that in the past required a transcranial procedure have become resectable through the endoscopic endonasal approach. As larger tumors are being resected from the anterior skull base, the resultant skull base defects have also become more complex and consequently, techniques have emerged to address their reconstruction. Typically, reconstruction of skull base defects with cerebrospinal fluid (CSF) leaks is generally repaired in a multilayered approach, often with a pedicled mucosal flap.1 2 3 4 5
In 2006, the Hadad and Bassagasteguy nasoseptal flap (NSF) was introduced and since its inception, it has quickly become the workhorse reconstructive pedicled flap in endoscopic skull base surgery.6 In one systematic review, the use of pedicled flaps in skull base reconstruction has reduced the rate of postoperative CSF leaks from 15.6 to 6.7%, compared with free mucosal grafts.7 The NSF, however, is not without associated morbidity. The NSF donor site can take up to 12 weeks for complete remucosalization.8 In one study, donor site complications from NSF harvest included anterior septal perforation, prolonged crusting for greater than 6 months, squamous metaplasia, and nasal saddling.9 Several published reports have shown significant changes in postoperative endoscopic examinations, increased crusting, and increased sinonasal morbidity in the short-term period which may be attributed to the use of a NSF.10 11 12 13 14
Previous authors have described techniques to limit the postoperative morbidity associated with NSF harvest. Kimple et al described the use of a free mucosal graft harvested from a resected middle turbinate to graft the anterior septal cartilage of the NSF donor site.15 Kasemsiri et al reported the use of a “reverse flap” in which an anteriorly based posterior septal mucosa flap of the septectomy site was rotated 180 degrees to the opposite side to cover the bare anterior septal cartilage.16 Both studies showed improved time to remucosalization and reduced crusting in the early postoperative period.15 16 In this study, we present a novel method for reconstruction of the anterior septum after NSF harvest. The technique utilizes the posterior nasal septal mucosa on the side contralateral to the NSF, at the site of the posterior septectomy for transnasal, transsphenoidal approaches to the skull base, which would otherwise be discarded.
Methods
This study was approved by the Institutional Review Board of the University of California, Los Angeles, Office of Research Administration. A retrospective chart review was performed for all patients undergoing endonasal endoscopic skull base surgery between November 1, 2014 and August 30, 2015 who had a free mucosal graft reconstruction of the NSF donor site. Demographics and patient characteristics including gender, age at time of surgery, comorbidities, tumor pathology, side of NSF, and side of mucosal graft harvest. Each patient was followed up for ∼1 week and 6 weeks after surgery. The main outcomes evaluated were graft take.
Surgical Technique
First, NSF is elevated on the desired side as described by Hadad et al and placed into the nasopharynx or maxillary sinus for later use in reconstruction.6 The exposed septal bone and cartilage are left intact after elevation of the NSF. At this point, the contralateral nasal cavity is inspected. With the tip of a marking pen, the mucosa of the posterior septum anterior to the sphenoid ostium is marked on this side to allow for identification of the mucosal side of the graft after removal from the nasal cavity. Using monopolar electrocautery, the superior cut is made starting at the level of the sphenoid ostium and carried anteriorly until approximately the anterior edge of the middle turbinate. The posterior incision is made anterior to the face of the sphenoid sinus and down to the level of the inferior aspect of the middle turbinate. The anterior incision is made inferiorly from the anterior aspect of the superior incision down to the level of the inferior aspect of the middle turbinate. The inferior incision is made from the sphenoid ostium, along the floor of the nose, to the anterior edge of the inferior aspect of the middle turbinate. After the incisions are made, the mucosa is elevated off the underlying septal cartilage and bone in the submucoperiochondrial plane using a Cottle elevator (Fig. 1) and removed from the nasal cavity with Blakesley forceps. The graft should be ∼2 by 2 cm in size. The mucosal side of the graft is once again marked with ink to allow for ease of identification (Fig. 2). The graft is set aside for later use for septal reconstruction. The septectomy is then performed, removing the posterior septal bone and keel of the posterior septum, and the intersinus septum of the sphenoid sinuses. The septectomy is not taken completely to the anterior aspect of the mucosal graft harvest, leaving ∼5 mm width of exposed septal cartilage/bone.
Fig. 1.
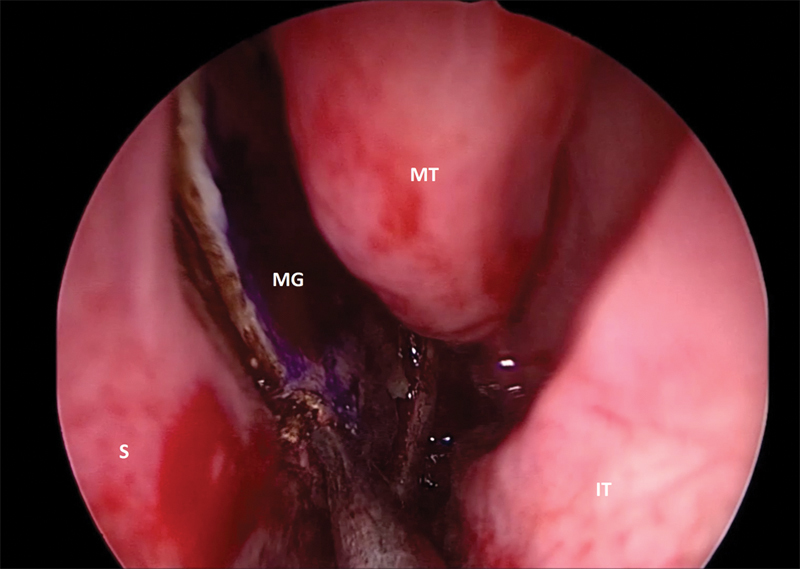
Still image of the left-sided posterior septal free mucosal graft in the process of harvest. IT, inferior turbinate; MG, mucosal graft; MT, middle turbinate; S, septum.
Fig. 2.
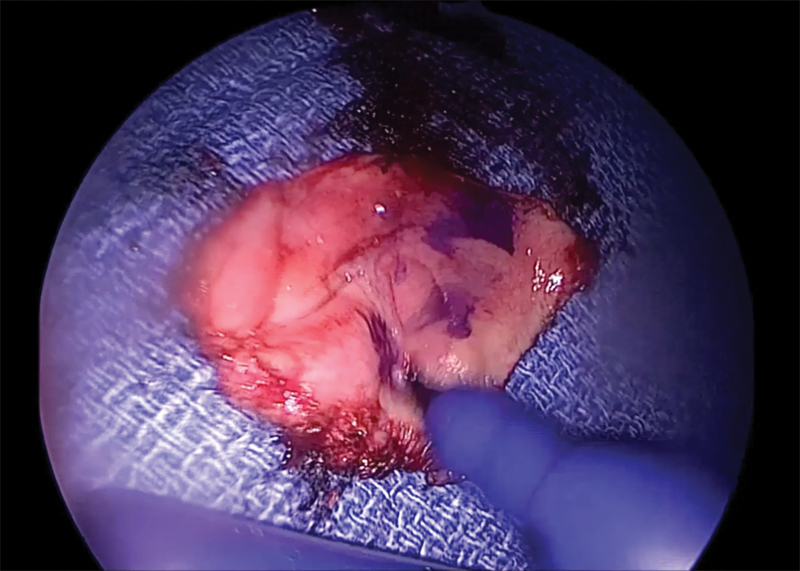
Still image of the posterior septal free mucosal graft after removal from the nose, in process of being marked on the mucosal side.
Placement of the graft is performed after the skull base portion of the surgical case has been completed. The graft is placed into the nasal cavity, at the anterior aspect of the exposed septal cartilage on the NSF side, with care taken to ensure the mucosal side of the graft is not faced toward the septal cartilage. With a Cottle elevator or ball-tipped probe, the edges of the graft are then unfurled and placed flat against the septal cartilage. The anterior aspect of the graft is positioned to directly oppose the cut edge of the mucocutaneous junction at the anterior septum. The graft is then secured to the septum using 4–0 plain gut suture on a Keith needle in a quilting fashion (Fig. 3). Additionally, absorbable nasal packing material is placed on along the graft to ensure apposition to the cartilage.
Fig. 3.
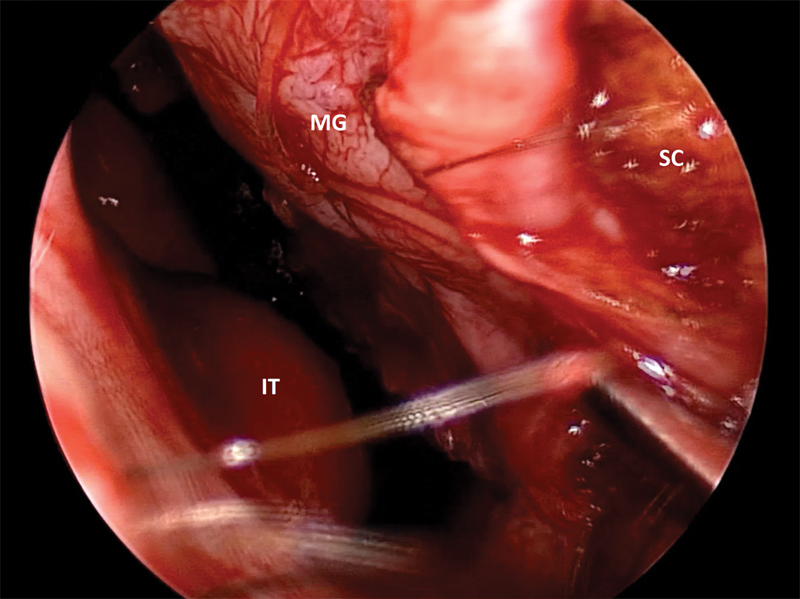
Still image of placement of the mucosal graft onto anterior septal nasoseptal flap donor site. Secured to septum with a 4–0 plain gut suture in quilting fashion. IT, inferior turbinate; MG, mucosal graft; SC, septal cartilage.
Patients were discharged with antibiotics for nasal packing toxic shock syndrome prophylaxis and nasal saline spray to be used starting on postoperative day 2, with two sprays on each side three times a day. The first postoperative follow-up was performed 1 to 2 weeks after surgery whenever possible. During this visit, residual absorbable nasal packing was removed and the graft was inspected. At this time, patients are instructed to begin gentle nasal saline irrigations twice daily. The second postoperative follow-up was performed ∼6 weeks thereafter, with nasal endoscopy and debridement performed if necessary. Subsequent follow-up visits were performed on an as needed basis.
Results
A total of 15 patients were identified for inclusion in this series. All patients underwent transnasal, transsphenoidal approaches for resection of a sellar or parasellar tumor. Table 1 shows the demographics, patient characteristics, and outcomes. The average age of these patients was 53 years. Ten of the patients were female and five were male. The majority of the patients (13) underwent transnasal, transsphenoidal resection of a pituitary adenoma, with one case of meningioma and one case of a craniopharyngioma. All but one of the patients had their NSF harvested from the right side. The choice of side being predominantly the right side was due to surgeon's preference. The patient who had the left-sided NSF harvest was due to the presence of a large septal spur which necessitated the use of the left nasal septal mucosa.
Table 1. Demographics, patient characteristics, and outcomes.
| Number of patients | N = 15 |
|---|---|
| Age at time of surgery (range) | 53 y (16–79 y) |
| Gender | |
| Male | 5 |
| Female | 10 |
| Tumor pathology | |
| Pituitary adenoma | 13 |
| Craniopharyngioma | 1 |
| Meningioma | 1 |
| Nasoseptal flap side | |
| Right | 14 |
| Left | 1 |
| Mucosal graft harvest side | |
| Right | 1 |
| Left | 14 |
| Graft success rate | 100% |
Upon follow-up, each patient was examined with nasal endoscopy. All free mucosal grafts were inspected and any residual nasal packing and crusting removed. There were no graft failures in this group. At the 6-week follow-up, all patients showed near complete remucosalization of the NSF donor site, with no or minimal crusting (Fig. 4). No anterior septal perforations were identified. There were no complications of the graft harvest site or the graft site during the perioperative or postoperative period.
Fig. 4.
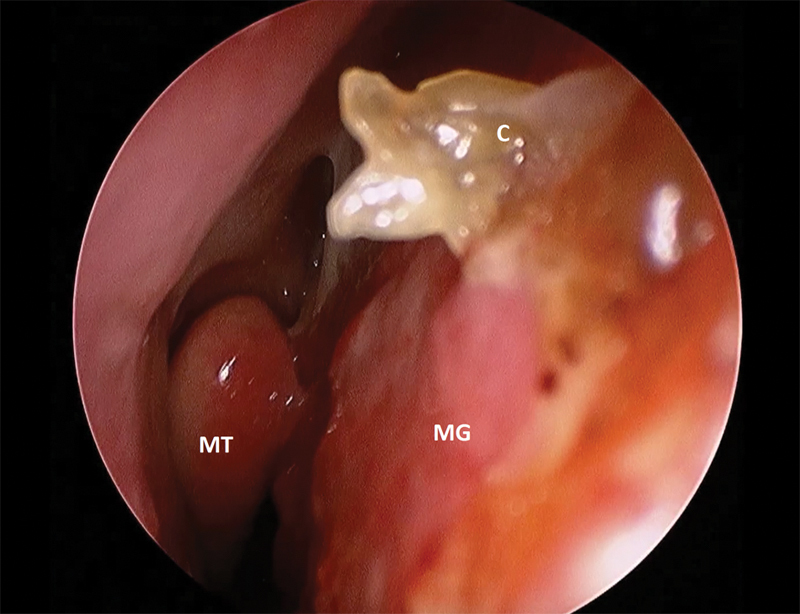
Postoperative week 6 image of a septal mucosal graft. A small crust noted anteriorly along the edge of the graft. C, crust; MG, mucosal graft; MT, middle turbinate.
Discussion
Quality of life after endoscopic endonasal skull base surgery has become an important topic of investigation in recent years as these approaches are becoming more popular. Multiple studies evaluating sinonasal morbidity after endoscopic endonasal skull base surgery have found that there is a 3- to 4-month period of increased sinonasal morbidity associated with these procedures.11 13 14 17 18 After this early period of increased sinonasal morbidity, improvements in sinonasal symptoms compared with preoperative levels following endoscopic endonasal skull base surgery have been shown.14 18 19 The most common sinonasal complaints after endoscopic endonasal skull base surgery included nasal crusting, nasal discharge, nasal airflow blockage, and disturbances in olfaction.14 17
The NSF has revolutionized reconstruction of the skull base in endoscopic endonasal approaches. Its use, however, leaves behind a large area of exposed cartilage and bone which can take 6 to 12 weeks to heal completely.8 With the absence of native mucosa with its mucociliary function, the stagnant blood and secretions on the exposed cartilage create a nidus for crusting which may lead to patient discomfort from nasal fullness, pain, poor nasal airflow, propensity for infection, and need for frequent debridements.15 Several studies have investigated the sinonasal morbidity associated with use of the NSF with conflicting results. Hanson et al found significant endoscopy score differences in postoperative patients after NSF at 90 days after surgery; however, no differences were noted in quality-of-life questionnaire scores.10 De Almeida et al, in a prospective cohort study, found that patients with NSF reconstruction did have a slightly shorter time to absence of crusting, but this finding was not statistically significant.13 Three other investigations, in contrast, all noted significant improvements in subjective sinonasal morbidity in patients who did not have a NSF utilized compared with those who did.11 12 20
Several alterations to the traditional NSF have been proposed to reduce the postoperative nasal morbidity associated with its use. The nasoseptal “rescue” flap has been described in which the posterior-superior aspect of the NSF and the posterior septal branch pedicle are elevated and reflected inferiorly off of the nasal septum at the posterior septectomy site, while the rest of the flap is kept in place to reduce the nasal morbidity in the event the NSF may not be required.21 22 Use of various mucosal grafting techniques at the anterior NSF donor site has also been previously shown to reduce crusting and allow for faster remucosalization of the anterior septal donor site.15 16 In one technique, a resected middle turbinate is used in ordered to obtain a free mucosal graft which is placed onto the NSF donor site.15 In these situations, middle turbinectomy was performed for surgical access; however, this is not always necessary for lesions of the sella, planum sphenoidale, and upper third of the clivus.23 24 Additionally, resection of the middle turbinate may result in additional morbidity, such as altered nasal airflow, reduced nasal air humidification, and epistaxis from the turbinectomy site.25 26 The “reverse flap” is another mucosal flap technique aimed at grafting the anterior NSF donor site, but to allow the flap to reach the anterior edge of the donor site, the posterior septectomy may need to be extended anteriorly, possibly causing increased alteration of nasal airflow.16
In this study, we introduce a previously undescribed method for reconstruction of the anterior septum with a free mucosal graft harvested from the contralateral nasal septal mucosa, at the site of the posterior septectomy. This process is advantageous to previously described techniques in that it utilizes mucosa which would otherwise be discarded, allows for preservation of the middle turbinate, thus avoiding any associated morbidity, and allows for placement of the graft with direct apposition to the anterior mucosal edge. In addition, during the trial of this technique, there were several instances in which the tumor resection did not result in a CSF leak, and thus did not require a NSF for reconstruction. In these instances, the free mucosal graft harvested earlier in the case was utilized to reconstruct the skull base defect and the NSF was replaced and secured to the anterior septum with a plain gut suture. The harvesting of the free mucosal graft from the posterior septum prevents the need to harvest a separate free mucosal graft at a later time and allows for versatility and adaptability at the conclusion of the case, depending on the reconstructive needs of the skull base defect. Though our technique does leave a small area (∼5 mm in width) of exposed septal cartilage or bone at the anterior aspect of the septectomy, this area remucosalizes quickly due to its small size and has not presented any significant problems in the postoperative follow-up period. It is our opinion that the anterior septum, if left exposed, would have a more significant effect on the sinonasal morbidity following NSF harvest due to its position anteriorly, leading to greater exposure to airflow and crusting in this area, with subsequent nasal obstruction and discomfort.
Sinonasal morbidity, such as nasal obstruction, discomfort, and/or disturbances in smell/taste, is possible from any nasal surgery, and as mentioned previously in this discussion, can be expected in the short term following endoscopic endonasal surgery. In our patient cohort, all patients reported some degree of nasal obstruction, headaches, and/or smell disturbance in the first follow-up visit, likely secondary to postoperative edema and residual nasal packing which were removed at that time. It is also difficult to determine if the sinonasal morbidity was secondary to the endoscopic endonasal surgery as a whole, or if it was related to the septal grafting technique alone, based on this study design. However, on subsequent follow-up visits, most patients reported resolution of nasal obstruction and smell disturbance. We do believe that with more rapid remucosalization of the anterior septum, there will be a faster return to baseline in terms of nasal morbidity, but this needs to be studied further. After the first two follow-up visits, patients returned on an as needed basis, thus long-term outcomes (outside of first 2–3 months following surgery) are not available.
The limitations of this study include its small study sample, retrospective design, and lack of long-term follow-up. Future analysis of a prospective cohort, possibly with a control group, may allow for improved delineation of the advantages of this technique. However, from previous published reports with prospective data and similar technical process, it can be inferred that this technique may lead to improved early postoperative sinonasal symptoms.15
Conclusions
We present a novel technique for anterior septal reconstruction of the NSF harvest site following its use in endoscopic endonasal skull base surgical procedures. This technique utilizes mucosa which would otherwise be discarded and allows for reconstruction at the most anterior aspect of the donor site. Our results show that it is a reliable method with a 100% graft take rate in this small series, with minimal complexity and added time to the surgical procedure. This adjunct procedure may reduce short-term nasal morbidity in patients undergoing transnasal, transsphenoidal approaches to the skull base.
Note
This paper was presented as a podium presentation at the North American Skull Base Society Meeting in Scottsdale, AZ on February 12–14, 2016.
References
- 1.Zuniga M G, Turner J H, Chandra R K. Updates in anterior skull base reconstruction. Curr Opin Otolaryngol Head Neck Surg. 2016;24(1):75–82. doi: 10.1097/MOO.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 2.Chin D, Harvey R J. Endoscopic reconstruction of frontal, cribiform and ethmoid skull base defects. Adv Otorhinolaryngol. 2013;74:104–118. doi: 10.1159/000342285. [DOI] [PubMed] [Google Scholar]
- 3.Patel M R, Stadler M E, Snyderman C H. et al. How to choose? Endoscopic skull base reconstructive options and limitations. Skull Base. 2010;20(6):397–404. doi: 10.1055/s-0030-1253573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eloy J A, Patel S K, Shukla P A, Smith M L, Choudhry O J, Liu J K. Triple-layer reconstruction technique for large cribriform defects after endoscopic endonasal resection of anterior skull base tumors. Int Forum Allergy Rhinol. 2013;3(3):204–211. doi: 10.1002/alr.21089. [DOI] [PubMed] [Google Scholar]
- 5.Clavenna M J, Turner J H, Chandra R K. Pedicled flaps in endoscopic skull base reconstruction: review of current techniques. Curr Opin Otolaryngol Head Neck Surg. 2015;23(1):71–77. doi: 10.1097/MOO.0000000000000115. [DOI] [PubMed] [Google Scholar]
- 6.Hadad G, Bassagasteguy L, Carrau R L. et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope. 2006;116(10):1882–1886. doi: 10.1097/01.mlg.0000234933.37779.e4. [DOI] [PubMed] [Google Scholar]
- 7.Harvey R J, Parmar P, Sacks R, Zanation A M. Endoscopic skull base reconstruction of large dural defects: a systematic review of published evidence. Laryngoscope. 2012;122(2):452–459. doi: 10.1002/lary.22475. [DOI] [PubMed] [Google Scholar]
- 8.Kassam A B Thomas A Carrau R L et al. Endoscopic reconstruction of the cranial base using a pedicled nasoseptal flap Neurosurgery 200863101ONS44–ONS52., discussion ONS52–ONS53 [DOI] [PubMed] [Google Scholar]
- 9.Soudry E, Psaltis A J, Lee K H, Vaezafshar R, Nayak J V, Hwang P H. Complications associated with the pedicled nasoseptal flap for skull base reconstruction. Laryngoscope. 2015;125(1):80–85. doi: 10.1002/lary.24863. [DOI] [PubMed] [Google Scholar]
- 10.Hanson M, Patel P M, Betz C, Olson S, Panizza B, Wallwork B. Sinonasal outcomes following endoscopic anterior skull base surgery with nasoseptal flap reconstruction: a prospective study. J Laryngol Otol. 2015;129 03:S41–S46. doi: 10.1017/S002221511500047X. [DOI] [PubMed] [Google Scholar]
- 11.Pant H, Bhatki A M, Snyderman C H. et al. Quality of life following endonasal skull base surgery. Skull Base. 2010;20(1):35–40. doi: 10.1055/s-0029-1242983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson C F, Suh J D, Liu Y, Bergsneider M, Wang M B. Modifications to the endoscopic approach for anterior skull base lesions improve postoperative sinonasal symptoms. J Neurol Surg B Skull Base. 2014;75(1):65–72. doi: 10.1055/s-0033-1356492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Almeida J R, Snyderman C H, Gardner P A, Carrau R L, Vescan A D. Nasal morbidity following endoscopic skull base surgery: a prospective cohort study. Head Neck. 2011;33(4):547–551. doi: 10.1002/hed.21483. [DOI] [PubMed] [Google Scholar]
- 14.Balaker A E, Bergsneider M, Martin N A, Wang M B. Evolution of sinonasal symptoms following endoscopic anterior skull base surgery. Skull Base. 2010;20(4):245–251. doi: 10.1055/s-0030-1249248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimple A J, Leight W D, Wheless S A, Zanation A M. Reducing nasal morbidity after skull base reconstruction with the nasoseptal flap: free middle turbinate mucosal grafts. Laryngoscope. 2012;122(9):1920–1924. doi: 10.1002/lary.23325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kasemsiri P, Carrau R L, Otto B A. et al. Reconstruction of the pedicled nasoseptal flap donor site with a contralateral reverse rotation flap: technical modifications and outcomes. Laryngoscope. 2013;123(11):2601–2604. doi: 10.1002/lary.24088. [DOI] [PubMed] [Google Scholar]
- 17.Awad A J, Mohyeldin A, El-Sayed I H, Aghi M K. Sinonasal morbidity following endoscopic endonasal skull base surgery. Clin Neurol Neurosurg. 2015;130:162–167. doi: 10.1016/j.clineuro.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 18.McCoul E D, Anand V K, Bedrosian J C, Schwartz T H. Endoscopic skull base surgery and its impact on sinonasal-related quality of life. Int Forum Allergy Rhinol. 2012;2(2):174–181. doi: 10.1002/alr.21008. [DOI] [PubMed] [Google Scholar]
- 19.Harrow B R, Batra P S. Sinonasal quality of life outcomes after minimally invasive resection of sinonasal and skull-base tumors. Int Forum Allergy Rhinol. 2013;3(12):1013–1020. doi: 10.1002/alr.21200. [DOI] [PubMed] [Google Scholar]
- 20.Alobid I, Enseñat J, Mariño-Sánchez F. et al. Expanded endonasal approach using vascularized septal flap reconstruction for skull base tumors has a negative impact on sinonasal symptoms and quality of life. Am J Rhinol Allergy. 2013;27(5):426–431. doi: 10.2500/ajra.2013.27.3932. [DOI] [PubMed] [Google Scholar]
- 21.Rivera-Serrano C M, Snyderman C H, Gardner P. et al. Nasoseptal “rescue” flap: a novel modification of the nasoseptal flap technique for pituitary surgery. Laryngoscope. 2011;121(5):990–993. doi: 10.1002/lary.21419. [DOI] [PubMed] [Google Scholar]
- 22.Rawal R B, Kimple A J, Dugar D R, Zanation A M. Minimizing morbidity in endoscopic pituitary surgery: outcomes of the novel nasoseptal rescue flap technique. Otolaryngol Head Neck Surg. 2012;147(3):434–437. doi: 10.1177/0194599812443042. [DOI] [PubMed] [Google Scholar]
- 23.Guthikonda B, Nourbakhsh A, Notarianni C, Vannemreddy P, Nanda A. Middle turbinectomy for exposure in endoscopic endonasal transsphenoidal surgery: when is it necessary? Laryngoscope. 2010;120(12):2360–2366. doi: 10.1002/lary.21153. [DOI] [PubMed] [Google Scholar]
- 24.Nyquist G G, Anand V K, Brown S, Singh A, Tabaee A, Schwartz T H. Middle turbinate preservation in endoscopic transsphenoidal surgery of the anterior skull base. Skull Base. 2010;20(5):343–347. doi: 10.1055/s-0030-1253582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsakiropoulou E, Vital V, Constantinidis J, Kekes G. Nasal air-conditioning after partial turbinectomy: myths versus facts. Am J Rhinol Allergy. 2015;29(2):e59–e62. doi: 10.2500/ajra.2015.29.4151. [DOI] [PubMed] [Google Scholar]
- 26.Cassano M, Cassano P. Epistaxis after partial middle turbinectomy: the role of sphenopalatine artery ligation. Am J Otolaryngol. 2012;33(1):116–120. doi: 10.1016/j.amjoto.2011.04.007. [DOI] [PubMed] [Google Scholar]


