Abstract
Introduction The placement of intramammary marker clips has proven to be helpful for tumor localization in patients undergoing neoadjuvant chemotherapy and breast-conserving surgery. The purpose of our study was to investigate the feasibility of using a clip marker system for breast cancer localization and its influence on the imaging assessment of treatment responses after neoadjuvant chemotherapy.
Patients and Methods Between March and June 2015, a total of 25 patients (n = 25), with a suspicion of invasive breast cancer with diameters of at least 2 cm (cT2), underwent preoperative sonographically guided core needle biopsy using a single-use breast biopsy system (HistoCore™) and intramammary clip marking using a directly adapted clip system based on the established O-Twist Marker™, before their scheduled preoperative neoadjuvant chemotherapy. Localization of the intramammary marker clip was controlled by sonography and digital breast tomosynthesis.
Results Sonography detected no dislocation of intrammammary marker clips in 20 of 25 patients (80 %), while digital breast tomosynthesis showed accurate placement without dislocation in 24 patients (96 %) (p < 0.05). There was no evidence of significant clip migration during preoperative follow-up imaging after neoadjuvant chemotherapy. No complication related to the clip marking was noted and there was no difficulty in evaluating the treatment response to neoadjuvant chemotherapy. Among the breast-conserving surgeries performed, no cases were identified in which intraoperative loss of the marker clip had occurred.
Conclusion Our study underscores the importance of intramammary marking clip systems before neoadjuvant chemotherapy. Placement of marker clips is advised to facilitate accurate tumor bed localization. With regard to digital breast tomosynthesis, its development continues to improve the quality of diagnostics and the therapy of breast cancer particularly for small breast cancer tumors or in neoadjuvant chemotherapy setting.
Key words: breast cancer, neoadjuvant chemotherapy, intramammary clip marker, digital breast tomosynthesis (DBT)
Zusammenfassung
Einleitung Die Platzierung intramammärer Clips zur Markierung eines Mammakarzinoms bei Patientinnen, bei denen eine neoadjuvante Chemotherapie und brusterhaltenden Therapie geplant sind, ist heutzutage ein etabliertes Verfahren. Ziel dieser Studie war es, den Einsatz eines intramammären Clipmarkierungs-Systems unter neoadjuvanter Chemotherapie zu überprüfen, und dessen möglichen Einfluss auf bildgebende Verfahren zur Beurteilung der Ansprechrate nach neoadjuvanter Chemotherapie.
Patientinnen und Methoden Im Rahmen dieser Studie wurde bei 25 Patientinnen zwischen März und Juni 2015 bei Verdacht auf ein Mammakarzinom mit einem Durchmesser von größer 2 cm (cT2), eine sonografisch gesteuerte Stanzbiopsie (HistoCore™) mit gleichzeitiger adaptierter Clipmarkierung (O-Twist Marker™) in das Tumorzentrum vor einer geplanten neoadjuvanten Chemotherapie durchgeführt. Anschließend wurde eine Lokalisationsbestimmung der intramammären Clipmarkierung sowohl durch Sonografie als auch durch eine digitale Brusttomosynthese durchgeführt.
Ergebnisse Sonografisch war bei 20 von 25 Patientinnen (80 %) keine Dislozierung der intrammammären Clips festzustellen, wohingegen die digitale Tomosynthese der Brust eine exakte Platzierung ohne Dislozierung bei 24 Patientinnen (96 %) nachwies (p < 0,05). Nach neoadjuvanter Chemotherapie ergab sich kein Anhalt für eine signifikante Dislozierung in der präoperativen Verlaufskontrolle. Des Weitern gab es weder Komplikationen im Zusammenhang mit der Clipmarkierung noch Schwierigkeiten bei der Bestimmung der Ansprechraten nach der neoadjuvanten Chemotherapie. Unter allen Patientinnen, die sich einer brusterhaltenden Therapie unterzogen, gab es keine Fälle mit intraoperativem Verlust des Markierungsclips.
Schlussfolgerung Unsere Studie hebt die Bedeutung einer intramammären Clipmarkierung vor einer neoadjuvanten Chemotherapie hervor. Eine Platzierung von Markierungsclips wird empfohlen, um die präzise Lokalisierung von Brusttumoren zu erleichtern. Was die digitale Tomosynthese der Brust anbetrifft, wird die kontinuierliche Weiterentwicklung dieses bildgebenden Verfahrens die Qualität der Diagnostik und Therapie des Mammakarzinoms weiter verbessern, besonders bei kleinen Tumoren bzw. bei neoadjuvanter Chemotherapie.
Schlüsselwörter: Brustkrebs, neoadjuvante Chemotherapie, intramammäre Clipmarkierung, digitale Tomosynthese der Brust (DBT), Mammakarzinom
Introduction
Neoadjuvant chemotherapy has been the accepted standard of care for patients with operable or inoperable breast cancers. The benefits of neoadjuvant chemotherapy performed prior to surgery are
reduction of mortality
improvement of surgical options, such as conversion to breast-conserving surgery in operable patients, as well as surgery in previously inoperable patients
early collection of information on the treatment response and tumor biology of the breast cancer 1, 2, 3, 4, 5
As new chemotherapeutic agents have been developed, patients who have undergone neoadjuvant chemotherapy have shown a positive response and often even pathologic complete response (pCR) can be achieved. The placement of intramammary marker clips has proven to be helpful and safe for tumor localization in patients undergoing neoadjuvant chemotherapy and breast-conserving surgery because a pathologic complete response in a patient without a marker clip would not allow to accurately locate and excise any residual cancerous tissue, or reconstruct the breast with a satisfactory cosmetic result 6, 7, 8.
This difficulty can be remedied using a marker clip to locate the primary breast tumor during sonographically guided core needle biopsy prior to surgical therapy or neoadjuvant chemotherapy 9, 10, 11, 12. Based on the findings of our previous studies and using a marker clip system we had helped to develop and establish 13, 14, we investigated the accuracy of an innovative clip marking method in patients with breast cancer 15. Our previously published results confirmed that this innovation had precisely adapted the stylet length of the marking system to the single-use breast biopsy system (HistoCore™). Formerly, when using the O-Twist Marker™ clip system, a pre-fabricated sliding spacer was placed directly around the stylet, and the notch length of the spacer used during marker clip placement (pushed forward through the coaxial biopsy needle in situ) had to be varied for every intervention, depending on the respective length of the biopsy needle. This approach was imprecise and semi-subjective because it was not possible to adjust the fitted spacer precisely using only the marking ring engraved at intervals of one centimeter. Our innovation offered the possibility of placing a precise marker clip for every biopsy needle length without requiring a spacer 15.
This creates a target point for preoperative sonographically guided wire marking, which is particularly useful in patients with pathologic complete response after neoadjuvant chemotherapy.
The response after neoadjuvant chemotherapy can be evaluated by different imaging techniques 12. In the view of those imaging techniques we had investigated in a recent study the accuracy of different imaging techniques when determining the precise position of marker clips placed directly in the center of intramammary lesions 16. In particular, we had compared the accuracy of sonography versus digital breast tomosynthesis to locate the above mentioned intramammary marker clip placed under sonographic guidance and had shown that the use of digital breast tomosynthesis could improve the accuracy when locating intramammary marker clips compared to sonography 16.
This time the purpose of our current study was to investigate the feasibility of using the clip marker system for tumor localization and its influence on the imaging assessment of treatment responses after neoadjuvant chemotherapy.
Material and Methods
Study population
One-hundred and three patients (n = 103) with a suspicion of invasive breast cancer with diameters of at least 2 cm (cT2) were investigated at the University Breast Center Franconia between March and June 2015, using complementary breast diagnostics consisting of clinical examination, mammography (Selenia Dimensions3D™ [Hologic™]) and sonography (2-D, Acuson Antares, 13 MHz [Siemens™]).
The decision to perform sonographically guided core needle biopsy in combination with clip marking was made subjectively by the examining radiologist. A total of 25 patients (n = 25) underwent preoperative sonographically guided clip marking to accurately localize the malignant lesion before their scheduled preoperative neoadjuvant chemotherapy and were enrolled in this study. The neoadjuvant chemotherapy regimen for all enrolled patients was four cycles of combined epirubicin (90 mg/m2 BSA [body surface area]) and cyclophosphamide (600 mg/m2 BSA), q21d, followed by twelve cycles of paclitaxel (80 mg/m2 BSA), q7d.
Sonographically guided core needle biopsy and clip marking
Invasive breast cancer was confirmed histologically in all 25 patients by sonographically guided core needle biopsy using a single-use breast biopsy system (HistoCore™, BIP™ Biomedizinische Instrumente & Produkte GmbH, Germany) 13. Intramammary clip marking was additionally done using a directly adapted clip system based on the established O-Twist Marker™ system (BIP™ Biomedizinische Instrumente & Produkte GmbH, Germany) 14, 15. All sonographically guided core needle biopsies and clip markings were performed by the same two experienced radiologists to exclude potential inter-observer variability. The single-use breast biopsy system (HistoCore™) was used with a 12-gauge, 10-cm outer cannula and a needle advance of 18 or 25 mm. After careful disinfection of the skin and administration of a local anesthetic, the single-use breast biopsy system (combination of a coaxial cannula [11 gauge] and the core biopsy needle [12 gauge]) was placed over the focal tumor. Core needle biopsy was carried out under sonographic control tangentially to the linear 13.0-MHz transducer. The length of the needle was documented before and after the intervention on pictures. Four or more core needle biopsy specimens were obtained to secure sufficient material for histological diagnosis and molecular-genetic testing. Using the coaxial needle (11 gauge) and the adapted clip system (O-Twist Marker™), a clip was placed directly in the puncture site, i.e. in the middle of the tumorous lesion, under “real-time” sonographic guidance for subsequent control investigations of clip localization.
Localization control of marker clips using sonography and digital breast tomosynthesis
Localization of the intramammary marker clip was controlled by sonography (2-D, Acuson Antares, 13 MHz [Siemens™]) and digital breast tomosynthesis (Selenia Dimensions 3D™ [Hologic™]) approximately 30 minutes after the intervention. A compression bandage was used in all patients to minimize hematoma formation. As part of our study, digital breast tomosynthesis with the same radiation dose was performed in each patient (after informed consent) instead of control mammography (two orthogonal planes, cranio-caudal and medio-lateral oblique view).
Post-procedural measurements of dislocation of the intramammary marker clips in mm (along the x-, y- and z-axis) were performed as previously published by us 16, and were also carried out by the same two radiologists to reduce inter-observer variability.
After completion of neoadjuvant chemotherapy follow-up sonography was performed before elective surgery to evaluate treatment response according to revised RECIST guideline (version 1.1) 17.
After surgical excision, specimen mammography was also done to evaluate clip retrieval and to assess specimen margin. A pathologist confirmed the results.
The same two radiologists reviewed the medical records, and all images from the time of clip insertion to surgery were reviewed to confirm the localization of clips, clip migration, the presence of complications such as hemorrhage or infection, and the effect of clips on treatment assessment. Clip migration was defined as the clip being located outside the proven malignancy at a distance of more than 10 mm.
Statistical analysis
SPSS 18.0 was used for statistical analysis. Data were initially analyzed descriptively and subsequently assessed for statistically relevant differences between investigated groups. The level of significance was p < 0.05.
Results
Comparison of sonography versus digital breast tomosynthesis to locate the intramammary marker clip
All included patients (n = 25) presented with breast lesion of a clinical T2 tumor stage. Lesion diameters ranged from 2.2 to 3.6 cm (median 2.7 cm) and patient age range was 31–77 years (median 54 years). After placing the marker clip in the center of each tumor, the localization of the marker clip was controlled using both sonography (Fig. 1) and digital breast tomosynthesis (Fig. 2).
Fig. 1.
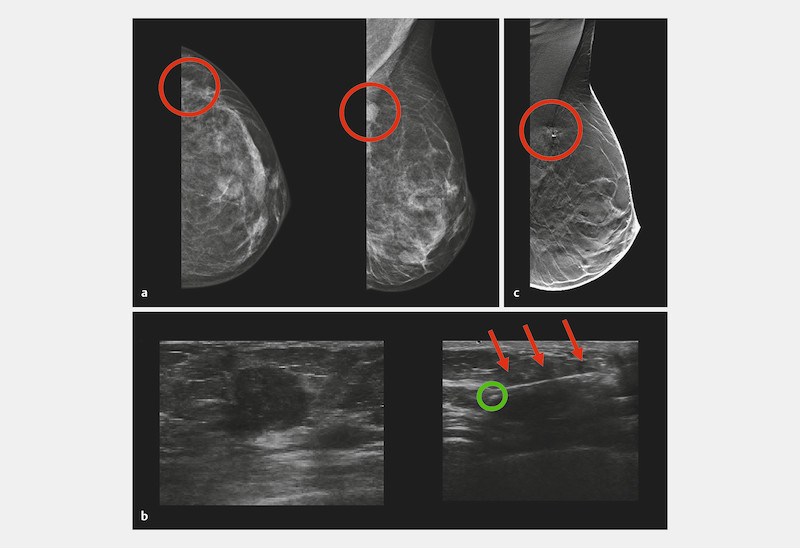
Detection of an intramammary lesion by mammography and sonography, sonographically guided core needle biopsy and clip marking, and localization of the intramammary marker clip using digital breast tomosynthesis. a Mammogram (cranial-caudal and mediolateral-oblique view) of the left breast of a 48-year-old woman showing a breast cancer lesion (cT2) (red circles). b Corresponding sonogram and sonographic localization of the intramammary marker clip. The biopsy needle tip is sited directly above the focal tumor (red arrows, 3 × magnification) after placement of the marker clip (green circle). c Postprocedural digital breast tomosynthesis was performed after clipping and showed the marker clip in the center of the intramammary lesion (red circle).
Fig. 2.
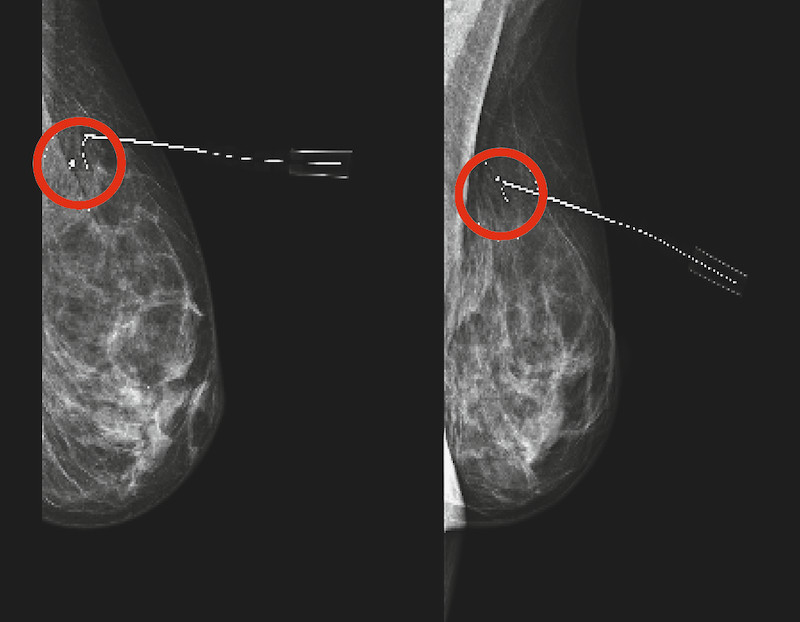
Preoperative follow-up imaging after neoadjuvant chemotherapy. Mammogram after sonographically guided wire marking of the intramammary lesion after neoadjuvant chemotherapy. The clip was located in the intramammary lesion which had decreased in size (red circle). There was no evidence of clip migration or other complications.
On sonography, the inserted clip appeared as a hyperechoic structure with or without posterior shadowing and on digital breast tomosynthesis as a ring structure. By sonography (measurement of the tumor without the echo-rich margin) no dislocation of the marker clips was seen in 20 of 25 patients (80 %), while in four patients (20 %) sonography control indicated a maximum dislocation of 6 mm along the x-, y- or z-axis (Table 1). Digital breast tomosynthesis showed precise placement without dislocation of the marker clip in 24 patients (96 %); a maximum dislocation of 2 mm along the x-, y- or z-axis was found in one patient (4 %) (Table 1). The difference in the accuracy of the two imaging techniques to locate marker clips was statistically significant (p < 0.05).
Table 1 Post-procedurally measured dislocation of the intramammary marker clip in mm (along the x-, y- and z-axis); dislocation was observed in five patients using sonography and in one patient using digital breast tomosynthesis (DBT). The intratumorous location of the marker clip was confirmed by specimen x-ray (SX) and histopathological examination (Histo.) after neoadjuvant chemotherapy and breast conserving surgery (out of a total of 25 examined patients).
| Patients | Sonography | DBT | SX. | Histo. | ||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | |||
| 1. | 2 | 2 | 3 | 0 | 0 | 0 | ✓ | ✓ |
| 2. | 5 | 4 | 3 | 0 | 0 | 0 | ✓ | ✓ |
| 3. | 4 | 6 | 5 | 0 | 0 | 0 | ✓ | ✓ |
| 4. | 5 | 5 | 6 | 0 | 0 | 0 | ✓ | ✓ |
| 5. | 5 | 5 | 6 | 0 | 0 | 0 | ✓ | ✓ |
| 6. | 0 | 0 | 0 | 1 | 2 | 2 | ✓ | ✓ |
Radiologic evaluation after neoadjuvant chemotherapy
The mean period between clip marking and preoperative follow-up imaging after neoadjuvant chemotherapy was 196 days (± 24 days). There was no evidence of significant clip migration during preoperative follow-up imaging, detected by sonography and mammography after sonographically guided wire marking of the intramammary lesion after neoadjuvant chemotherapy (Fig. 2).
Furthermore, no complication related to the clip marking was noted during the follow-up examinations, and no patient complained of pain during sonography.
Moreover, there was no difficulty in evaluating the treatment response to neoadjuvant chemotherapy using sonography. We observed an overall complete response rate of 32 % (n = 8), a partial response rate of 64 % (n = 16), and a stable disease in one case (4 %).
The intratumorous location of the marker clip was confirmed both by specimen x-ray done intraoperatively and by the subsequent histopathological examination of the tumor.
Postoperative pathological evaluation and exclusion of intraoperative loss of clips
All patients underwent breast-conserving surgery, and specimen x-rays (Fig. 3) were correlated with imaging findings and histopathological examination of the specimens. Pathologic response of the breast tumor after neoadjuvant chemotherapy was reported in all but one case (4 %). Pathologic complete response of the breast tumors was defined as no residual invasive tumor (ypT0) 18 and we observed a pathologic complete response rate of 24 % (n = 6). There was no difficulty in the pathological evaluation of a specimen due to the inserted marker clip. Among the 25 surgical procedures performed during the study period, no cases were identified in which intraoperative loss of the marker clip had occurred.
Fig. 3.
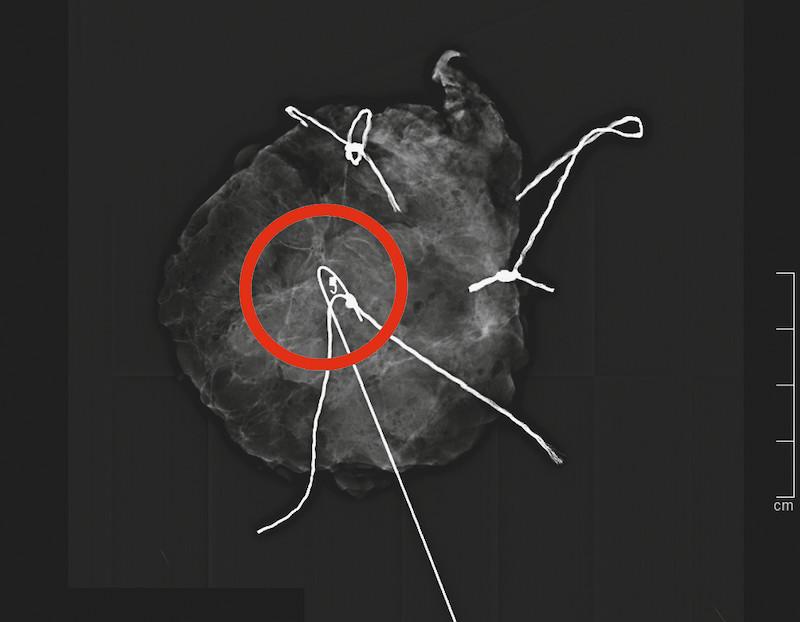
Intratumorous localization of the marker clip by specimen x-ray. Specimen x-ray (with radiopaque threads for specimen orientation) was performed immediately after breast-conserving surgery, and the marker clip was visualized in the proven intramammary lesion without evidence of clip migration (red circle). The pathologic result was invasive breast cancer of no special type, and a clear tumor margin was observed.
Discussion
The use of neoadjuvant chemotherapy is a powerful tool, alongside oncoplastic techniques, for offering women increasing options for breast-conserving surgery. In operable breast cancer, neoadjuvant chemotherapy also allows measuring in vivo response to systemic therapy. This response can be evaluated by different imaging techniques. However, neoadjuvant therapy concepts represent new challenges for breast surgeons, radiologists and pathologists, as there is no target for preoperative, sonographically guided wire marking in patients in pathologic complete response.
In this context the aim of this study was to investigate the accuracy of a marker clip system and different imaging techniques when determining the precise position of marker clips placed directly in the center of intramammary lesions before and after neoadjuvant chemotherapy. Reports have shown that clip deployment following stereotactic core-needle biopsy is an accurate method for marking the biopsy site 9, 10, 19. Other studies reported that clips may be deployed off the biopsy site or migrate from the biopsy site 10, 20. There are many reported causes of clip migration, which for example include the clip migration in the biopsy track, clip displacement by a hematoma, change in the clip site due to resorption of air at the biopsy cavity, or as mentioned above changes in the clip site after neoadjuvant chemotherapy 21. To improve the accuracy of clip localization, several generations of clips have been developed. The marker clip system, which combines a single-use core biopsy needle with a precisely adapted marker clip for the placement through a coaxial needle in situ, allows clips to be placed accurately in intramammary lesions 15.
Based on the findings of our previous studies, here we investigated the accuracy of this innovative clip marking method in patients with breast cancer (cT2) scheduled to undergo neoadjuvant chemotherapy.
The migration of surgical clips and related complications can be a major limitation of surgical clip insertion. There was no evidence of significant clip migration during preoperative follow-up after completion of neoadjuvant chemotherapy, despite the long time period from clipping to surgery of approximately 6 months. Furthermore, no complication related to the marker clip was noted during the follow-up examinations, and no patient complained of pain during examination or sonography. Our results confirmed that the examined marking clip system meets the requirements of neoadjuvant therapy concepts for breast cancer. It creates a target point for preoperative sonographically guided wire marking, which is particularly useful in patients with pathologic complete response after neoadjuvant chemotherapy 15.
There have been previously published studies examining intramammary marker clips inserted by sonographically guided semi-automated methods to facilitate localization of breast cancer after neoadjuvant chemotherapy 22, 23. This method seems to be superior to automated methods 9, 10 as there is no need for repeating needle insertion into the intramammary lesion with less complications. Nonetheless, these and others studies usually performed a two-step clip marking procedure; since we can perform on-site clip insertion immediately after core needle biopsy for either a benign or malignant lesion, both medical costs and procedure time is lower than those of the two-step clip marking procedure.
Control imaging using sonography and digital breast tomosynthesis to verify the position of the marker clip additionally confirmed the high diagnostic accuracy of digital breast tomosynthesis both before and after neoadjuvant chemotherapy. Although sonography is a well-established diagnostic method, the range of indications and the value of digital breast tomosynthesis has not yet been precisely determined 24, 25, 26, 27. Digital image acquisition, image processing and image reproduction allow many sequences of images to be acquired within a short time. The compilation of sequentially acquired tomograms is used to create three-dimensional images of the breast, so-called digital breast tomosynthesis 25. The radiation exposure parameters for every plane are selected to ensure that total radiation exposure corresponds to the radiation dose of two-plane mammography. Various reconstruction algorithms are used to display the breast as a series of slices at different depths or as a freely rotatable 3-dimensional image. Digital breast tomosynthesis thus redresses one of the limitations of mammography, namely, the reduction of the three-dimensional breast to a two-dimensional image 25. Digital breast tomosynthesis should therefore be able to avoid false-positive or false-negative findings which are the result of superimpositions created with mammography. This should improve detection rates in mammographically denser breasts 24. Digital breast tomosynthesis also appears to be superior to both sonography and mammography for the determination of the size of breast tumors 24. As shown earlier by us 16, our current findings confirm that determination of the localization of marker clips placed after sonographically guided core needle biopsy is significantly more precise using digital breast tomosynthesis compared to sonography. One reason for this could be because digital breast tomosynthesis is standardized compared to sonography, where the application is semi-subjective, irrespective of whether it is 2-D, 3-D, or 4-D imaging.
Our present study has several limitations. Firstly, only patients who had agreed to marker clip insertion and digital breast tomosynthesis instead of mammography were selected. Therefore, a selection bias may exist. Secondly, the number of subjects was limited at only 25, a number too small to provide a reliable overall generalization from the study results. Further studies are needed for continued assessment of this procedure. Thirdly, our investigation is lacking data on the further adjuvant treatment or survival rates.
Conclusions
We believe that our study underscores the importance of intramammary marking clip systems before neoadjuvant chemotherapy for optimal local control in breast-conserving surgery. Early placement of marker clips in the tumor bed and one-step clipping procedure are advised to facilitate accurate tumor bed localization and will provide valuable information for both the radiologist and breast surgeon.
With regard to digital breast tomosynthesis, its development continues to improve the quality of diagnostics and the therapy of breast cancer particularly for small breast cancer tumors or as shown here in neoadjuvant chemotherapy setting with subsequent pathologic (complete) response.
Footnotes
Conflict of Interest None.
References
- 1.Fisher B, Brown A, Mamounas E. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15:2483–2493. doi: 10.1200/JCO.1997.15.7.2483. [DOI] [PubMed] [Google Scholar]
- 2.Bear H D, Anderson S, Smith R E. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2006;24:2019–2027. doi: 10.1200/JCO.2005.04.1665. [DOI] [PubMed] [Google Scholar]
- 3.Mauri D, Pavlidis N, Ioannidis J P. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:188–194. doi: 10.1093/jnci/dji021. [DOI] [PubMed] [Google Scholar]
- 4.Cortazar P, Zhang L, Untch M. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 5.Boughey J C, McCall L M, Ballman K V.Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) Prospective Multicenter Clinical Trial Ann Surg 2014260608–614.discussion 614–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oh J L, Nguyen G, Whitman G J. Placement of radiopaque clips for tumor localization in patients undergoing neoadjuvant chemotherapy and breast conservation therapy. Cancer. 2007;110:2420–2427. doi: 10.1002/cncr.23068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaufmann M, von Minckwitz G, Mamounas E P. Recommendations from an international consensus conference on the current status and future of neoadjuvant systemic therapy in primary breast cancer. Ann Surg Oncol. 2012;19:1508–1516. doi: 10.1245/s10434-011-2108-2. [DOI] [PubMed] [Google Scholar]
- 8.Dash N, Chafin S H, Johnson R R. Usefulness of tissue marker clips in patients undergoing neoadjuvant chemotherapy for breast cancer. AJR Am J Roentgenol. 1999;173:911–917. doi: 10.2214/ajr.173.4.10511147. [DOI] [PubMed] [Google Scholar]
- 9.Liberman L, Dershaw D D, Morris E A. Clip placement after stereotactic vacuum-assisted breast biopsy. Radiology. 1997;205:417–422. doi: 10.1148/radiology.205.2.9356622. [DOI] [PubMed] [Google Scholar]
- 10.Rosen E L, Vo T T. Metallic clip deployment during stereotactic breast biopsy: retrospective analysis. Radiology. 2001;218:510–516. doi: 10.1148/radiology.218.2.r01fe39510. [DOI] [PubMed] [Google Scholar]
- 11.Schulz-Wendtland R, Heywang-Kobrunner S H, Aichinger U. [Do tissue marker clips after sonographically or stereotactically guided breast biopsy improve follow-up of small breast lesions and localisation of breast cancer after chemotherapy?] Rofo. 2002;174:620–624. doi: 10.1055/s-2002-28278. [DOI] [PubMed] [Google Scholar]
- 12.Schulz-Wendtland R. Neoadjuvant chemotherapy—monitoring: clinical examination, ultrasound, mammography, MRI, elastography: only one, only few or all? Eur J Radiol. 2012;81 01:S147–S148. doi: 10.1016/S0720-048X(12)70061-X. [DOI] [PubMed] [Google Scholar]
- 13.Schulz-Wendtland R, Dilbat G, Bani M R. HistoCore® – a new single-use breast biopsy system of the next generation in daily clinical routine. Senologie. 2010;7:265–268. [Google Scholar]
- 14.Sittek H, Heske N, Kessler M. [O-twist marker for post-interventional marking in imaging of suspected breast lesions] Radiologe. 2005;45:223–229. doi: 10.1007/s00117-005-1177-2. [DOI] [PubMed] [Google Scholar]
- 15.Schulz-Wendtland R, Dankerl P, Dilbat G. Evaluation of newly adapted clip marker system in ultrasound-guided core needle biopsy for suspicion of breast cancer. Geburtsh Frauenheilk. 2013;73:1135–1138. doi: 10.1055/s-0033-1351086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schulz-Wendtland R, Dankerl P, Dilbat G. Comparison of sonography versus digital breast tomosynthesis to locate intramammary marker clips. Geburtsh Frauenheilk. 2015;75:72–76. doi: 10.1055/s-0034-1396164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisenhauer E A, Therasse P, Bogaerts J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 18.von Minckwitz G, Untch M, Blohmer J U. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 19.Burbank F, Forcier N. Tissue marking clip for stereotactic breast biopsy: initial placement accuracy, long-term stability, and usefulness as a guide for wire localization. Radiology. 1997;205:407–415. doi: 10.1148/radiology.205.2.9356621. [DOI] [PubMed] [Google Scholar]
- 20.Brenner R J. Percutaneous removal of postbiopsy marking clip in the breast using stereotactic technique. AJR Am J Roentgenol. 2001;176:417–419. doi: 10.2214/ajr.176.2.1760417. [DOI] [PubMed] [Google Scholar]
- 21.Esserman L E, Cura M A, DaCosta D. Recognizing pitfalls in early and late migration of clip markers after imaging-guided directional vacuum-assisted biopsy. Radiographics. 2004;24:147–156. doi: 10.1148/rg.241035052. [DOI] [PubMed] [Google Scholar]
- 22.Youn I, Choi S H, Kook S H. Ultrasonography-guided surgical clip placement for tumor localization in patients undergoing neoadjuvant chemotherapy for breast cancer. J Breast Cancer. 2015;18:44–49. doi: 10.4048/jbc.2015.18.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baron L F, Baron P L, Ackerman S J. Sonographically guided clip placement facilitates localization of breast cancer after neoadjuvant chemotherapy. AJR Am J Roentgenol. 2000;174:539–540. doi: 10.2214/ajr.174.2.1740539. [DOI] [PubMed] [Google Scholar]
- 24.Houssami N, Skaane P. Overview of the evidence on digital breast tomosynthesis in breast cancer detection. Breast. 2013;22:101–108. doi: 10.1016/j.breast.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 25.Schulz-Wendtland R, Wenkel E, Lell M. Experimental phantom lesion detectability study using a digital breast tomosynthesis prototype system. Rofo. 2006;178:1219–1223. doi: 10.1055/s-2006-926933. [DOI] [PubMed] [Google Scholar]
- 26.Schulz-Wendtland R, Hermann K P, Wacker T. [Current situation and future perspectives of digital mammography] Radiologe. 2008;48:324–334. doi: 10.1007/s00117-008-1639-4. [DOI] [PubMed] [Google Scholar]
- 27.Schulz-Wendtland R, Wenkel E, Wacker T. [Quo vadis? Trends in digital mammography] Geburtsh Frauenheilk. 2009;69:108–117. [Google Scholar]


