Abstract
Objectives Rathke's cleft cysts (RCC) are benign cystic lesions of the sella resulting from incomplete obliteration of Rathke's cleft. Symptomatic lesions often require surgical decompression, which is often amenable to a transnasal, transsphenoidal (TNTS) approach. We report our experience with marsupialization of RCC and describe a novel technique to promote re-epithelization of the cyst cavity.
Design Retrospective review.
Setting Tertiary academic medical center.
Participants Patients who underwent TNTS for RCC between 2007 and 2015.
Main Outcome Measures Demographics, lesion characteristics, and reconstruction and treatment outcomes.
Results In total, 52 patients were identified. The mean age was 41 ± 18 years. The mean RCC size was 13 ± 5 mm. Intraoperative cerebrospinal fluid (CSF) leak was encountered in 14 (27%) patients; all were repaired. There were six complications (12%) and no deaths. Mean follow-up was 20 ± 18 months, with five (10%) recurrences. RCC size was associated with intraoperative CSF leak (p = 0.04). In 12 patients, the marsupialized cyst cavity was lined with a free mucosal graft (FMG) to promote healing and re-epithelialization.
Conclusions The TNTS approach is safe and effective in surgical decompression of RCC. Lining the exposed cyst cavity with an FMG is a simple intervention without added morbidity that may promote formation of an epithelialized tract.
Level of Evidence Not applicable.
Keywords: Rathke's cleft cyst, marsupialization, free mucosal grafting
Introduction
Rathke's cleft cysts (RCC) are cystic lesions formed within the persistence of Rathke's cleft, which is the embryonic remnant between what would later become the anterior and posterior lobes of the pituitary gland. In normal embryonic development, the pituitary gland is formed from the union of Rathke's pouch derived from both the oral cavity (stomodeum) and the brain (diencephalon). Normally, the space between obliterates, and failure of obliteration, leads to the formation of RCC.
The vast majority of RCCs are asymptomatic, with one report citing up to nearly a 4% incidence found in routine autopsies.1 However, when symptomatic, they account for 10% of symptomatic sellar lesions and can lead to headache, visual changes, and endocrinopathies from compression of surrounding structures.2 They may also occur concurrently with pituitary adenomas, with the cyst itself often ruptured.3 4
The mainstay of the management of RCC is surgery, wherein a transnasal microscopic or transnasal transsphenoidal (TNTS) approach is usually favored. The goal is cyst decompression with evacuation of its contents. Historically, RCC was managed through fenestration, where a small opening (much like a “punch”) is created directly through the anterior pituitary gland and into the cyst cavity.5 Other surgeons have advocated for extracapsular dissection with complete removal of the cyst wall (gross total resection) in addition to decompression; however, this appears to be associated with a higher risk of postoperative endocrinopathies and cerebrospinal fluid (CSF) leak, as well as placing adjacent structures (i.e., optic chiasm and carotid artery) at risk.6 The more recently utilized technique is marsupialization, where the cyst wall is widely opened and the cavity is allowed to drain into the sphenoid sinus. In theory, marsupialization effectively decompresses the cyst and provides a dependent drainage pathway and at the same time avoids inadvertent injury to surrounding structures. By working only on the anterior surface of the cyst, the risk of intraoperative CSF leak is also decreased.
In this study, we present our institution's experience with marsupialization of RCC via a TNTS approach and describe a novel, simple, and economical method of reconstructing the marsupialized cavity.
Methods
Institutional Review Board approval was obtained for this study. We performed a retrospective chart review of all patients who underwent TNTS at a tertiary academic medical center found to have RCC on histopathologic analysis between January 1, 2007, and September 1, 2015. Patient demographics (age, gender, past medical history, length of follow-up), lesion characteristics (size), reconstruction techniques (marsupialization, cyst removal), and outcomes (postoperative endocrinopathy, worsening vision, CSF leak, complications, recurrence) were collected. Statistical analysis for determining relationships between continuous variables was performed using two-tailed Student's t-tests, while those involving categorical data were compared using Fisher's exact tests. Multivariate logistic regression was also used to determine independent predictors of a given event. A significance level of p < 0.05 was used in all cases.
Marsupialization
For all cases involving marsupialization, a TNTS approach allowed for wide access to the sella (Fig. 1). Sphenoid sinus mucosa and bone overlying the sella were removed. An inferiorly based dural incision is made sharply with a pituitary blade, and a transverse incision is made in the anterior pituitary capsule. Gentle blunt dissection posterior to the gland provides excellent access to the contents of the RCC, which are bluntly delivered and removed piecemeal. The cyst cavity is thus exteriorized and ready for reconstruction.
Fig. 1.
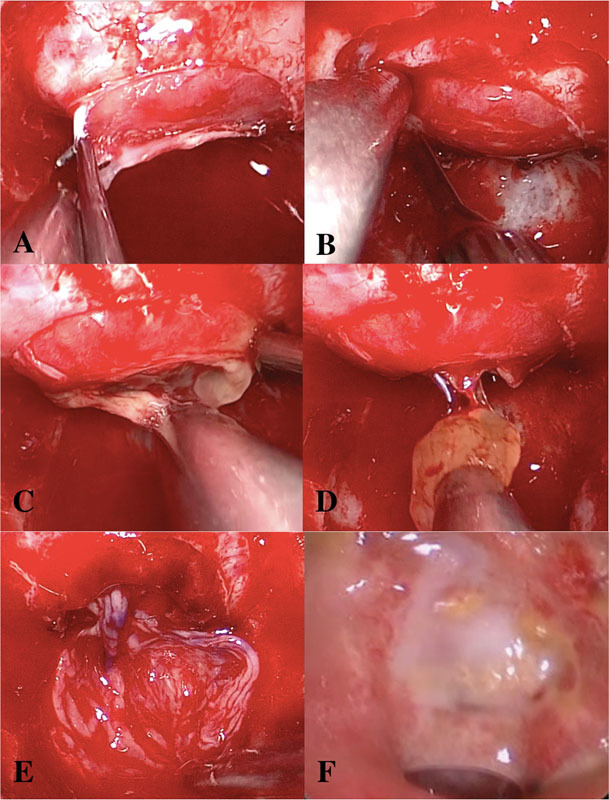
Marsupialization of Rathke's cleft cyst with free mucosal graft reconstruction. (A) After wide exposure of the sella is obtained, an inferiorly based dural incision is made sharply. (B) A transverse incision through the anterior pituitary capsule is made sharply. (C) The cyst cavity is entered and its contents are expressed through blunt dissection and gentle suctioning at the surface. (D) Solid components can be removed with suction or fine graspers. (E) Once the cyst is completely marsupialized, a free mucosal graft (blue coloring marks mucosal surface up) is placed into the inferior portion of the tract and additionally provides coverage of mucosa-denuded sellar bone. (F) At 8 weeks postoperatively, the marsupialized cavity has healed well and remucosalized.
Free Mucosal Grafting Technique
In several cases, instead of leaving the marsupialized cyst simply open, a free mucosal graft (FMG) was placed along the anteroinferior edge of the cyst tract. The FMG is harvested from the posterior septum (i.e., opposite the side of the nasoseptal flap, if indicated) during posterior septectomy as part of the TNTS. Prior to removal of septal bone anterior to the sphenoid sinus, a mucosal incision is outlined with electrocautery, and mucosa is elevated bluntly and then harvested using nontraumatic grasping forceps. The procedure adds no added morbidity and utilizes mucosa that would otherwise have been sacrificed in the TNTS approach.
Results
In total, 52 patients were identified and included in the analysis. There were 31 (60%) females and 21 (40%) males. The mean age at diagnosis was 41 ± 18 years (range: 13–78). The mean RCC size was 13 ± 5 mm (range: 5–24). Intraoperative CSF leak was encountered in 14 (27%) patients; all were repaired with fat grafting with no persistent leak thereafter. There were six complications (12%; three postoperative bleeding, one carotid injury leading to stroke, one postoperative CSF leak, one postoperative apnea requiring reintubation), and no deaths. The mean follow-up period was 20 ± 18 months (range: 1–65), with only five (10%) recurrences. Two (4%) patients developed new postoperative endocrine dysfunction (both requiring growth hormone replacement). Only one patient reported worsening of vision postoperatively. Of the 52 surgeries performed, 40 (77%) cases were managed by cyst marsupialization. In 12 patients, the marsupialized cyst cavity was lined with an FMG to promote healing and re-epithelialization. Our institution's results correlate with those from previous reports utilizing a TNTS approach to the management of RCC (Table 1).
Table 1. Patient characteristics and surgical outcomes of endoscopic resection of Rathke's cleft cysts.
| Study | n | Mean age, y | Symptomatic recurrence rate, % | New permanent postoperative endocrine dysfunction, % |
Postoperative visual worsening, % | Postoperative CSF leak, % | Mean follow-up (range), mo |
|---|---|---|---|---|---|---|---|
| Frank et al7 | 22 | 37 | 5 | 5 | 0 | 9 | 33 (8–60) |
| Koutourousiou et al8 | 14 | 43 | 0 | 14 | 0 | 0 | 31 (11–48) |
| Madhok et al9 | 35 | 34 | 3 | 0 | 0 | 0 | 19 (1–60) |
| Jayarao et al10 | 2 | NR | 50 | 0 | 0 | NR | NR |
| Xie et al11 | 23 | 43 | 9 | 0 | 4 | 13 | (3–36) |
| Jahangiri et al12 | 9 | 46 | 11 | 0 | 0 | 0 | 21 |
| Mendelson et al13 | 11 | 43 | 18 | 0 | 0 | 0 | 24 (9–60) |
| Solari et al14 | 29 | 40 | 3 | 7 | 0 | 7 | NR |
| UCLA | 52 | 41 | 2 | 4 | 2 | 2 | 20 (1–65) |
Abbreviations: CSF, cerebrospinal fluid; NR, not reported, UCLA, University of California, Los Angeles.
On univariate analysis, the only risk factor associated with intraoperative CSF leak was RCC size (p = 0.02). Multivariate analysis further demonstrated that RCC size was the only independent predictor of intraoperative CSF leak (p = 0.04). On univariate and multivariate analysis, there was no association between any factor and recurrence (p > 0.05). There was no association between RCC size and postoperative endocrine dysfunction (p = 0.981) or worsening vision (p = 0.297). Additionally, the rate of intraoperative CSF leak was lower when marsupialization was employed for the management of RCC as compared with other techniques (p < 0.0001), but there was no difference in recurrence between techniques (p = 0.32).
Discussion
RCC, although frequently asymptomatic and/or incidentally discovered, may present with debilitating symptoms, such as headache or visual loss.2 For most cases, surgical decompression of RCC using an endoscopic, endonasal approach has gained in favor over the last several years as it affords direct access to a narrow and potentially deep surgical corridor, is minimally invasive without the use of any external incisions, and allows for two-surgeon, four-handed instrumentation. This technique additionally offers advantages when compared with the microscopic, endonasal approach by allowing for a dynamic view and the ability to access deeper or angled spaces. In a meta-analysis of more than 500 cases, Mendelson et al15 found that 18% of RCC cases were approached through the endoscopic, endonasal route. Though less frequently employed, the recurrence and postoperative endocrinopathy rate was lower than that of the microscopic approach, corroborating with our results that it is safe and efficacious.15 In this study, we report our institution's experience with surgical marsupialization of RCC via an endoscopic TNTS approach, which is the largest single-institution series to date.
Previous studies have found that RCC size is correlated with preoperative visual abnormalities, but not postoperative endocrine dysfunction16; in this study, we found no such association. A study on open resection of RCC by Shin et al17 identified size as a predictor for recurrence, but this was not found in our cohort. The extent of resection has also previously been found to correlate with postoperative complications, with complete cyst removal resulting in a higher risk of postoperative CSF leak as compared with marsupialization.6 18 19 This finding is consistent with those from this study, where we found that intraoperative CSF leak is more common with attempted cyst removal when compared with marsupialization. With pseudocapsular dissection and cyst removal, the surrounding microvasculature is more heavily manipulated, risking devascularization of the pituitary gland. In addition, dissection of the cyst wall off dura, such as the diaphragma sellae, places it on significantly more traction, increasing the risk of an accidental tear and resulting CSF leak. On the other hand, some studies have found decreased recurrence rates with gross total resection.20 As this study appears to show no difference in recurrence rates between marsupialization and gross total resection, we would thus strongly advocate for marsupialization as the primary technique for surgical treatment of RCC.
In 12 cases, the FMG reconstruction technique was employed to promote re-epithelialization of the marsupialized RCC tract in the sella. We designed an FMG from the posterior nasal septum covering bone that would otherwise be removed during wide bilateral sphenoidotomies. Based on limited follow-up data in the outpatient setting, the graft appears to provide a minor stenting effect for the inferior cyst opening and at the same time covers a portion of the inferior sellar bone where mucosa was necessarily stripped. A larger-sample, longer follow-up evaluation of the benefits of this technique is underway.
Two major limitations exist for this study and for research surrounding RCC. First, the follow-up period, though having several patients extended more than 5 years postoperatively, is ultimately still, on average, limited to within 3 years postoperatively. There have been several reports indicating that RCC may occur much later, within an interval of up to 5 to 10 years postoperatively.21 22 Due to the rather recent adoption of the endoscopic TNTS technique, no series to date has follow-up to that time range, and longer surveillance is necessary. Second, although symptomatic RCC is a rather rare condition, there are a large number of patients with asymptomatic RCC who do not require treatment. With this in mind, the true relationship between lesion factors, such as size, and outcomes is unknown and unable to be explored. Based on our results, the endoscopic TNTS approach to marsupialization of RCC is a low-morbidity, clinically sound option for symptomatic management of these lesions.
Conclusion
The endoscopic TNTS approach is safe and effective in surgical decompression of RCC. Cyst size appears to be an independent predictor of intraoperative CSF leak. Lining the marsupialized cyst cavity with an FMG harvested from the nasal septum is a simple intervention without added surgical morbidity that may promote maintenance of an epithelial lined tract to prevent recurrences. Long-term follow-up is necessary to determine the true clinical impact of the FMG technique.
Financial Disclosures
None.
Conflicts of Interest None.
Note
This study was presented orally at the North American Skull Base Society 25th Annual Meeting, February 13, 2016, in Scottsdale, Arizona.
References
- 1.Teramoto A, Hirakawa K, Sanno N, Osamura Y. Incidental pituitary lesions in 1,000 unselected autopsy specimens. Radiology. 1994;193(1):161–164. doi: 10.1148/radiology.193.1.8090885. [DOI] [PubMed] [Google Scholar]
- 2.Ross D A Norman D Wilson C B Radiologic characteristics and results of surgical management of Rathke's cysts in 43 patients Neurosurgery 1992302173–178., discussion 178–179 [DOI] [PubMed] [Google Scholar]
- 3.Guo S Y, Cai X Q, Ma J, Wang W Y, Lu G. Diagnosis of concomitant pituitary adenoma and Rathke's cleft cyst with magnetic resonance imaging. Int J Surg. 2015;18:191–195. doi: 10.1016/j.ijsu.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Ikeda H, Ohhashi G. Demonstration of high coincidence of pituitary adenoma in patients with ruptured Rathke's cleft cyst: results of a prospective study. Clin Neurol Neurosurg. 2015;139:144–151. doi: 10.1016/j.clineuro.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 5.Fager C A, Carter H. Intrasellar epithelial cysts. J Neurosurg. 1966;24(1):77–81. doi: 10.3171/jns.1966.24.1.0077. [DOI] [PubMed] [Google Scholar]
- 6.Benveniste R J, King W A, Walsh J, Lee J S, Naidich T P, Post K D. Surgery for Rathke cleft cysts: technical considerations and outcomes. J Neurosurg. 2004;101(4):577–584. doi: 10.3171/jns.2004.101.4.0577. [DOI] [PubMed] [Google Scholar]
- 7.Frank G Sciarretta V Mazzatenta D Farneti G Modugno G C Pasquini E Transsphenoidal endoscopic approach in the treatment of Rathke's cleft cyst Neurosurgery 2005561124–128., discussion 129 [DOI] [PubMed] [Google Scholar]
- 8.Koutourousiou M, Grotenhuis A, Kontogeorgos G, Seretis A. Treatment of Rathke's cleft cysts: experience at a single centre. J Clin Neurosci. 2009;16(7):900–903. doi: 10.1016/j.jocn.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Madhok R, Prevedello D M, Gardner P, Carrau R L, Snyderman C H, Kassam A B. Endoscopic endonasal resection of Rathke cleft cysts: clinical outcomes and surgical nuances. J Neurosurg. 2010;112(6):1333–1339. doi: 10.3171/2009.10.JNS09348. [DOI] [PubMed] [Google Scholar]
- 10.Jayarao M Devaiah A K Chin L S Utility and safety of the flexible-fiber CO2 laser in endoscopic endonasal transsphenoidal surgery World Neurosurg 201176(1–2):149–155. [DOI] [PubMed] [Google Scholar]
- 11.Xie T, Hu F, Yu Y, Gu Y, Wang X, Zhang X. Endoscopic endonasal resection of symptomatic Rathke cleft cysts. J Clin Neurosci. 2011;18(6):760–762. doi: 10.1016/j.jocn.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Jahangiri A, Potts M, Kunwar S, Blevins L, El-Sayed I H, Aghi M K. Extended endoscopic endonasal approach for suprasellar Rathke's cleft cysts. J Clin Neurosci. 2014;21(5):779–785. doi: 10.1016/j.jocn.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 13.Mendelson Z S, Husain Q, Kanumuri V V, Eloy J A, Liu J K. Endoscopic transsphenoidal surgery of Rathke's cleft cyst. J Clin Neurosci. 2015;22(1):149–154. doi: 10.1016/j.jocn.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Solari D, Cavallo L M, Somma T. et al. Endoscopic endonasal approach in the management of Rathke's cleft cysts. PLoS ONE. 2015;10(10):e0139609. doi: 10.1371/journal.pone.0139609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mendelson Z S, Husain Q, Elmoursi S, Svider P F, Eloy J A, Liu J K. Rathke's cleft cyst recurrence after transsphenoidal surgery: a meta-analysis of 1151 cases. J Clin Neurosci. 2014;21(3):378–385. doi: 10.1016/j.jocn.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Nishioka H, Haraoka J, Izawa H, Ikeda Y. Magnetic resonance imaging, clinical manifestations, and management of Rathke's cleft cyst. Clin Endocrinol (Oxf) 2006;64(2):184–188. doi: 10.1111/j.1365-2265.2006.02446.x. [DOI] [PubMed] [Google Scholar]
- 17.Shin J L, Asa S L, Woodhouse L J, Smyth H S, Ezzat S. Cystic lesions of the pituitary: clinicopathological features distinguishing craniopharyngioma, Rathke's cleft cyst, and arachnoid cyst. J Clin Endocrinol Metab. 1999;84(11):3972–3982. doi: 10.1210/jcem.84.11.6114. [DOI] [PubMed] [Google Scholar]
- 18.Higgins D M, Van Gompel J J, Nippoldt T B, Meyer F B. Symptomatic Rathke cleft cysts: extent of resection and surgical complications. Neurosurg Focus. 2011;31(1):E2. doi: 10.3171/2011.5.FOCUS1175. [DOI] [PubMed] [Google Scholar]
- 19.Fan J, Peng Y, Qi S, Zhang X A, Qiu B, Pan J. Individualized surgical strategies for Rathke cleft cyst based on cyst location. J Neurosurg. 2013;119(6):1437–1446. doi: 10.3171/2013.8.JNS13777. [DOI] [PubMed] [Google Scholar]
- 20.Kim J E, Kim J H, Kim O L. et al. Surgical treatment of symptomatic Rathke cleft cysts: clinical features and results with special attention to recurrence. J Neurosurg. 2004;100(1):33–40. doi: 10.3171/jns.2004.100.1.0033. [DOI] [PubMed] [Google Scholar]
- 21.Mukherjee J J, Islam N, Kaltsas G. et al. Clinical, radiological and pathological features of patients with Rathke's cleft cysts: tumors that may recur. J Clin Endocrinol Metab. 1997;82(7):2357–2362. doi: 10.1210/jcem.82.7.4043. [DOI] [PubMed] [Google Scholar]
- 22.Kasperbauer J L, Orvidas L J, Atkinson J L, Abboud C F. Rathke cleft cyst: diagnostic and therapeutic considerations. Laryngoscope. 2002;112(10):1836–1839. doi: 10.1097/00005537-200210000-00024. [DOI] [PubMed] [Google Scholar]


