Abstract
Objectives Bilateral anterior skull base (ASB) defects following endoscopic endonasal tumor resection are most commonly repaired utilizing multilayered reconstruction with a vascularized mucosal flap. Single-layer closure of large ASB defects has been described in the literature but this technique has yet to gain a widespread use. We report our experience with a series of patients who underwent reconstruction of large ASB defects using a single-layer intradural graft, without nasoseptal flaps. We also compared the use of acellular dermal matrix (AlloDerm, LifeCell, Branchburg, New Jersey, United States) or collagen matrix xenograft (Duramatrix, Stryker, Kalamazoo, Michigan, United States) as the graft biomaterial.
Design A retrospective case series.
Setting Tertiary academic medical center.
Main Outcome Measures Postoperative cerebrospinal fluid leak, the number of postoperative debridements, the number of postoperative infections, and time to remucosalization.
Results Two patients were reconstructed with AlloDerm and three with Duramatrix, with all patients receiving postoperative external beam radiation. There were no postoperative cerebrospinal fluid leaks identified in these patients during follow-up. The AlloDerm group showed increased postsurgical crusting, the number of clinically apparent postoperative infections, and an increased time to remucosalization.
Conclusions Single-layer repair without a vascularized mucosal flap is a viable method of skull base repair for large ASB defects. We found repair with Duramatrix was superior, with less graft crusting and infection, requiring fewer debridements.
Keywords: anterior cranial fossa, skull base, skull base neoplasm, paranasal sinus neoplasms, reconstructive surgical procedures, biocompatible materials
Introduction
Endonasal endoscopic surgical techniques have advanced greatly over the past three decades. Traditionally, skull base tumors had required craniofacial or craniotomy approaches for resection and reconstruction, which have been associated with high morbidity. In recent years, attention has been placed on expanded endonasal endoscopic approaches to resection of these skull base tumors. These approaches are quickly becoming the standard of care and have yielded comparable disease-free outcomes compared with open approaches, with reduced morbidity.1 2 One study comparing endoscopic and open approaches for resection of anterior skull base and sinonasal tumors showed that endoscopic approaches led to fewer surgical and medical complications with comparable disease-free survival rates.2
Advances in the field of rhinology and endoscopic skull base surgery worldwide have allowed for safe and effective removal of even large anterior skull base tumors. Defects can span the entire anterior skull base, from the sphenoid sinus to frontal sinus, which can be technically challenging to reconstruct, especially when there is a lack of available donor sites due to the tumor resection or from prior surgery. Reconstruction is critical to prevent postoperative cerebrospinal fluid (CSF) leaks and complications such as meningitis, pneumocephalus, seizure, or even death. These large skull base defects are typically reconstructed using a multilayered approach, utilizing some combination of an intradural layer, with an extradural layer composed of a vascularized flap, most commonly a pedicled nasoseptal flap.1 3 4 5 6
In 2007, Germani et al described a single-layer reconstructive approach using AlloDerm (LifeCell, Branchburg, New Jersey, United States), an acellular dermal allograft, to reconstruct large anterior cranial skull base defects without a vascularized mucosal flap overlay.7 Although this reconstructive approach produced excellent outcomes in a small series, large skull base defect reconstruction without a vascularized mucosal flap has not been readily adopted, and no other authors have published on its use for large defects. In this study, we describe our experience with large anterior skull base defect reconstruction with the primary reconstructive material being a biomaterial graft, used in a single-layer reconstruction. Theoretical benefits of single-layer reconstruction utilizing biomaterials compared with multilayer repair have several advantages; it is easily performed, reduces the load of foreign material introduced as much as possible, reduces operative procedure times, and reduces the cost of additional procedures or using multilayered artificial reconstructive materials. It is additionally appealing because this method can be performed when a nasoseptal flap or other pedicled flap is not available, and can avoid potential donor-site morbidity associated with mucosal grafts. We also compare the outcomes after reconstruction with two different types of graft materials, including AlloDerm and Duramatrix (Stryker, Kalamazoo, Michigan, United States), a bovine collagen matrix xenograft.
Methods
This study was approved by the Institutional Review Board of the University of California, Los Angeles, Office of Research Administration. A retrospective chart review was performed of all patients who underwent transnasal endoscopic resection of an anterior skull base tumor with skull base reconstruction of a large defect without vascularized mucosal flap between January 2012 and September 2015. All patients were treated at an academic tertiary medical center (University of California, Los Angeles, California, United States). Five patients were identified in this time period who met these criteria. Demographic and clinical information were collected, including: gender, age at time of surgery, skull base tumor type, size of anterior skull base defect, biomaterial used for reconstruction, method of reconstruction, time period of follow-up, postoperative CSF leak, number of postoperative debridements, number of clinically apparent infections, postoperative radiation therapy, and time to remucosalization, if achieved. Preoperative and postoperative sinonasal outcome test (SNOT-22) questionnaire scores at 3, 6, and 12 months after surgery were collected, if available.
For all five patients, an underlay graft placement technique was utilized in reconstruction with the use of a single layer of biomaterial as the primary graft (AlloDerm or Duramatrix). In all cases, the graft was placed intradurally. The reconstructions were then secured in place using fibrin glue and then a layer of saline soaked Gelfoam (Pfizer, New York, New York, United States). This reconstruction was then supported inferiorly with bilateral Pope packs, fashioned from a Merocel sponge (Medtronic, Minneapolis, Minnesota, United States) placed inside of a single finger of a sterile glove and sutured at the open end. These packs were positioned into the sphenoid sinus on one end, with the length of the pack adjacent to the repair. The sponges were then expanded with sterile saline in situ. If necessary, the paranasal sinuses were then packed with Nasopore sponges (Stryker) and nasal trumpets were placed inferiorly to divert nasal airflow during the immediate postoperative period. Lumbar drains were not utilized in any of these patients. The nasal trumpets were removed before discharge. Fig. 1 depicts a postoperative MRI of the reconstruction. The Pope packs were removed at the first postoperative visit, approximately 2 weeks after surgery. All patients were discharged with broad-spectrum antibiotics for 2 weeks. No sinus irrigation was performed until the packing was removed.
Fig. 1.
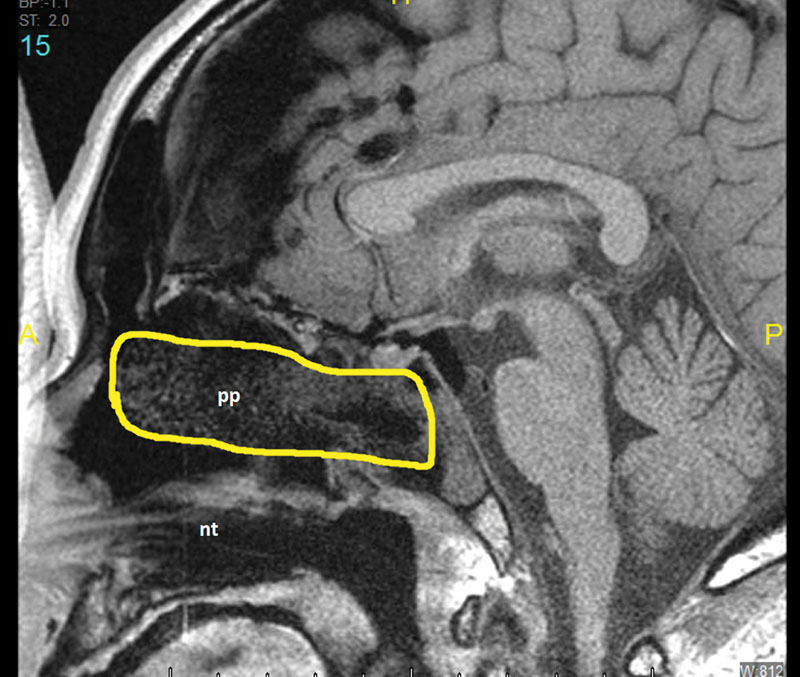
Sagittal MRI (T1 sequence) of reconstruction. MRI, magnetic resonance imaging; nt, nasal trumpet; pp, pope pack - nasal packing fashioned from merocel sponge placed inside a a finger of a sterile glove (highlighted).
Results
AlloDerm was used to reconstruct two patients and Duramatrix was used in three patients (Figs. 2 3 4 5). Patient demographics information is available in Table 1. All patients were treated for esthesioneuroblastoma which crossed the midline, requiring resection of both the left and right anterior skull base as part of the tumor resection. All had a complete endoscopic resection and they were free of evidence of recurrence during their respective follow-up periods. Patients were followed up for a range of 5 to 34 months. All defects had the largest diameter of greater than 2.0 cm which is consistent with the definition of a large skull base defect as defined in previous studies.7 The largest defect was 12 cm2 in area with the smallest defect being 4.5 cm2 in area. The average length of stay for patients was 3.2 days after surgery.
Fig. 2.
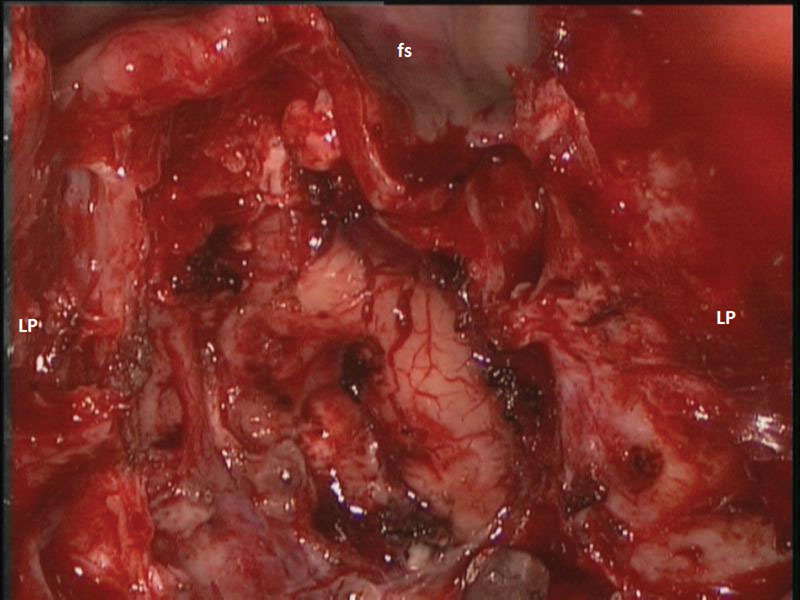
Intraoperative photograph of skull base defect for patient 1. fs, frontal sinus; LP, lamina papyracea.
Fig. 3.
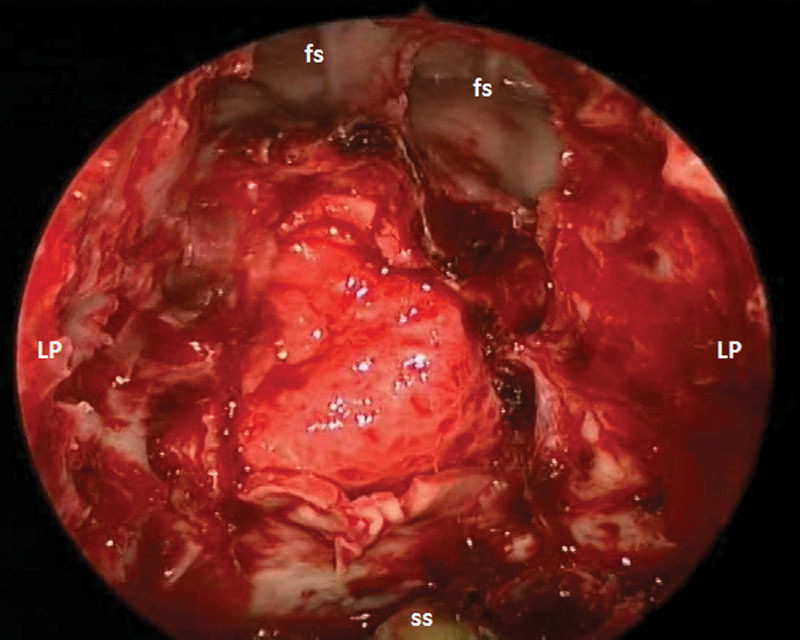
Intraoperative photograph of AlloDerm (LifeCell, Branchburg, New Jersey, United States) reconstruction of skull base defect for patient 1. fs, frontal sinus; LP, lamina papyracea; ss, sphenoid sinus.
Fig. 4.
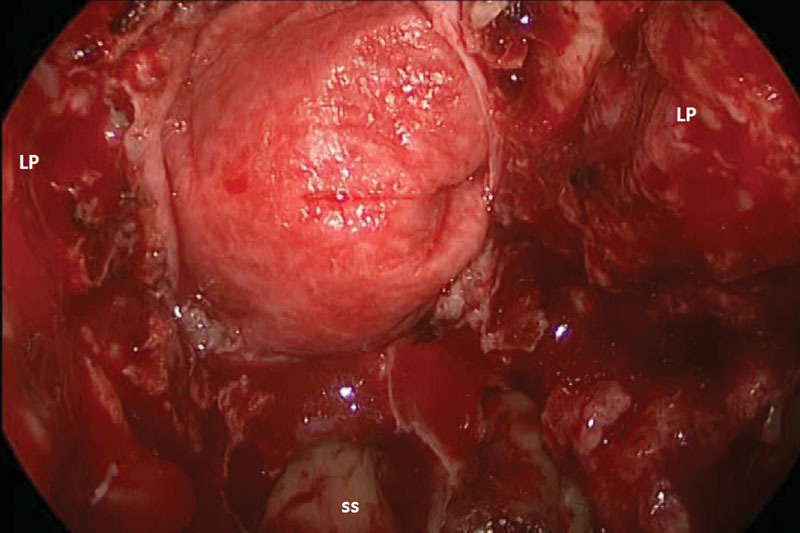
Intraoperative photograph of Duramatrix (Stryker, Kalamazoo, Michigan, United States) reconstruction of skull base defect for patient 3. LP, lamina papyracea; ss, sphenoid sinus.
Fig. 5.
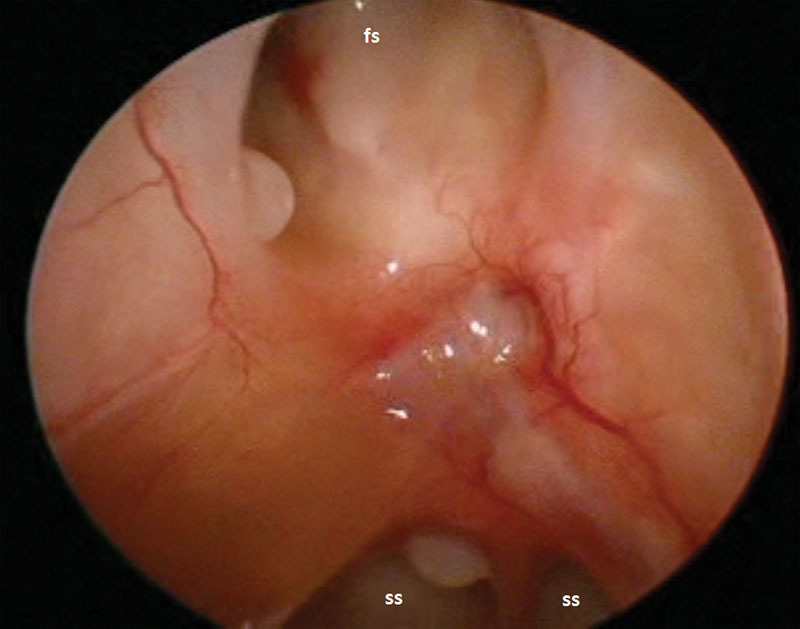
Postoperative photograph of Duramatrix (Stryker, Kalamazoo, Michigan, United States) reconstruction of skull base for patient 3, approximately 10 months after surgery. fs, frontal sinus; ss, sphenoid sinus.
Table 1. Patient demographics, diagnosis, follow-up period, and size of defect.
| Patient Number | Gender | Age at surgery (y) | Diagnosis | Follow-up period (mo) | Size of defect (cm) |
|---|---|---|---|---|---|
| 1 | Male | 70 | Esthesioneuroblastoma | 20 | 1.75 × 3.5 |
| 2 | Male | 72 | Esthesioneuroblastoma | 34 | 2.3 × 2.5 |
| 3 | Female | 37 | Esthesioneuroblastoma | 20 | 4 × 3 |
| 4 | Male | 52 | Esthesioneuroblastoma | 6 | 2.5 × 2 |
| 5 | Male | 53 | Esthesioneuroblastoma | 4 | 1.5 × 3 |
All five patients had successful skull base reconstruction, without the use of a lumbar drain, and without evidence of CSF leak perioperatively or postoperatively. All patients had adjuvant external beam radiation therapy. Patients 1 and 2 were reconstructed using AlloDerm and patients 3 to 5 were reconstructed using Duramatrix. Patient 3 required the use of abdominal fat to close off dead space created by mass effect from the anterior skull base tumor. Table 2 shows follow-up information including the number of debridements, the number of graft-site infections, culture results, and time to complete remucosalization. Graft-site infections were defined as clinically visualized purulence with positive bacterial or fungal cultures. Patients 1 and 2 underwent a total of 7 and 17 debridements of operative site crusting, respectively. In addition, these patients had 3 and 9 episodes of symptomatic graft-site infections, respectively, both with chronic infections with methicillin-sensitive Staphylococcus aureus. Patients 3 to 5 underwent 2, 3, and 2 debridements of the operative site, respectively, and had only 1 or 2 clinically graft-site infections, with culture showing two of these patients with fungal infections and one patient with methicillin-resistant S. aureus infection. Time to remucosalization was prolonged in the AlloDerm group, with both patients requiring at least 18 months for complete remucosalization of the reconstruction site. In the Duramatrix group, all three patients achieved complete remucosalization, requiring 2 to 10 months to achieve remucosalization of the operative site.
Table 2. Patient clinical information: Reconstructive graft material, time of continuous postoperative debridements, number of clinically apparent infections, and time to remucosalization.
| Patient number | Reconstructive material | Number of postoperative debridements | No. of graft infections | Culture results | Time to remucosalization (mo) |
|---|---|---|---|---|---|
| 1 | AlloDerm | 7 | 3 | MSSA | 18 |
| 2 | AlloDerm | 17 | 9 | MSSA, Serratia species | 20 |
| 3 | Duramatrix | 2 | 1 | Aspergillus fumigatus | 10 |
| 4 | Duramatrix | 3 | 2 | MRSA | 4 |
| 5 | Duramatrix | 2 | 1 | Aspergillus niger | 2 |
Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus.
Note: AlloDerm (LifeCell, Branchburg, New Jersey, United States) and Duramatrix (Stryker, Kalamazoo, Michigan, United States).
SNOT-22 questionnaire information was also collected for all five patients and is shown in Fig. 6. Preoperative SNOT-22 scores were available for four of the five patients. Of these four patients, all showed improvement in their SNOT-22 scores at 3 months after surgery, compared with their preoperative scores. For patients with a follow-up of a year or longer, SNOT-22 scores continued to improve up to a year following surgery.
Fig. 6.
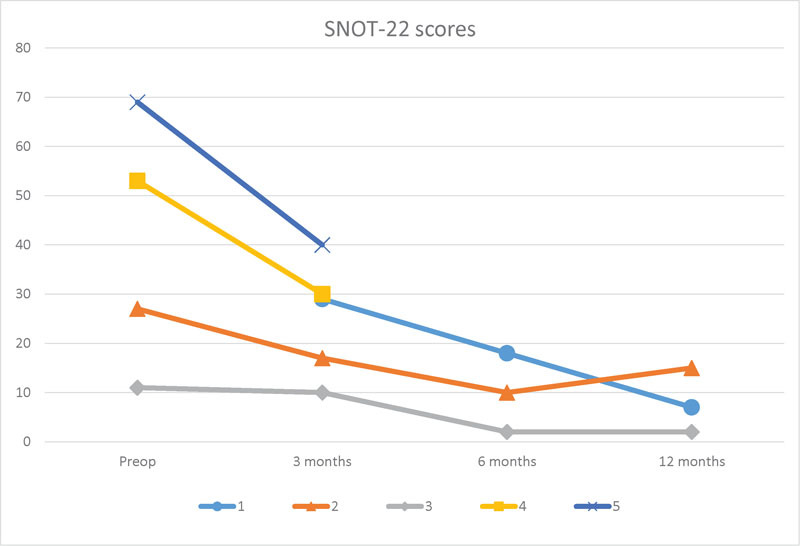
Graph of SNOT-22 scores for each patient. Preoperative SNOT-22 score was unavailable for patient 1. Patients 4 and 5 without postoperative SNOT-22 scores beyond 3 months. SNOT-22, sinonasal outcome test. Lower score on SNOT-22 denotes improved quality of life.
Discussion
As expanded endoscopic skull base approaches for large anterior skull base tumors have advanced, multiple techniques for reconstruction have been developed and discussed in the literature. Most commonly, skull base surgeons advocate the use of a multilayered approach in reconstructing large skull base defects.1 3 4 5 6 8 These recommendations typically include an inlay graft with fat, fascia, and/or biomaterial for water-tight closure, with an onlay layer which consists of a vascularized mucosal flap. A systematic review performed in 2012 by Harvey et al revealed a 6.7% leak rate for reconstructions using a vascularized flap, as opposed to nonvascularized free graft reconstructions with a leak rate of 15.6%, and this study has been the basis for many of the recommendations for use of a vascularized flap in large skull base defects.9 In the past, some have also advocated for a rigid layer for reconstruction to prevent frontal lobe sagging, or encephalocele, however, the need for this has not been supported in the literature.3 10 11
In terms of vascularized mucosal flaps, the nasoseptal flap has become the workhorse of endoscopic skull base reconstruction since its introduction in 2006 by Hadad et al.12 Other vascularized flaps have been introduced since, which have also shown similar success rates.3 4 13 14 A recent study evaluating patients who had undergone reconstruction with a nasoseptal flap showed significant differences between pre- and postoperative (at 3 months) endoscopy scores in both the flap harvest side and the nonharvest side, but there was no significant difference in the SNOT-22 quality of life scores.15 Pant et al compared patients who had undergone nasoseptal flap reconstruction versus those who did not have a nasoseptal flap reconstruction during endoscopic skull base surgery and found that the nasoseptal flap group had statistically significant higher SNOT-22 scores at 3 months after surgery.16 In our series, though with limited data, four of five patients showed decreases in SNOT-22 scores at 3 months after surgery compared with preoperative values, and these scores continued to drop for those with follow-up reaching 12 months.
Our series confirms the findings of Germani et al in applying a single-layered approach to reconstruction of large anterior skull base defects with successful outcomes.7 To date, this is the only study which has investigated the use of a single-layer technique for closure of large anterior skull base defects following expanded endonasal skull base resection. In their article, the authors describe an “inlay–onlay” technique, in which the graft is placed initially intracranially and intradurally, then the edges of the graft folded externally to drape over the bony edges extracranially.7 The results of their retrospective analysis showed 12 patients with large anterior skull base defects (larger than 2.0 cm) with 100% graft success and no CSF leaks noted in this subgroup with single-layer reconstruction using AlloDerm.7 In our reconstructions, we performed a solely intradural inlay technique for all of our graft placements with successful reconstruction and no postoperative CSF leaks, even after adjuvant external beam radiation therapy.
In comparing the outcomes of using two different graft materials, AlloDerm and Duramatrix, we found that the patients reconstructed using AlloDerm, were subject to longer periods of postoperative crusting and recurrent infection. The Duramatrix group had a shorter time period after surgery of continuous debridement, reduced number of postoperative infections, and reduced time to remucosalization. In fact, the first two reconstructions performed were done so with AlloDerm, but due to the extensive crusting and multiple local graft infections requiring treatment, the decision was made to trial Duramatrix for the primary reconstructive material. The reason for these discrepancies is unknown but further study with more patients is warranted to truly investigate this difference. This may be related to inherent differences in the structure of the graft materials. It is also possible that there may have been a “learning curve” phenomenon involved, with improvements seen in the latter cases compared with the earlier cases, but we do not believe this to be the case, as the only change between the AlloDerm and Duramatrix groups were the graft material and no changes were made to the perioperative and intraoperative care otherwise, or postoperative patient instructions.
With these findings in mind, one advantage of using a mucosal graft overlay may be improved times to remucosalization of the operative site, which in our series took up to 20 months to achieve. In addition, we did see a high rate of local graft infection, especially with the AlloDerm reconstructions. These infections were treated with antibiotics if culture proven on bacterial cultures with symptomatic exacerbations, and with debridement of crusts when cultures showed fungal infection. Improving remucosalization times would likely reduce the number of local graft infections, which often arise from stagnant mucus and drainage over the nonfunctional graft surface during the healing period. However, utilizing a pedicled flap or free mucosal graft which is harvested from another site introduces the possibility of additional donor-site morbidity which could affect the short-term quality of life.15 16 In addition, adjuvant radiotherapy after surgery may have also contributed to infection rates and prolonged remucosalization times.
There was also close follow-up required for the patients in the AlloDerm group, due to postoperative crusting and recurrent graft infections. Both patients required multiple debridements and treatments with antibiotics during their healing process. However, with the Duramatrix group, each patient only required two or three debridements postoperatively which is not beyond the expected range following this type of procedure. The additional time and cost of debridements and treatment of local infections seen with AlloDerm are potential reasons for surgeons to consider an alternative biomaterial. It is our belief that the graft material may have played a role in this phenomenon but it is difficult to draw solid conclusions based on our small series.
Previous authors have commented on the use of single-layer closure with AlloDerm and have noted that these closures should be limited to defects which have adequate bony and dural edges for placement of the graft in a watertight fashion.4 In addition, we have found that large intracranial dead space from tumor mass effect can decrease the amount of downward force necessary to secure the intradural graft in place and to create a watertight seal. In our experience with patient 3, we found placing a small amount of intracranial fat can be used to fill this space and allow for a watertight closure of the skull base defect with a single-layer of the biomaterial.
The advantages of a single-layer closure using biomaterials are that it can potentially eliminate nasoseptal flap or fascia lata donor-site morbidity and complications. In addition, a single layer repair is relatively straightforward to place, and it can be customized to any size defect, and it is readily available for use. This approach can also be utilized if there are not any local pedicled mucosal flaps available for use due to involvement with the disease process or a compromised pedicle from prior surgical procedures. Often with esthesioneuroblastomas, the superior septum is involved which may compromise the use of a nasoseptal flap in some instances.
Conclusions
Single layer repair of large, bilateral anterior skull base defects can be successfully performed using biomaterials following expanded endonasal endoscopic surgical approaches. In addition, we noted that Duramatrix may be superior to AlloDerm in this use, with reduced postoperative crusting, reduced number of infections, and decreased time to remucosalization. This technique should be part of the armamentarium of skull base surgeons and considered in select cases as an effective alternative to established reconstructive methods.
Footnotes
Conflicts of Interest Authors have no conflicts of interest to disclose.
References
- 1.Zuniga M G, Turner J H, Chandra R K. Updates in anterior skull base reconstruction. Curr Opin Otolaryngol Head Neck Surg. 2016;24(1):75–82. doi: 10.1097/MOO.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 2.Suh J D, Ramakrishnan V R, Chi J J, Palmer J N, Chiu A G. Outcomes and complications of endoscopic approaches for malignancies of the paranasal sinuses and anterior skull base. Ann Otol Rhinol Laryngol. 2013;122(1):54–59. doi: 10.1177/000348941312200110. [DOI] [PubMed] [Google Scholar]
- 3.Chin D, Harvey R J. Endoscopic reconstruction of frontal, cribiform and ethmoid skull base defects. Adv Otorhinolaryngol. 2013;74:104–118. doi: 10.1159/000342285. [DOI] [PubMed] [Google Scholar]
- 4.Patel M R, Stadler M E, Snyderman C H. et al. How to choose? Endoscopic skull base reconstructive options and limitations. Skull Base. 2010;20(6):397–404. doi: 10.1055/s-0030-1253573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eloy J A, Patel S K, Shukla P A, Smith M L, Choudhry O J, Liu J K. Triple-layer reconstruction technique for large cribriform defects after endoscopic endonasal resection of anterior skull base tumors. Int Forum Allergy Rhinol. 2013;3(3):204–211. doi: 10.1002/alr.21089. [DOI] [PubMed] [Google Scholar]
- 6.Clavenna M J, Turner J H, Chandra R K. Pedicled flaps in endoscopic skull base reconstruction: review of current techniques. Curr Opin Otolaryngol Head Neck Surg. 2015;23(1):71–77. doi: 10.1097/MOO.0000000000000115. [DOI] [PubMed] [Google Scholar]
- 7.Germani R M, Vivero R, Herzallah I R, Casiano R R. Endoscopic reconstruction of large anterior skull base defects using acellular dermal allograft. Am J Rhinol. 2007;21(5):615–618. doi: 10.2500/ajr.2007.21.3080. [DOI] [PubMed] [Google Scholar]
- 8.Bernal-Sprekelsen M, Rioja E, Enseñat J. et al. Management of anterior skull base defect depending on its size and location. BioMed Res Int. 2014;2014:346873. doi: 10.1155/2014/346873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harvey R J, Parmar P, Sacks R, Zanation A M. Endoscopic skull base reconstruction of large dural defects: a systematic review of published evidence. Laryngoscope. 2012;122(2):452–459. doi: 10.1002/lary.22475. [DOI] [PubMed] [Google Scholar]
- 10.Eloy J A, Shukla P A, Choudhry O J, Singh R, Liu J K. Assessment of frontal lobe sagging after endoscopic endonasal transcribriform resection of anterior skull base tumors: is rigid structural reconstruction of the cranial base defect necessary? Laryngoscope. 2012;122(12):2652–2657. doi: 10.1002/lary.23539. [DOI] [PubMed] [Google Scholar]
- 11.Battaglia P Turri-Zanoni M Castelnuovo P Prevedello D M Carrau R L Brain herniation after endoscopic transnasal resection of anterior skull base malignancies Neurosurgery 20151103457–462., discussion 462 [DOI] [PubMed] [Google Scholar]
- 12.Hadad G, Bassagasteguy L, Carrau R L. et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope. 2006;116(10):1882–1886. doi: 10.1097/01.mlg.0000234933.37779.e4. [DOI] [PubMed] [Google Scholar]
- 13.Zanation A M, Snyderman C H, Carrau R L, Kassam A B, Gardner P A, Prevedello D M. Minimally invasive endoscopic pericranial flap: a new method for endonasal skull base reconstruction. Laryngoscope. 2009;119(1):13–18. doi: 10.1002/lary.20022. [DOI] [PubMed] [Google Scholar]
- 14.Patel M R, Taylor R J, Hackman T G. et al. Beyond the nasoseptal flap: outcomes and pearls with secondary flaps in endoscopic endonasal skull base reconstruction. Laryngoscope. 2014;124(4):846–852. doi: 10.1002/lary.24319. [DOI] [PubMed] [Google Scholar]
- 15.Hanson M, Patel P M, Betz C, Olson S, Panizza B, Wallwork B. Sinonasal outcomes following endoscopic anterior skull base surgery with nasoseptal flap reconstruction: a prospective study. J Laryngol Otol. 2015;129 03:S41–S46. doi: 10.1017/S002221511500047X. [DOI] [PubMed] [Google Scholar]
- 16.Pant H, Bhatki A M, Snyderman C H. et al. Quality of life following endonasal skull base surgery. Skull Base. 2010;20(1):35–40. doi: 10.1055/s-0029-1242983. [DOI] [PMC free article] [PubMed] [Google Scholar]


