Abstract
Plasma concentration, splanchnic and renal exchange, and urinary excretion of 20 amino acids were studied in obese subjects during prolonged (5-6 wk) starvation. Splanchnic amino acid uptake was also investigated in postabsorptive and briefly (36-48 hr) fasted subjects.
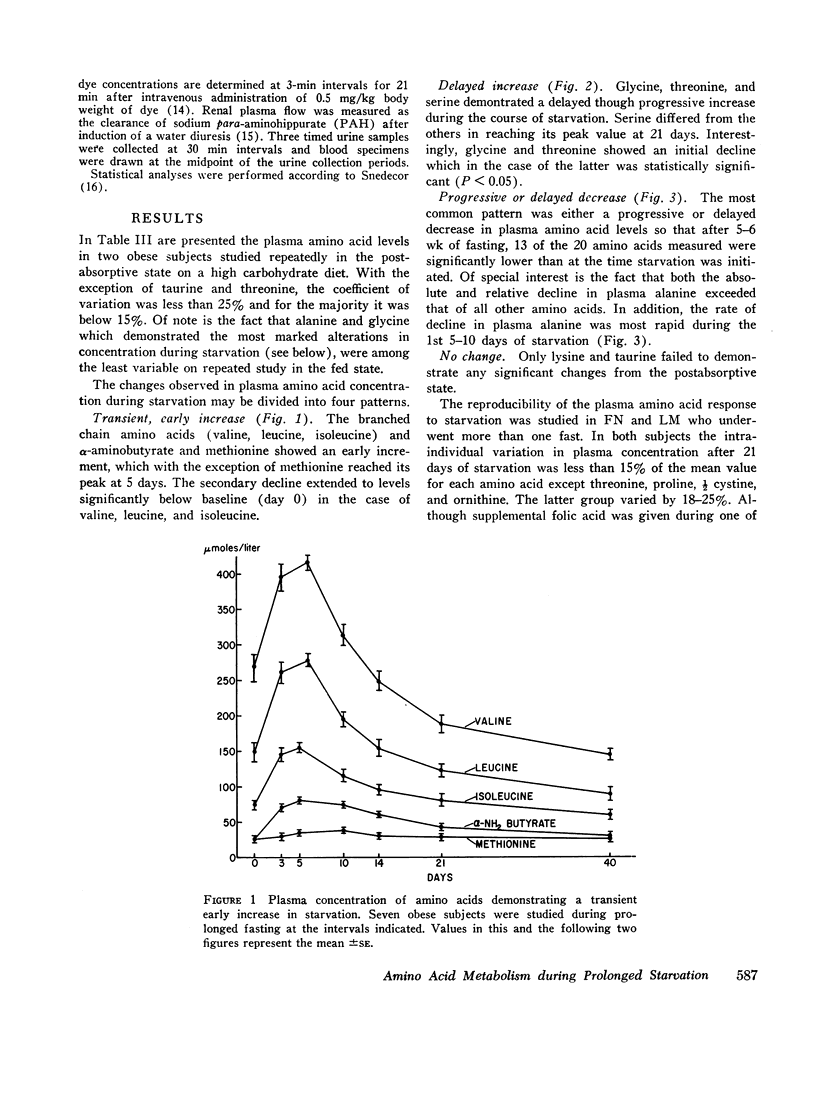
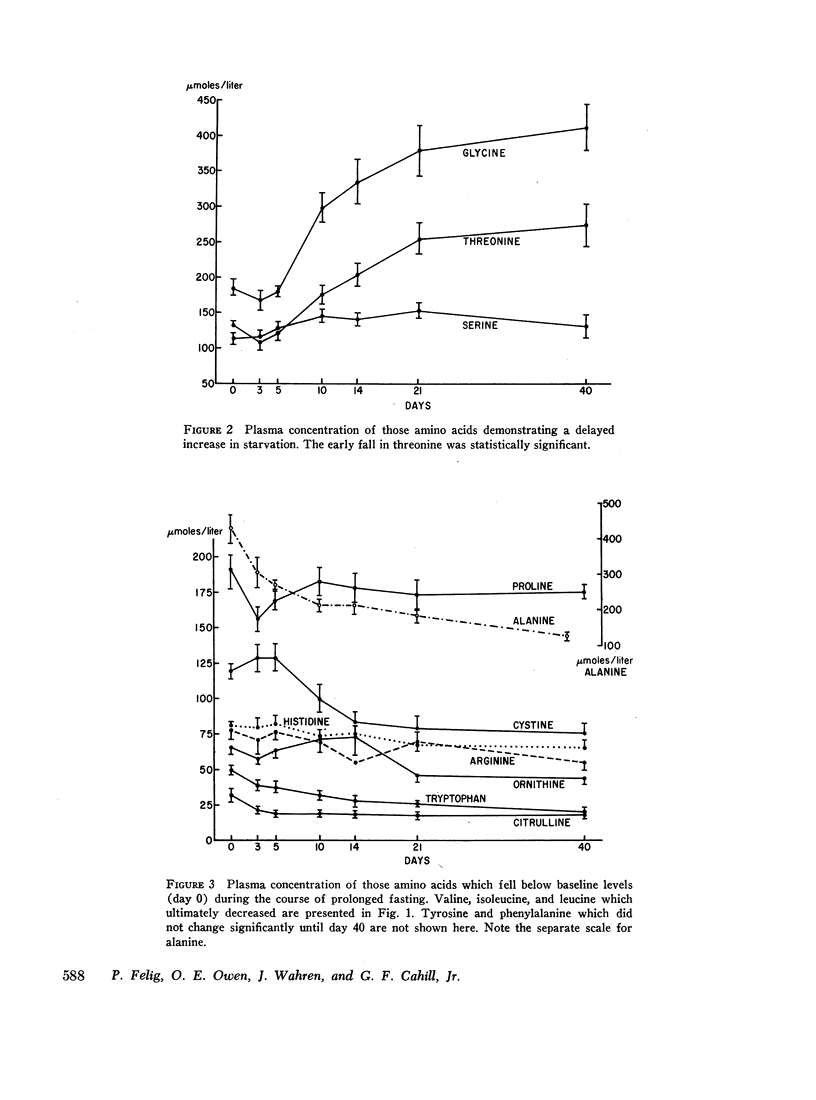
A transient increase in plasma valine, leucine, isoleucine, methionine, and α-aminobutyrate was noted during the 1st wk of starvation. A delayed, progressive increase in glycine, threonine, and serine occurred after the 1st 5 days. 13 of the amino acids ultimately decreased in starvation, but the magnitude of this diminution was greatest for alanine which decreased most rapidly during the 1st week of fasting.
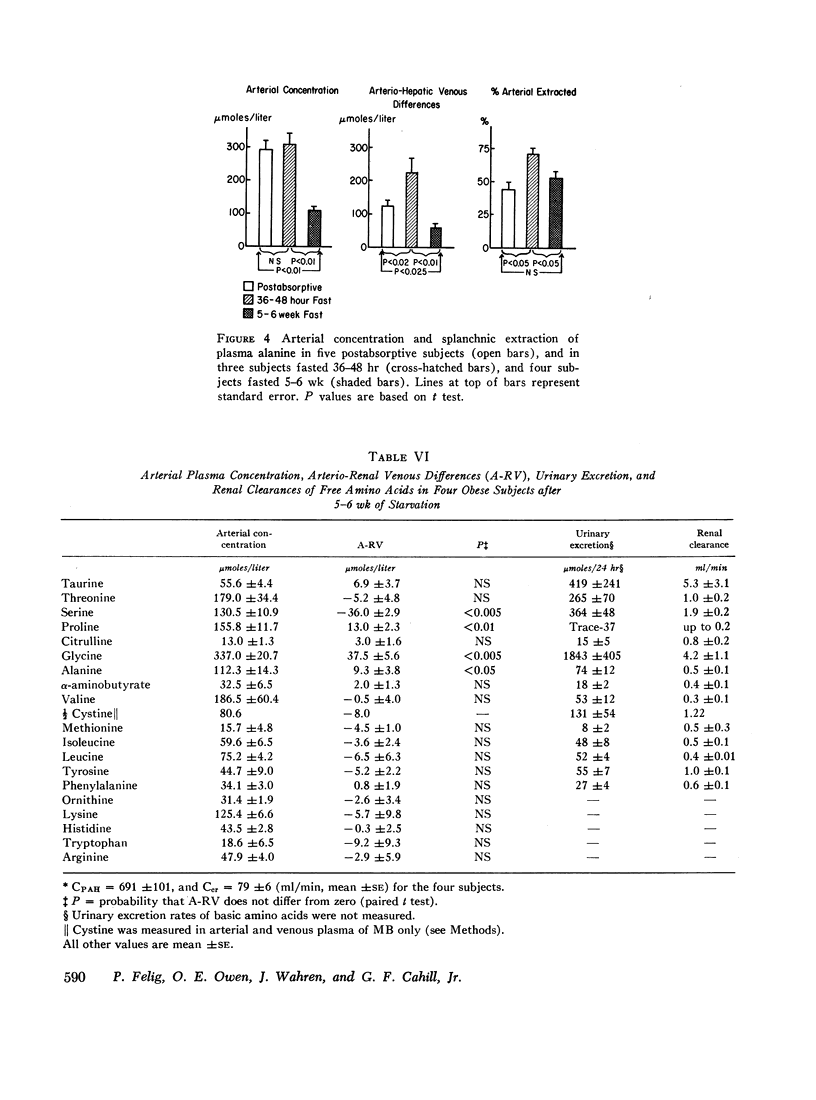
In all subjects alanine was extracted by the splanchnic circulation to a greater extent than all other amino acids combined. Brief fasting resulted in an increased arterio-hepatic venous difference for alanine due to increased fractional extraction. After 5-6 wk of starvation, a marked falloff in splanchnic alanine uptake was attributable to the decreased arterial concentration. Prolonged fasting resulted in increased glycine utilization by the kidney and in net renal uptake of alanine.
It is concluded that the marked decrease in plasma alanine is due to augmented and preferential splanchnic utilization of this amino acid in early starvation resulting in substrate depletion. Maintenance of the hypoalaninemia ultimately serves to diminish splanchnic uptake of this key glycogenic amino acid and is thus an important component of the regulatory mechanism whereby hepatic gluconeogenesis is diminished and protein catabolism is minimized in prolonged fasting. The altered renal extraction of glycine and alanine is not due to increased urinary excretion but may be secondary to the increased rate of renal gluconeogenesis observed in prolonged starvation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adibi S. A. Influence of dietary deprivations on plasma concentration of free amino acids of man. J Appl Physiol. 1968 Jul;25(1):52–57. doi: 10.1152/jappl.1968.25.1.52. [DOI] [PubMed] [Google Scholar]
- Block W. D., Markovs M. E., Steele B. F. Comparison between free amino acid levels in plasma deproteinated with picric acid and with sulfosalicylic acid. Proc Soc Exp Biol Med. 1966 Aug-Sep;122(4):1089–1091. doi: 10.3181/00379727-122-31333. [DOI] [PubMed] [Google Scholar]
- CAESAR J., SHALDON S., CHIANDUSSI L., GUEVARA L., SHERLOCK S. The use of indocyanine green in the measurement of hepatic blood flow and as a test of hepatic function. Clin Sci. 1961 Aug;21:43–57. [PubMed] [Google Scholar]
- Cahill G. F., Jr, Herrera M. G., Morgan A. P., Soeldner J. S., Steinke J., Levy P. L., Reichard G. A., Jr, Kipnis D. M. Hormone-fuel interrelationships during fasting. J Clin Invest. 1966 Nov;45(11):1751–1769. doi: 10.1172/JCI105481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsten A., Hallgren B., Jagenburg R., Svanborg A., Werkö L. Amino acids and free fatty acids in plasma in diabetes. I. The effect of insulin on the arterial levels. Acta Med Scand. 1966 Mar;179(3):361–370. doi: 10.1111/j.0954-6820.1966.tb05471.x. [DOI] [PubMed] [Google Scholar]
- Carlsten A., Hallgren B., Jagenburg R., Svanborg A., Werkö L. Arterio-hepatic venous differences of free fatty acids and amino acids. Studies in patients with diabetes or essential hypercholesterolemia, and in healthy individuals. Acta Med Scand. 1967 Feb;181(2):199–207. doi: 10.1111/j.0954-6820.1967.tb07246.x. [DOI] [PubMed] [Google Scholar]
- DENTON A. E., ELVEHJEM C. A. Availability of amino acids in vivo. J Biol Chem. 1954 Jan;206(1):449–454. [PubMed] [Google Scholar]
- DENTON A. E., GERSHOFF S. N., ELVEHJEM C. A. New method for cannulating the portal vein of dogs. J Biol Chem. 1953 Oct;204(2):731–735. [PubMed] [Google Scholar]
- DICKINSON J. C., ROSENBLUM H., HAMILTON P. B. ION EXCHANGE CHROMATOGRAPHY OF THE FREE AMINO ACIDS IN THE PLASMA OF THE NEWBORN INFANT. Pediatrics. 1965 Jul;36:2–13. [PubMed] [Google Scholar]
- DRENICK E. J., SWENDSEID M. E., BLAHD W. H., TUTTLE S. G. PROLONGED STARVATION AS TREATMENT FOR SEVERE OBESITY. JAMA. 1964 Jan 11;187:100–105. doi: 10.1001/jama.1964.03060150024006. [DOI] [PubMed] [Google Scholar]
- De Barnola F. V. The effect of insulin on plasma free amino acids. Acta Physiol Lat Am. 1965;15(3):260–265. [PubMed] [Google Scholar]
- FREEDMAN A. D., KOHN L. PYRUVATE METABOLISM AND CONTROL: FACTORS AFFECTING PYRUVIC CARBOXYLASE ACTIVITY. Science. 1964 Jul 3;145(3627):58–60. doi: 10.1126/science.145.3627.58. [DOI] [PubMed] [Google Scholar]
- Feigin R. D., Klainer A. S., Beisel W. R. Circadian periodicity of blood amino-acids in adult men. Nature. 1967 Jul 29;215(5100):512–514. doi: 10.1038/215512b0. [DOI] [PubMed] [Google Scholar]
- IVY J. H., SVEC M., FREEMAN S. Free plasma levels and urinary excretion of eighteen amino acids in normal and diabetic dogs. Am J Physiol. 1951 Oct;167(1):182–192. doi: 10.1152/ajplegacy.1951.167.1.182. [DOI] [PubMed] [Google Scholar]
- MCMURRAY W. C., RATHBUN J. C., MOHYUDDIN F., KOEGLER S. J. CITRULLINURIA. Pediatrics. 1963 Sep;32:347–357. [PubMed] [Google Scholar]
- Owen O. E., Morgan A. P., Kemp H. G., Sullivan J. M., Herrera M. G., Cahill G. F., Jr Brain metabolism during fasting. J Clin Invest. 1967 Oct;46(10):1589–1595. doi: 10.1172/JCI105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts R. F., Stone W. J. Renal metabolism of alanine. J Clin Invest. 1967 Apr;46(4):530–538. doi: 10.1172/JCI105554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross B. D., Hems R., Krebs H. A. The rate of gluconeogenesis from various precursors in the perfused rat liver. Biochem J. 1967 Mar;102(3):942–951. doi: 10.1042/bj1020942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHARFF R., WOOL I. G. CONCENTRATION OF AMINO ACIDS IN RAT MUSCLE AND PLASMA. Nature. 1964 May 9;202:603–604. doi: 10.1038/202603a0. [DOI] [PubMed] [Google Scholar]
- SHRAGO E., LARDY H. A., NORDLIE R. C., FOSTER D. O. METABOLIC AND HORMONAL CONTROL OF PHOSPHOENOLPYRUVATE CARBOXYKINASE AND MALIC ENZYME IN RAT LIVER. J Biol Chem. 1963 Oct;238:3188–3192. [PubMed] [Google Scholar]
- STEIN W. H. A chromatographic investigation of the amino acid constituents of normal urine. J Biol Chem. 1953 Mar;201(1):45–58. [PubMed] [Google Scholar]
- STEIN W. H., MOORE S. The free amino acids of human blood plasma. J Biol Chem. 1954 Dec;211(2):915–926. [PubMed] [Google Scholar]
- Saunders S. J., Truswell A. S., Barbezat G. O., Wittman W., Hansen J. D. Plasma free aminoacid pattern in protein-calorie malnutrition. Reappraisal of its diagnostic value. Lancet. 1967 Oct 14;2(7520):795–797. doi: 10.1016/s0140-6736(67)92233-7. [DOI] [PubMed] [Google Scholar]
- Schimassek H., Gerok W. Control of the levels of free amino acids in plasma by the liver. Biochem Z. 1965 Dec 31;343(4):407–415. [PubMed] [Google Scholar]
- WHEELER P., MORGAN A. F. The absorption by immature and adult rats of amino acids from raw and autoclaved fresh pork. J Nutr. 1958 Jan 10;64(1):137–150. doi: 10.1093/jn/64.1.137. [DOI] [PubMed] [Google Scholar]
- Williamson D. H., Lopes-Vieira O., Walker B. Concentrations of free glucogenic amino acids in livers of rats subjected to various metabolic stresses. Biochem J. 1967 Aug;104(2):497–502. doi: 10.1042/bj1040497. [DOI] [PMC free article] [PubMed] [Google Scholar]


