Abstract
The mechanism responsible for the hyperdynamic circulatory state in hyperthyroidism has not been defined. Although certain cardiac manifestations resemble those caused by excessive adrenergic stimulation, recent evidence suggests that thyroid hormone exerts an effect on the heart that is independent of the adrenergic system. Since the inotropic and chronotropic effects of norepinephrine appear to be mediated by activation of adenyl cyclase, the possibility that thyroxine and triiodothyronine are also capable of activating adenyl cyclase was examined in the particulate fraction of cat heart homogenates.
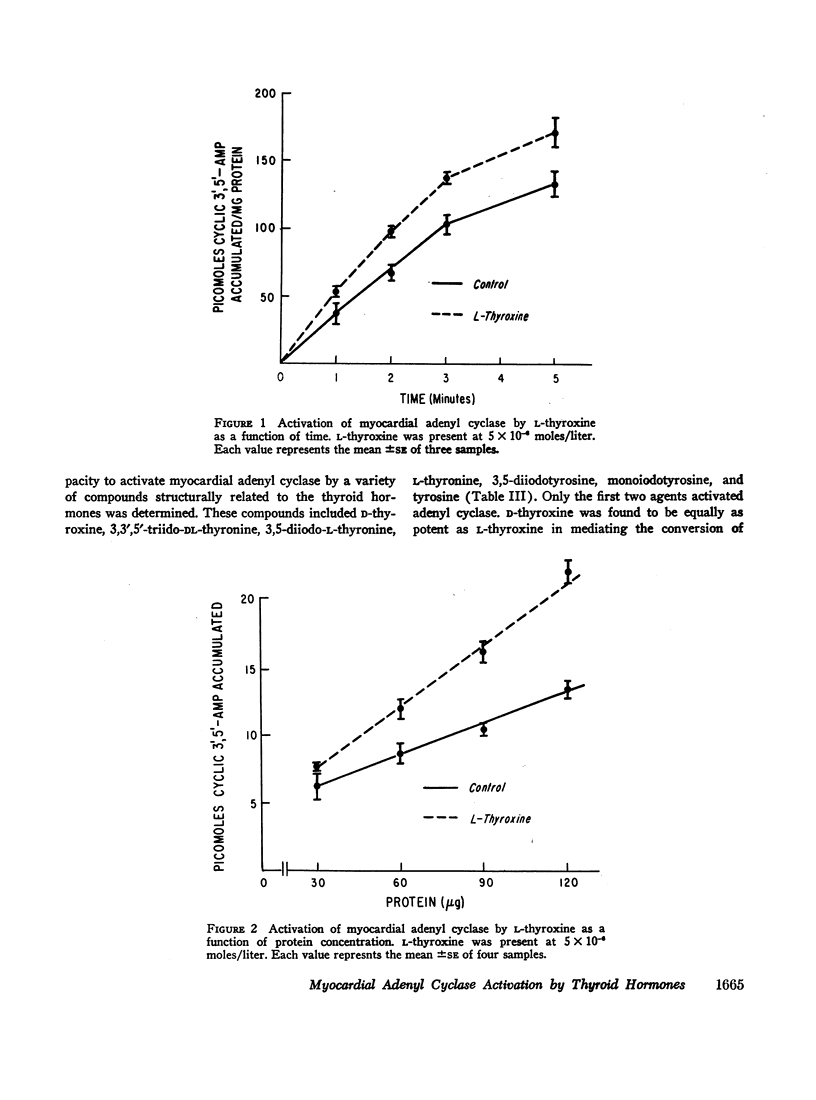
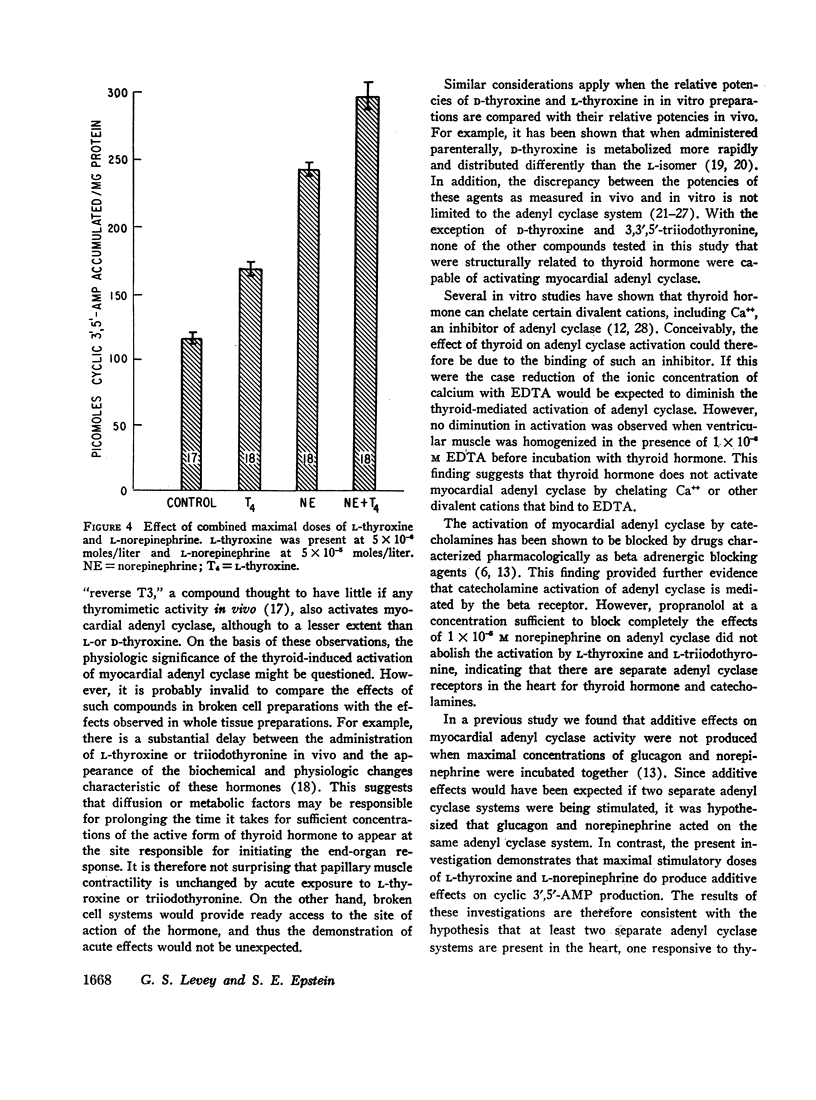
L-thyroxine and L-triiodothyronine increased the conversion of adenosine triphosphate-32P (ATP-32P) to cyclic 3′,5′-adenosine monophosphate-32P (3′,5′-AMP-32P) by 60 and 45% respectively (P < 0.01). A variety of compounds structurally related to the thyroid hormones, but devoid of thyromimetic activity did not activate adenyl cyclase: these included 3,5-diiodo-L-thyronine, L-thyronine, 3,5-diiodotyrosine, monoiodotyrosine, and tyrosine. D-thyroxine activated adenyl cyclase and half maximal activity was identical to that of the L-isomer. Although the beta adrenergic blocking agent propranolol abolished norepinephrine-induced activation of adenyl cyclase, it failed to alter activation caused by thyroxine. When maximal concentrations of L-thyroxine (5 × 10-6 moles/liter) and norepinephrine (5 × 10-5 moles/liter) were incubated together, an additive effect on cyclic 3′,5′-AMP production resulted.
This investigation demonstrates: (a) thyroid hormone is capable of activating myocardial adenyl cyclase in vitro and (b) this effect is not mediated by the beta adrenergic receptor. Moreover, the additive effects of norepinephrine and thyroxine suggest that at least two separate adenyl cyclase systems are present in the heart, one responsive to norepinephrine, the other to thyroid hormone.
These findings are compatible with the hypothesis that the cardiac manifestations of the hyperthyroid state may, in part, be caused by the direct activation of myocardial adenyl cyclase by thyroid hormone.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buccino R. A., Spann J. F., Jr, Pool P. E., Sonnenblick E. H., Braunwald E. Influence of the thyroid state on the intrinsic contractile properties and energy stores of the myocardium. J Clin Invest. 1967 Oct;46(10):1669–1682. doi: 10.1172/JCI105658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOPER C., TAPLEY D. F. The effect of thyroxine and related compounds on oxidative phosphorylation. J Biol Chem. 1956 Sep;222(1):341–349. [PubMed] [Google Scholar]
- DUNNE P. B., TAPLEY D. F. Oxygen consumption by tissues from rats injected with L- or D-thyroxine. Nature. 1960 Feb 27;185:622–623. doi: 10.1038/185622b0. [DOI] [PubMed] [Google Scholar]
- KLEBANOFF S. J. An effect of thyroxine and related compounds on the oxidation of certain hydrogen donors by the peroxidase system. J Biol Chem. 1959 Sep;234:2437–2442. [PubMed] [Google Scholar]
- Krishna G., Weiss B., Brodie B. B. A simple, sensitive method for the assay of adenyl cyclase. J Pharmacol Exp Ther. 1968 Oct;163(2):379–385. [PubMed] [Google Scholar]
- LARDY H. Effect of thyroid hormones on enzyme systems. Brookhaven Symp Biol. 1955 Feb;7:90-7; discussion, 97-101. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levey G. S., Epstein S. E. Activation of adenyl cyclase by glucagon in cat and human heart. Circ Res. 1969 Feb;24(2):151–156. doi: 10.1161/01.res.24.2.151. [DOI] [PubMed] [Google Scholar]
- Levey G. S., Epstein S. E. Activation of cardiac adenyl cyclase by thyroid hormone. Biochem Biophys Res Commun. 1968 Dec 30;33(6):990–995. doi: 10.1016/0006-291x(68)90411-7. [DOI] [PubMed] [Google Scholar]
- MALEY G. F., LARDY H. A. Metabolic effects of thyroid hormones in vitro. II. Influence of thyroxine and triiodothyronine on oxidative phosphorylation. J Biol Chem. 1953 Sep;204(1):435–444. [PubMed] [Google Scholar]
- Margolius H. S., Gaffney T. E. The effects of injected norepinephrine and sympathetic nerve stimulation in hypothyroid and hyperthyroid dogs. J Pharmacol Exp Ther. 1965 Sep;149(3):329–335. [PubMed] [Google Scholar]
- Murayama M., Goodkind M. J. Effect of thyroid hormone on the frequency-force relationship of atrial myocardium from the guinea pig. Circ Res. 1968 Dec;23(6):743–751. doi: 10.1161/01.res.23.6.743. [DOI] [PubMed] [Google Scholar]
- PITTMAN C. S., BARKER S. B. The calorigenic effects of some thyroxine analogs on intact animals and on excised tissues. J Clin Invest. 1962 Apr;41:696–701. doi: 10.1172/JCI104527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PITTMAN J. A., TINGLEY J. O., NICKERSON J. F., HILL S. R., Jr Antimetabolic activity of 3,3',5'-triiodo-DL-thyronine in man. Metabolism. 1960 Mar;9:293–295. [PubMed] [Google Scholar]
- SELENKOW H. A., ASPER S. P., Jr Biological activity of compounds structurally related to thyroxine. Physiol Rev. 1955 Apr;35(2):426–474. doi: 10.1152/physrev.1955.35.2.426. [DOI] [PubMed] [Google Scholar]
- SOKOLOFF L., KAUFMAN S. Thyroxine stimulation of amino acid incorporation into protein. J Biol Chem. 1961 Mar;236:795–803. [PubMed] [Google Scholar]
- Sutherland E. W., Robison G. A. The role of cyclic-3',5'-AMP in responses to catecholamines and other hormones. Pharmacol Rev. 1966 Mar;18(1):145–161. [PubMed] [Google Scholar]
- TAPLEY D. F., DAVIDOFF F. F., HATFIELD W. B., ROSS J. E. Physiological disposition of D- and L-thyroxine in the rat. Am J Physiol. 1959 Nov;197:1021–1027. doi: 10.1152/ajplegacy.1959.197.5.1021. [DOI] [PubMed] [Google Scholar]
- TAPLEY D. F. The effect of thyroxine and other substances on the swelling of isolated rat liver mitochondria. J Biol Chem. 1956 Sep;222(1):325–339. [PubMed] [Google Scholar]
- WOLFF J., WOLFF E. C. The effect of thyroxine on isolated dehydrogenases. Biochim Biophys Acta. 1957 Nov;26(2):387–396. doi: 10.1016/0006-3002(57)90021-5. [DOI] [PubMed] [Google Scholar]
- Waldstein S. S. Thyroid-catecholamine interrelations. Annu Rev Med. 1966;17:123–132. doi: 10.1146/annurev.me.17.020166.001011. [DOI] [PubMed] [Google Scholar]
- Weinbach E. C., Garbus J. The rapid restoration of respiratory control to uncoupled mitochondria. J Biol Chem. 1966 Aug 25;241(16):3708–3713. [PubMed] [Google Scholar]
- Wilson W. R., Theilen E. O., Hege J. H., Valenca M. R. Effects of beta-adrenergic receptor blockade in normal subjects before, during, and after triiodothyronine-induced hypermetabolism. J Clin Invest. 1966 Jul;45(7):1159–1169. doi: 10.1172/JCI105422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Schoot J. B., Moran N. C. An experimental evaluation of the reputed influence of thyroxine on the cardiovascular effects of catecholamines. J Pharmacol Exp Ther. 1965 Sep;149(3):336–345. [PubMed] [Google Scholar]


