Abstract
Sensitization is a form of implicit learning produced by the exposure to a harmful stimulus. In humans and other mammals, sensitization following skin injury increases the responsiveness of peripheral nociceptors, and enhances the synaptic transmission of nociceptive input in the central nervous system (CNS). Here, we show that sensitization-related changes in the CNS are not restricted to nociceptive pathways and, instead, also affect other sensory modalities, especially if that modality conveys information relevant for the sensitized body part. Specifically, we show that after sensitizing the forearm using high-frequency electrical stimulation of the skin (HFS), visual stimuli projected onto the sensitized forearm elicit significantly enhanced brain responses. Whereas mechanical hyperalgesia was present both 20 and 45 minutes after HFS, the enhanced responsiveness to visual stimuli was present only 20 minutes after HFS. Taken together, our results indicate that sensitization involves both nociceptive-specific and multimodal mechanisms, having distinct time courses.
Keywords: Central Sensitization, Sensory Processing, EEG, HFS, Learning
Introduction
The intense activation of nociceptors, such as during skin injury, leads to a sustained enhancement of the responses to noxious stimuli delivered to the injured skin (referred to as “primary hyperalgesia”), but also to the surrounding uninjured skin (referred to as “secondary hyperalgesia”). Whereas primary hyperalgesia is commonly explained by increased responsiveness of peripheral nociceptors, i.e. peripheral sensitization, secondary hyperalgesia is considered a hallmark of central sensitization, which is defined as increased responsiveness of nociceptive neurons of the central nervous system to their normal or subthreshold afferent input [23].
Considering that the purpose of sensitization is to prevent further exposure of the injured body part to noxious stimuli, we postulated that in vertebrates such as humans, sensitization does not restrict itself to mechanisms acting on nociceptive pathways. Instead, it may also involve cortical mechanisms affecting other sensory modalities, if the information conveyed by that sensory system is relevant for the injured body part, for instance, visual input predicting imminent contact of an object with that body part. Supporting this possibility, patients with chronic pain often report hypersensitivity to a broad range of stimuli including non-somatosensory stimuli [11,44].
To test this hypothesis, we examined whether high frequency electrical stimulation of the skin (HFS), an experimental procedure known to induce a robust central sensitization of nociceptive pathways in healthy volunteers [17], also induces an enhancement of the cortical processing of visual stimuli projected onto the sensitized body part. Differently from previous studies, we did not explore the effects of shortly preceding or concomitant nociceptive stimulation [13,14,29]. Instead, we examined whether sustained after-effects on visual processing can be observed 20 minutes after the intense pain experienced during the application of HFS has ceased. During conditioning, HFS generates intense pain and/or discomfort, but these sensations do not persist after the end of the conditioning [18]. This ensures that the effects observed after HFS are not due merely to an effect of ongoing spontaneous pain.
Importantly, previous studies have shown that, in addition to a long lasting (> 6 hours) mechanical hyperalgesia ascribed to central sensitization of nociceptive pathways at the level of the spinal cord [28], HFS also induces an enhancement of the responses to non-painful vibrotactile stimuli conveyed via the dorsal columns [37]. This enhancement was found to be short-lasting, being present 20 minutes after HFS, but not 45 minutes after HFS. This suggests that the changes induced by HFS involve multiple mechanisms, having distinct time courses.
To characterize the effects of the intense activation of nociceptors on visual processing, we applied HFS to the skin of the left or right ventral forearm, and recorded EEG responses to brief visual stimuli generated using a visible laser pointing directly onto the sensitized skin (HFS area) or onto the skin of the contralateral forearm (control area), immediately before applying HFS, 20 minutes after applying HFS, and 45 minutes after applying HFS.
Materials and Methods
Participants
Twenty-six participants took part in the experiment (18 women, 8 men, median age 23, range 19-37). Participants were recruited among students and staff of the university and were naïve to the aims of the study. Informed written consent was obtained before the beginning of the study, which was approved by the Ethics Committee of the Université catholique de Louvain.
Experimental setup and stimuli
The experiment was conducted in a dimly lit room. Participants were comfortably sitting on a chair, the chin on a chin rest and their arms positioned palms up on a table in front of them.
Visual stimuli were presented using two green laser diodes (green laser module 6194SD1 series, Sean and Stephen Corporation, Taiwan, wavelength 532 nm) pointing to the middle of the left and right volar forearms, equidistant from the wrist and the cubital fossa. The two lasers were mounted on the upper part of the chin rest frame, onto which participants positioned their chin.
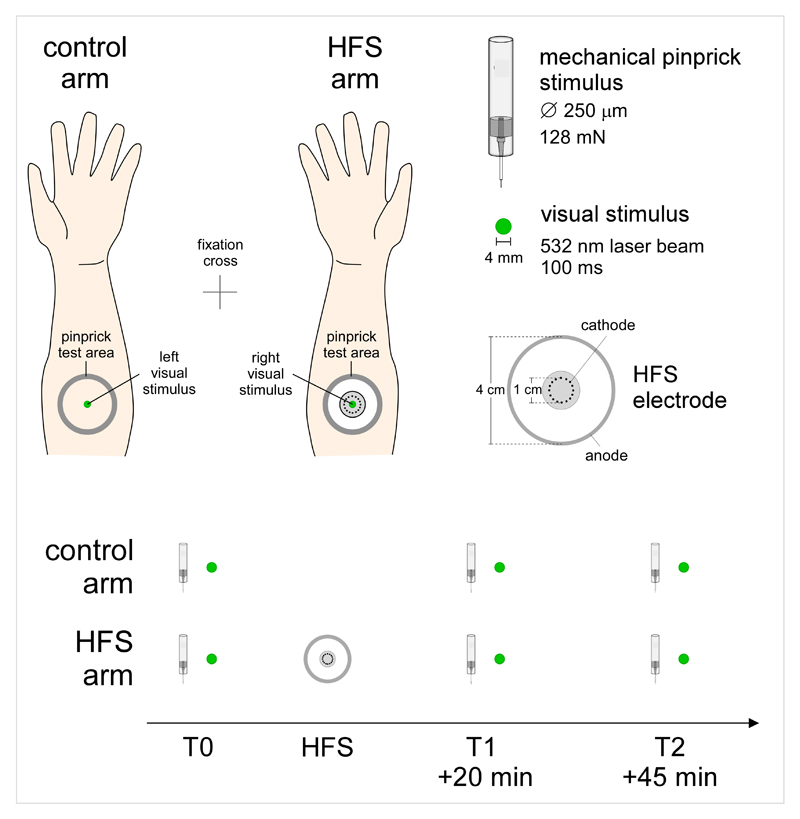
The area of the forearm where the visual stimuli were projected was marked onto the skin at the beginning of the experiment; and controlled before and after each session. The position of the arms was also marked on the table such as to ensure a constant position throughout the whole experiment. Participants underwent three sessions, one session before high frequency stimulation (HFS) of the skin (T0), and two sessions after HFS (T1: 20 minutes after HFS; T2: 45 minutes after HFS) (Figure 1).
Figure 1.
Experimental setup. Sensitization of the left or right volar forearm was induced using high frequency electrical stimulation of the skin (HFS). The stimulation consisted in five 1-s trains of 100 Hz stimuli (pulse width 2 ms), delivered to the skin using a specific electrode made of 16 blunt stainless steel pins with a diameter of 0.2 mm protruding 1 mm from the base. The pins were placed in a circle with a diameter of 10 mm and serve as cathode. A stainless steel reference electrode that served as anode was concentrically located with an inner diameter of 22 mm and an outer diameter of 40 mm. Visual stimuli were projected onto the skin of the left and right volar forearms using two green laser diodes. The two lasers were mounted on the upper part of a chin rest frame, onto which participants positioned their chin. Changes in mechanical pinprick sensitivity were tested using a 128 mN pinprick probe. Visual-evoked brain responses and mechanical pinprick sensitivity were assessed before applying HFS (T0), 20 minutes after applying HFS (T1) and 45 minutes after applying HFS (T2).
Eye movements were monitored in all participants using an infrared camera (EyeLinks 1000, SR Research Ltd. Kanata, Ontario, Canada) recording at a sampling rate of 1,000 Hz. Participants were instructed to fixate a cross equidistant from the two arms for the whole duration of the experimental sessions, and not to look directly at the arms. Online monitoring of eye gaze position was performed by one of the experimenters, to ensure that all participants maintained their gaze towards the fixation cross. In 8 of the 26 participants, the position of the eye gaze was recorded at 5 kHz using a CED Micro 1401-3 (Cambridge Electronic Design, CED, UK).
High frequency stimulation (HFS) of the volar forearm skin consisted of five trains of stimuli (pulse width 2 ms) lasting 1 second. During the trains, the stimuli were repeated at a 100 Hz frequency. The time interval between each train was 9 seconds. The entire procedure lasted 50 seconds. The intensity of stimulation was adjusted individually at twenty times the detection threshold of a single pulse. The side of HFS was balanced between the dominant and non-dominant arm across participants. Stimuli were generated by a constant-current electrical stimulator (Digitimer DS7A) and delivered to the skin using a specific electrode [17]. The electrode is made of 16 blunt stainless steel pins with a diameter of 0.2 mm protruding 1 mm from the base. The pins are placed in a circle with a diameter of 10 mm and serve as cathode. A stainless steel reference electrode that serves as anode is concentrically located and has an inner diameter of 22 mm and an outer diameter of 40 mm. Participants were unaware of the side of HFS at the beginning of the experiment.
Experimental sessions
Briefly presented visual stimuli generate a series of deflections in the EEG that are time locked to the occurrence of the stimulus and are referred to as visual evoked potentials (VEPs). Each of these deflections is characterized by a specific latency corresponding to a different (cortical) generator [4]. A total of 160 stimuli were applied in each visual evoked potential (VEP) session (80 per arm), alternatively on the right or left arm, with no more than 3 stimuli applied consecutively to the same arm. Each stimulus lasted 100 ms, and the inter-stimulus interval between two stimuli varied between 2 and 3 seconds.
Visual stimuli can also be presented in a periodic fashion, such as to elicit a steady-state visual-evoked potential (SS-VEP) related to the synchronous entrainment of neurons responding to the stimulus [31]. In the SS-VEP session, trains of visual stimuli were flashed simultaneously onto the two forearms using two different frequencies (7.04 Hz and 8.92 Hz). A total of 10 trains were applied, each lasting 10 s. The stimulation-free interval between two trains was 10 s. The frequency of the visual stimulus applied onto the control and HFS arm was balanced across participants.
To ensure that HFS successfully induced hyperalgesia on the conditioned arm, mechanical punctate stimuli were applied with a 128 mN pinprick probe having a flat cylindrical tip of 250 µm within an area of 4 cm2 at a distance of 2 cm distal and proximal relative to the center of the conditioning stimulation. In half of the participants, the mechanical punctate stimuli were applied before the recording of VEPs and SS-VEPs. In the remaining half, the assessment of mechanical hyperalgesia was performed after the recording of VEPs and SS-VEPs.
EEG recording
The EEG was recorded at a 1 kHz sampling rate using a 64-channel amplifier and digitizer (ASA-LAB EEG system; Advanced Neuro Technologies, The Netherlands). Scalp signals were acquired using 64 shielded electrodes, positioned according to the International 10-10 system (Waveguard; Advanced Neuro Technologies, The Netherlands). The ground electrode was positioned at FCz. A pair of bipolar electrodes placed at the upper-left and lower-right sides of the right eye were used to record artifacts related to eye movements. Analysis of the EEG data was carried out using Letswave 6 (http://www.nocions.org/letswave).
VEPs. The continuous EEG recordings were band-pass filtered using 0.5-30 Hz Butterworth zero phase filter, and segmented in 1.5 s epochs extending from -0.5 to +1 s relative to stimulus onset. EOG artifacts were subtracted using a validated method based on independent component analysis (ICA) [16]. In all datasets, ICs related to eye movements had a large EOG channel contribution and a frontal scalp distribution. Baseline correction was performed using the -0.5 to 0 s pre-stimulus interval. Epochs exceeding ± 100 µV were excluded. For each participant, separate average waveforms were computed for each session (T0, T1, T2) and arm (HFS and control arms).
SS-VEPs. Due to technical issues, data from three participants was not available therefore analysis of the SS-VEPs was based on the data obtained in 23 volunteers. A DC filter with a linear detrend was applied to remove slow drifts from the continuous EEG recordings. The signal was then segmented twice in order to obtain epoch durations corresponding to a multiple of the 7.04 Hz and 8.92 Hz stimulation cycles: from 0.994 to 9.939 s after the onset of the stimulation train to extract the 7.04 Hz SS-VEP and from 1.008 to 9.967 after the onset of the stimulation train to extract the 8.92 Hz SS-VEP. This segmentation ensured that the Fourier transform of the signals included frequency bins at the exact frequencies of stimulation and its harmonics. The first second of stimulation was excluded to avoid contamination of the signals by transient responses related to the onset of the visual stimulation. EOG artifacts were subtracted using a validated method based on independent component analysis (ICA) [16]. Epochs were then averaged and a Fast Fourier Transform (FFT) applied. The spectra obtained at occipital electrodes (Pz, POz, Oz, O1, O2, PO5, PO3, PO4, PO6, PO7, PO8) were averaged, and the baseline-corrected amplitudes at 7.04 Hz and 8.92 Hz and their first harmonic (14.08 Hz and 17.84 Hz) were extracted.
Eye gaze movements
Eye gaze movements were monitored in all participants, and analyzed offline in 8 participants. The X and Y signals were segmented from -0.5 to +1.5 after stimulus onset, low-pass filtered at 100 Hz. Trials containing eye blinks were eliminated. Each of the 160 epochs of the T0, T1 and T2 was visually inspected to detect saccades or microsaccades, in the whole epoch.
Statistical analysis
Statistical analyses were conducted using IBM Statistics SPSS 19 (Armonk, NY: IBM Corp.). Assumption of normality was tested using the Wilk-Shapiro test. The Greenhouse-Geisser correction was used in case of violation of sphericity. To characterize the effects of HFS at T1 (20 minutes after HFS) and T2 (45 minutes after HFS), two separate repeated-measures ANOVA were performed with the following within-subject factors: ‘time’ (T1 vs. T0 and T2 vs. T0) and ‘side’ (treated vs. control arm). The interaction between the two factors was used to isolate the effects of HFS. The analyses were performed on the ratings of the intensity of perception elicited by mechanical pinprick stimulation, the magnitude and latency of the vertex components of the VEPs, and the magnitude of the SS-VEPs.
Results
Mechanical hyperalgesia
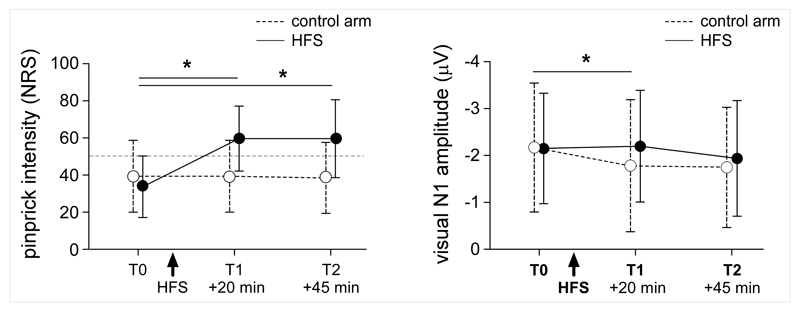
After HFS, the intensity of the percept elicited by mechanical pinprick stimulation was markedly increased at the HFS area, both 20 minutes after HFS (T1 ‘time’ x ‘arm’ interaction: F(1,25)=119.496 p<0.0001) and 45 minutes after HFS (T2 F(1,25)=96.335 p<0.001) (Fig. 2). This confirms that HFS induced a robust and long-lasting mechanical hyperalgesia related to the sensitization of nociceptive pathways.
Figure 2.
Mechanical hyperalgesia. Increased sensitivity to mechanical pinprick stimuli was observed at the HFS arm, both 20 minutes (T1) and 45 minutes (T2) after applying HFS. HFS also exerted a significant effect on the event-related potentials elicited by visual stimuli delivered to the HFS arm. The magnitude of the N1 wave of visual-evoked potentials elicited by stimulation of the HFS arm was significantly enhanced 20 minutes after applying HFS, but not 45 minutes after HFS. Asterisks indicate a p-values smaller than 0.05.
Visual event-related brain potentials (VEPs)
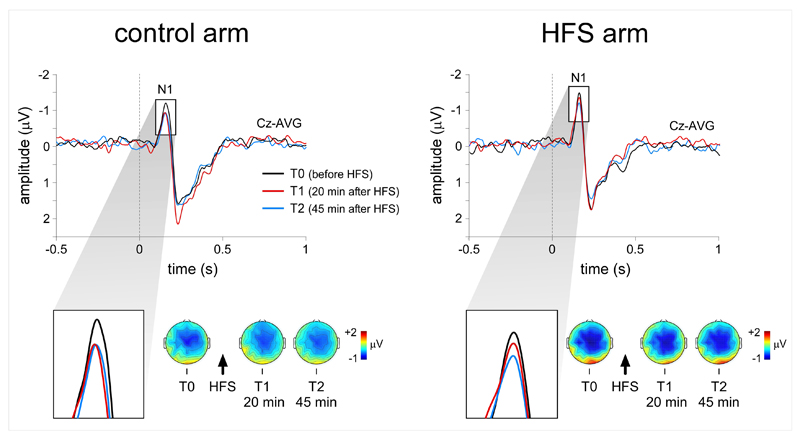
In all sessions and participants, the visual stimuli delivered to the left and right forearms elicited a clear negative wave, peaking approximately 150-160 ms after stimulation onset, maximal over the scalp vertex, and referred to as the N1 wave [41], see also [4] (Fig. 3). This wave was followed by a positive deflection (P2) peaking approximately 200-300 ms after stimulation. Both peaks were maximal at electrode Cz and were detected in all participants. Earlier occipito-temporal components were inconsistently identified (see also [42]). This was most likely due to the fact that, in the present study, the visual stimuli were presented in the far periphery of the visual field (the visual angle between the central fixation point and the visual stimulus was approximately 25°), and the fact that the visual stimulus consisted in a bright but small dot flashed onto the forearm and not a reversing checkerboard. For these reasons, analysis of the VEP waveforms was restricted to the magnitude and latency of the N1 and P2 waves measured at electrode Cz.
Figure 3.
Visual evoked potentials. Effect of HFS on the N1 wave of visual-evoked potentials elicited by stimuli projected onto the sensitized arm. The waveforms show the group-level average of the signals recorded at electrode Cz. The scalp maps show the topographical distribution of the N1 wave. HFS exerted a significant effect on the magnitude of the N1 wave of visual-evoked potentials. Twenty minutes after HFS, but not 45 minutes after HFS, the N1 wave was reduced for stimuli applied to the control arm, but not for stimuli applied to the HFS arm.
HFS had a significant effect on the magnitude of the N1 wave elicited by stimuli delivered to the HFS arm. Twenty minutes after HFS (T1), the repeated-measures ANOVA showed a significant interaction between the factors ‘time’ and ‘arm’ (F(1, 25)=5.393 p=0.029). In contrast, 45 minutes after HFS, there was no significant interaction between the two factors (F(1,25)=1.244 p=0.275). The N1 wave was clearly decreased after HFS at T1 for stimuli applied onto the control arm (paired t-test T1 vs. T0 t(25)=-2.823 p=0.009), and this was not the case for stimuli applied onto the sensitized arm (T1 vs. T0 t(25)=0.440 p=0.664) (Figs. 2 and 3). Effects were also observed on the magnitude of the P2 wave. At both T1 and T2 we observed a significant interaction between the factors ‘time’ and ‘arm’ (T1: F(1, 25)=6.566 p=0.017, T2: F(1, 25)=4.742 p=0.039). The amplitude of the P2 wave was increased after HFS at T1 for stimuli applied onto the control arm (paired t-test T1 vs. T0 t(25)=3.596 p=0.001), but not for stimuli applied onto the sensitized arm (paired t-test T1 vs. T0 t(25)=0.158 p=0.876) (Figure 3). No significant differences were observed at T2 for the HFS arm (paired t-test T2 vs. T0 t(25)=-0.851 p=0.403) as well as for control arm (paired t-test T2 vs. T0 t(25)=1.390 p=0.177). No interactions were observed for the latencies of the two peaks (N1 T1: F(1, 25)=0.237 p=0.631, T2 F(1, 25)= p=0.064 p=0.803; P2 T1: F(1, 25)=0.001 p=0.986, T2 F(1, 25)=0.821 p=0.374)
Steady-state visual-evoked (SS-VEPs)
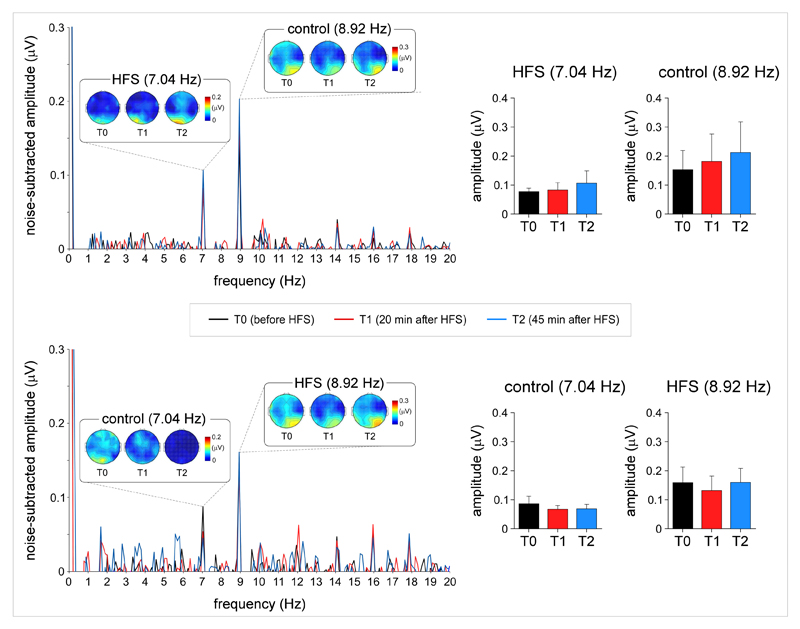
SS-VEPs were recorded by flashing simultaneously the visual stimuli onto both arms, using two different frequencies (7 and 9 Hz) to “frequency tag” the cortical activity triggered by the visual stimulation of each arm. The decision to record SS-VEPs in addition to VEPs was motivated by the fact that SS-VEPs elicited by flashing visual stimuli have a high signal-to-noise ratio, and predominantly reflect activity originating from low level visual areas [5,33]. In all sessions and participants, visual stimulation of the two arms generated a clear peak at 7 Hz and 9 Hz in the EEG frequency spectra, maximal over posterior occipital electrodes (Fig. 4). Contrasting with the effects of HFS on the magnitude of the VEPs, HFS did not appear to have any effect on the magnitude of the SS-VEPs. Both 20 minutes and 45 minutes after HFS, there was no significant ‘time’ x ‘arm’ interaction (base frequency T1: F(1,22)=0.984 p=0.332, T2: F(1,22)=0.071 p=0.792; first harmonic frequency F(1,22)=2.614 p=0.120, T2: F(1,22)=0.312 p=0.528).
Figure 4.
Steady-state visual-evoked potentials. Steady-state visual-evoked potentials (SS-VEPs) elicited by concomitant periodic visual stimulation of the HFS arm and control arm at 7 Hz or 9 Hz. The frequency of the stimulation applied onto the HFS arm or the control arm was balanced across participants. Contrary to the N1 wave of visual-evoked potentials, HFS did not appear to exert any significant effect on the magnitude of the SS-VEPs, whose scalp topographies were maximal over occipital regions.
Eye movements
To exclude the possibility that our results could be explained by participants overtly looking at the sensitized arm, we performed for all participants a monitoring of eye gaze with an infrared eye tracker system (EyeLink 1000, SR Research Mississauga, Ontario). Online visual inspection by the experimenter showed that all participants effectively maintained their gaze onto the fixation cross, and did not perform more gaze movements towards the treated or control arm after HFS. In 8 of the 26 participants these responses were additionally recorded and the gaze movements in these three sessions were analyzed off-line. The average number of saccades and/or microsaccades performed was 5 (± 3) out of 160 at T0, 7 (± 4) at T1, 7 (± 4) at T2.
Discussion
In this study we show that the intense activation of nociceptors does not only lead to a sensitization of nociceptive pathways, but also induces changes in the cortical processing of visual stimuli projected onto the sensitized body part. Specifically, we show that, in healthy volunteers, sensitization of nociceptive pathways induced by transcutaneous high frequency electrical stimulation (HFS) exerts an effect on the brain responses to visual stimuli projected onto the sensitized skin.
Short- vs. long-lasting effects of HFS
The effect of HFS on the magnitude of the N1 wave of VEPs was present 20 minutes after applying HFS, but not 45 minutes after applying HFS. In contrast, mechanical hyperalgesia was present both 20 minutes and 45 minutes after HFS. This is in line with our hypothesis that the intense activation of nociceptors has multiple effects on the function of the nervous system, and that these different effects have distinct time courses. Indeed, in previous studies we showed that HFS enhances the responses to non-painful tactile stimuli as well as warm stimuli, but that the duration of this enhancement is much shorter than the duration of the induced mechanical hyperalgesia [36–40]. We propose that the long-lasting effect of HFS on the responses to noxious mechanical stimuli are predominantly related to a specific effect of HFS on nociceptive pathways [35], whereas the shorter-lived effects of HFS on the responses to innocuous tactile or visual stimuli result from supraspinal and multimodal mechanisms leading to an enhancement of the responses to stimuli that are relevant for the sensitized body part.
Previous studies have already shown evidence for multisensory interactions between concurrent noxious and visual processing [29], as well as cross-modal effects of spatial attention between vision and nociception [1,2,8]. Furthermore, previous studies have also shown that spatial attention can increase the magnitude of the negative vertex component elicited by stimuli belonging to several modalities (e.g. [9,10,20,41], see [3,12,21,34] for reviews and commentary). Therefore, a possible explanation to the “crossmodal sensitization” observed in the present study could be that HFS-related sensations induce cross-modal links in endogenous or exogenous spatial attention. However, this explanation seems unlikely. HFS elicits a strong painful sensation when it is applied, but it is not associated with any spontaneous ongoing after-sensation [18,32]. Therefore, because the effect of HFS on the responses to visual stimuli delivered onto the sensitized arm was observed 20-30 minutes after HFS, it seems unlikely that this effect of HFS on visual processing was merely a consequence of endogenous spatial attention. Moreover, crossmodal links in exogenous spatial attention are typically observed with a short temporal delay between the cue and the ‘target’ stimulus (usually milleseconds or seconds e.g. [7,8]). Supporting the view that these effects are not due to spatial attention is also the finding that brain responses to electrical stimuli delivered to the sensitized arm are not increased immediately after HFS, but only after a time interval of 20-30 minutes [39]. Instead, an effect on spatial attention of the sensation perceived while applying HFS would be expected to be maximal immediately after experiencing HFS, and to then to decay rapidly over time.
Studies have shown that, after HFS, activity-dependent long-term potentiation (LTP) is observed for nociceptive neurons in lamina I [15], lamina II [22,30], and deep dorsal horn neurons. However, after injury, several forms of LTP can be observed in other parts of the central nervous system, for instance, in the thalamus and in the cingulate cortex [19] (see [45] for a review). Because there is no reason to consider that these supra-spinal changes in synaptic efficacy only involve neurons responding to nociceptive input, these previously described changes could contribute to our observation that HFS enhances the N1 wave of VEPs.
Effects of HFS on early vs. late stages of visual processing
Most previous studies have shown that the presentation of a transient visual stimulus in the left or right visual hemifield elicits a complex negative wave with a maximum over posterior areas contralateral to the stimulated hemifield as well as the scalp vertex. This topography has been interpreted as reflecting activity originating from multiple cortical sources [4,6]. It is important to emphasize that, in most of the previous studies, the eccentricity of the visual stimuli was much smaller than the eccentricity of the visual stimuli delivered in the present study. For example, the visual stimuli used by [4,6] were presented 4° from the central fixation point. Van Voorhis et al. [41] used a 20° visual angle which was more similar to the one used in the present study, and observed an N1 wave which was maximal over the scalp vertex. The relative contribution of activity originating from contralateral visual areas vs. other higher-order areas could be reduced and/or become more variable when stimuli are presented far in the periphery of the visual field. Another important distinguishing feature of our study is that the visual stimulus consisted in a bright but small green dot flashed onto the forearm, and not a checkerboard. Probably for these reasons, in the present study, the N1 wave received a less prominent contribution of activity originating from contralateral visual areas and, hence, was predominantly reflected higher-order activity most consistently identified at the scalp vertex.
In contrast, SS-VEPs elicited by periodically flashing the visual stimulus at a constant frequency elicited a periodic EEG response which was clearly maximal over occipital recording sites, and most probably reflected activity originating predominantly from low-level visual areas [5,33].
The dissociation between the effect of HFS on the magnitude of the late N1 and P2 waves of VEPs and the absence of an effect of HFS on the magnitude of SS-VEPs suggests that this “cross-modal sensitization” modulates higher-order rather than low-level visual processes [4,24].
The magnitude of the N1 wave elicited by visual stimuli delivered to the sensitized arm was increased after HFS. In contrast, the magnitude of the later P2 wave was decreased after HFS. Similar mirror effects on the negative and positive components of the vertex potential have been described in previous studies [25–27,43], and have been explained by the fact that multiple components having distinct time courses may modulate the magnitude of the N1 wave. Specifically, components contributing to the negativity of the N1 wave may overlap with the later P2 wave. If such components are enhanced, for example, after HFS, this would lead to an increased negativity of the N1 wave and, conversely, a decreased positivity of the P2 wave. Of note, the occurrence of a “mismatch negativity” (MMN) response can lead to a similar mirror effect on the magnitude of the auditory N1-P2.
Conclusion
Taken together, our findings show that the mechanisms underlying central sensitization extend well beyond the nociceptive system. Specifically, our findings show that, at the level of the central nervous system, the sensitization induced by the sustained and intense activation of nociceptors involves at least two different mechanisms having distinct time courses. One of these mechanisms appears to selectively affect the responses to noxious mechanical stimuli delivered to the sensitized body part and could be largely explained by a specific enhancement of the responsiveness of spinal nociceptive neurons [35]. This mechanical hypersensitivity has been well described in previous studies, and has been shown to be very long lasting as it is still present several hours after the application of HFS [28]. The other mechanism, identified in the present study, is supraspinal as it leads to a crossmodal enhancement of the responses to visual stimuli projected onto the sensitized body part. This mechanism is also shorter-lived, as it modulated the responses to visual stimulation 20 minutes after HFS, but not 45 minutes after HFS.
Acknowledgments
A.M. and E.V.D.B received support from an ERC starting grant (‘PROBING-PAIN’). L.F. is supported by the FRS-FNRS. The authors would like to thank Valéry Legrain for his suggestions.
Footnotes
No conflicts of interest are declared.
References
- [1].De Paepe AL, Crombez G, Legrain V. From a Somatotopic to a Spatiotopic Frame of Reference for the Localization of Nociceptive Stimuli. PloS one. 2015;10(8):e0137120. doi: 10.1371/journal.pone.0137120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].De Paepe AL, Crombez G, Spence C, Legrain V. Mapping nociceptive stimuli in a peripersonal frame of reference: Evidence from a temporal order judgment task. Neuropsychologia. 2014;56:219–228. doi: 10.1016/j.neuropsychologia.2014.01.016. [DOI] [PubMed] [Google Scholar]
- [3].Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual review of neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- [4].Di Russo F, Martinez A, Sereno MI, Pitzalis S, Hillyard SA. Cortical sources of the early components of the visual evoked potential. Human brain mapping. 2002;15(2):95–111. doi: 10.1002/hbm.10010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Di Russo F, Pitzalis S, Aprile T, Spitoni G, Patria F, Stella A, Spinelli D, Hillyard SA. Spatiotemporal analysis of the cortical sources of the steady-state visual evoked potential. Human brain mapping. 2007;28(4):323–334. doi: 10.1002/hbm.20276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Di Russo F, Spinelli D, Morrone MC. Automatic gain control contrast mechanisms are modulated by attention in humans: evidence from visual evoked potentials. Vision research. 2001;41(19):2435–2447. doi: 10.1016/s0042-6989(01)00134-1. [DOI] [PubMed] [Google Scholar]
- [7].Driver J, Spence C. Cross-modal links in spatial attention. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 1998;353(1373):1319–1331. doi: 10.1098/rstb.1998.0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Favril L, Mouraux A, Sambo CF, Legrain V. Shifting attention between the space of the body and external space: Electrophysiological correlates of visual-nociceptive crossmodal spatial attention. Psychophysiology. 2014 doi: 10.1111/psyp.12157. [DOI] [PubMed] [Google Scholar]
- [9].Franz M, Nickel MM, Ritter A, Miltner WH, Weiss T. Somatosensory spatial attention modulates amplitudes, latencies, and latency jitter of laser-evoked brain potentials. Journal of neurophysiology. 2015;113(7):2760–2768. doi: 10.1152/jn.00070.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Garcia-Larrea L, Lukaszewicz AC, Mauguiere F. Somatosensory responses during selective spatial attention: The N120-to-N140 transition. Psychophysiology. 1995;32(6):526–537. doi: 10.1111/j.1469-8986.1995.tb01229.x. [DOI] [PubMed] [Google Scholar]
- [11].Geisser ME, Glass JM, Rajcevska LD, Clauw DJ, Williams DA, Kileny PR, Gracely RH. A psychophysical study of auditory and pressure sensitivity in patients with fibromyalgia and healthy controls. The journal of pain : official journal of the American Pain Society. 2008;9(5):417–422. doi: 10.1016/j.jpain.2007.12.006. [DOI] [PubMed] [Google Scholar]
- [12].Hillyard SA, Vogel EK, Luck SJ. Sensory gain control (amplification) as a mechanism of selective attention: electrophysiological and neuroimaging evidence. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 1998;353(1373):1257–1270. doi: 10.1098/rstb.1998.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hofle M, Hauck M, Engel AK, Senkowski D. Viewing a needle pricking a hand that you perceive as yours enhances unpleasantness of pain. Pain. 2012;153(5):1074–1081. doi: 10.1016/j.pain.2012.02.010. [DOI] [PubMed] [Google Scholar]
- [14].Hofle M, Pomper U, Hauck M, Engel AK, Senkowski D. Spectral signatures of viewing a needle approaching one's body when anticipating pain. The European journal of neuroscience. 2013 doi: 10.1111/ejn.12304. [DOI] [PubMed] [Google Scholar]
- [15].Ikeda H, Heinke B, Ruscheweyh R, Sandkuhler J. Synaptic plasticity in spinal lamina I projection neurons that mediate hyperalgesia. Science. 2003;299(5610):1237–1240. doi: 10.1126/science.1080659. [DOI] [PubMed] [Google Scholar]
- [16].Jung TP, Makeig S, Humphries C, Lee TW, McKeown MJ, Iragui V, Sejnowski TJ. Removing electroencephalographic artifacts by blind source separation. Psychophysiology. 2000;37(2):163–178. [PubMed] [Google Scholar]
- [17].Klein T, Magerl W, Hopf HC, Sandkuhler J, Treede RD. Perceptual correlates of nociceptive long-term potentiation and long-term depression in humans. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24(4):964–971. doi: 10.1523/JNEUROSCI.1222-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Klein T, Magerl W, Rolke R, Treede RD. Human surrogate models of neuropathic pain. Pain. 2005;115(3):227–233. doi: 10.1016/j.pain.2005.03.021. [DOI] [PubMed] [Google Scholar]
- [19].Koga K, Descalzi G, Chen T, Ko HG, Lu J, Li S, Son J, Kim T, Kwak C, Huganir RL, Zhao MG, et al. Coexistence of two forms of LTP in ACC provides a synaptic mechanism for the interactions between anxiety and chronic pain. Neuron. 2015;85(2):377–389. doi: 10.1016/j.neuron.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Legrain V, Guerit JM, Bruyer R, Plaghki L. Attentional modulation of the nociceptive processing into the human brain: selective spatial attention, probability of stimulus occurrence, and target detection effects on laser evoked potentials. Pain. 2002;99(1-2):21–39. doi: 10.1016/s0304-3959(02)00051-9. [DOI] [PubMed] [Google Scholar]
- [21].Legrain V, Mancini F, Sambo CF, Torta DM, Ronga I, Valentini E. Cognitive aspects of nociception and pain: bridging neurophysiology with cognitive psychology. Neurophysiologie clinique = Clinical neurophysiology. 2012;42(5):325–336. doi: 10.1016/j.neucli.2012.06.003. [DOI] [PubMed] [Google Scholar]
- [22].Liu X, Sandkuhler J. Characterization of long-term potentiation of C-fiber-evoked potentials in spinal dorsal horn of adult rat: essential role of NK1 and NK2 receptors. Journal of neurophysiology. 1997;78(4):1973–1982. doi: 10.1152/jn.1997.78.4.1973. [DOI] [PubMed] [Google Scholar]
- [23].Loeser JD, Treede RD. The Kyoto protocol of IASP Basic Pain Terminology. Pain. 2008;137(3):473–477. doi: 10.1016/j.pain.2008.04.025. [DOI] [PubMed] [Google Scholar]
- [24].Mouraux A, Iannetti GD. Nociceptive laser-evoked brain potentials do not reflect nociceptive-specific neural activity. Journal of neurophysiology. 2009;101(6):3258–3269. doi: 10.1152/jn.91181.2008. [DOI] [PubMed] [Google Scholar]
- [25].Näätänen R, Kreegipuu K. The Mismatch Negativity (MMN) In: OH online, editor. The Oxford Book of Event-Related Potential Components. Oxford University Press; 2011. [Google Scholar]
- [26].Naatanen R, Paavilainen P, Rinne T, Alho K. The mismatch negativity (MMN) in basic research of central auditory processing: a review. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2007;118(12):2544–2590. doi: 10.1016/j.clinph.2007.04.026. [DOI] [PubMed] [Google Scholar]
- [27].Naatanen R, Pakarinen S, Rinne T, Takegata R. The mismatch negativity (MMN): towards the optimal paradigm. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2004;115(1):140–144. doi: 10.1016/j.clinph.2003.04.001. [DOI] [PubMed] [Google Scholar]
- [28].Pfau DB, Klein T, Putzer D, Pogatzki-Zahn EM, Treede RD, Magerl W. Analysis of hyperalgesia time courses in humans after painful electrical high-frequency stimulation identifies a possible transition from early to late LTP-like pain plasticity. Pain. 2011;152(7):1532–1539. doi: 10.1016/j.pain.2011.02.037. [DOI] [PubMed] [Google Scholar]
- [29].Pomper U, Hofle M, Hauck M, Kathmann N, Engel AK, Senkowski D. Crossmodal bias of visual input on pain perception and pain-induced beta activity. NeuroImage. 2013;66:469–478. doi: 10.1016/j.neuroimage.2012.10.040. [DOI] [PubMed] [Google Scholar]
- [30].Randic M, Jiang MC, Cerne R. Long-term potentiation and long-term depression of primary afferent neurotransmission in the rat spinal cord. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1993;13(12):5228–5241. doi: 10.1523/JNEUROSCI.13-12-05228.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Regan D. Human brain electrophysiology. Evoked potentials and evoked magnetic fields in science and medicine. New York: 1989. [Google Scholar]
- [32].Schmelz M. Translating nociceptive processing into human pain models. Experimental brain research. 2009;196(1):173–178. doi: 10.1007/s00221-009-1809-2. [DOI] [PubMed] [Google Scholar]
- [33].Srinivasan R, Bibi FA, Nunez PL. Steady-state visual evoked potentials: distributed local sources and wave-like dynamics are sensitive to flicker frequency. Brain topography. 2006;18(3):167–187. doi: 10.1007/s10548-006-0267-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Torta DM. Understanding the mechanisms through which spatial attention acts on nociception. Journal of neurophysiology. 2015;114(5):2561–2563. doi: 10.1152/jn.00450.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].van den Broeke EN, Lambert J, Huang G, Mouraux A. Central Sensitization of Mechanical Nociceptive Pathways Is Associated with a Long-Lasting Increase of Pinprick-Evoked Brain Potentials. Frontiers in human neuroscience. 2016;10:531. doi: 10.3389/fnhum.2016.00531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].van den Broeke EN, Mouraux A. Enhanced brain responses to C-fiber input in the area of secondary hyperalgesia induced by high-frequency electrical stimulation of the skin. Journal of neurophysiology. 2014;112(9):2059–2066. doi: 10.1152/jn.00342.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Van Den Broeke EN, Mouraux A. High frequency electrical stimulation of human skin induces heterotopical mechanical and heat hyperalgesia and enhanced responses to vibrotactile input. Journal of neurophysiology. 2014 doi: 10.1152/jn.00651.2013. [DOI] [PubMed] [Google Scholar]
- [38].van den Broeke EN, van Heck CH, Ceelen LA, van Rijn CM, van Goor H, Wilder-Smith OH. The effect of high-frequency conditioning stimulation of human skin on reported pain intensity and event-related potentials. Journal of neurophysiology. 2012;108(8):2276–2281. doi: 10.1152/jn.00391.2012. [DOI] [PubMed] [Google Scholar]
- [39].van den Broeke EN, van Heck CH, van Rijn CM, Wilder-Smith OH. Neural correlates of heterotopic facilitation induced after high frequency electrical stimulation of nociceptive pathways. Molecular pain. 2011;7:28. doi: 10.1186/1744-8069-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].van den Broeke EN, van Rijn CM, Biurrun Manresa JA, Andersen OK, Arendt-Nielsen L, Wilder-Smith OH. Neurophysiological correlates of nociceptive heterosynaptic long-term potentiation in humans. Journal of neurophysiology. 2010;103(4):2107–2113. doi: 10.1152/jn.00979.2009. [DOI] [PubMed] [Google Scholar]
- [41].Van Voorhis S, Hillyard SA. Visual evoked potentials and selective attention to points in space. Perception & psychophysics. 1977;22(1):54–62. [Google Scholar]
- [42].Vibell J, Klinge C, Zampini M, Spence C, Nobre AC. Temporal order is coded temporally in the brain: early event-related potential latency shifts underlying prior entry in a cross-modal temporal order judgment task. Journal of cognitive neuroscience. 2007;19(1):109–120. doi: 10.1162/jocn.2007.19.1.109. [DOI] [PubMed] [Google Scholar]
- [43].Wang AL, Mouraux A, Liang M, Iannetti GD. The enhancement of the N1 wave elicited by sensory stimuli presented at very short inter-stimulus intervals is a general feature across sensory systems. PloS one. 2008;3(12):e3929. doi: 10.1371/journal.pone.0003929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wilbarger JL, Cook DB. Multisensory hypersensitivity in women with fibromyalgia: implications for well being and intervention. Archives of physical medicine and rehabilitation. 2011;92(4):653–656. doi: 10.1016/j.apmr.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhuo M. Long-term potentiation in the anterior cingulate cortex and chronic pain. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2014;369(1633):20130146. doi: 10.1098/rstb.2013.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]