Summary
Most cancer radiation therapy accelerators purchased today have gantry-mounted imagers, typically used to image the patient prior to treatment. We imaged prostate cancer patients during their treatment. Combining images with marker segmentation software and a 2- to 3-dimensional reconstruction method, we were able to measure prostate motion during the treatment to within submillimeter accuracy. Because intrafraction prostate monitoring method uses widely available clinical equipment, intratreatment prostate motion monitoring could become routine.
Purpose
Most linear accelerators purchased today are equipped with a gantry-mounted kilovoltage X-ray imager which is typically used for patient imaging prior to therapy. A novel application of the X-ray system is kilovoltage intrafraction monitoring (KIM), in which the 3-dimensional (3D) tumor position is determined during treatment. In this paper, we report on the first use of KIM in a prospective clinical study of prostate cancer patients undergoing intensity modulated arc therapy (IMAT).
Methods and Materials
Ten prostate cancer patients with implanted fiducial markers undergoing conventionally fractionated IMAT (RapidArc) were enrolled in an ethics-approved study of KIM. KIM involves acquiring kV images as the gantry rotates around the patient during treatment. Post-treatment, markers in these images were segmented to obtain 2D positions. From the 2D positions, a maximum likelihood estimation of a probability density function was used to obtain 3D prostate trajectories. The trajectories were analyzed to determine the motion type and the percentage of time the prostate was displaced ≥3, 5, 7, and 10 mm. Independent verification of KIM positional accuracy was performed using kV/MV triangulation.
Results
KIM was performed for 268 fractions. Various prostate trajectories were observed (ie, continuous target drift, transient excursion, stable target position, persistent excursion, high-frequency excursions, and erratic behavior). For all patients, 3D displacements of ≥3, 5, 7, and 10 mm were observed 5.6%, 2.2%, 0.7% and 0.4% of the time, respectively. The average systematic accuracy of KIM was measured at 0.46 mm.
Conclusions
KIM for prostate IMAT was successfully implemented clinically for the first time. Key advantages of this method are (1) submillimeter accuracy, (2) widespread applicability, and (3) a low barrier to clinical implementation. A disadvantage is that KIM delivers additional imaging dose to the patient.
Introduction
Tumors move during radiation therapy treatment, resulting in reduced geometric and dosimetric accuracy. In standard clinical practice, this motion is not monitored during treatment. In prostate radiation therapy, the probability of biochemical and local control decreases and rectal toxicity increases when the rectum is distended during planning computed tomography (CT) simulations (1). In 2008, Kupelian et al (2) demonstrated that daily image guidance eliminates the error due to rectal distention. In 2010, Sandler et al (3) found that real-time motion monitoring using electromagnetic (EM) guidance and gating with a reduced planning target volume (PTV) margin resulted in reduced patient morbidity. From these previous data, it can be argued that real-time tumor localization and adaptation can improve clinical outcomes. Real-time adaptation is enabled by real-time localization. Hence, the introduction of a novel real-time tumor localization modality that is widely available may be beneficial for prostate cancer outcomes.
Several real-time tumor localization imaging modalities have been investigated; for example, ultrasonography (4), megavoltage (MV) imaging (5), combined MV and kV (6), Calypso EM guidance (7), and Navotek radioactive fiducial implant (8). However, some of these techniques are either still under development, not readily available, or are expensive.
Kilovoltage intrafraction monitoring (KIM) is a novel intrafraction real-time tumor localization modality. It involves a single gantry-mounted kV X-ray imager (which is widely available on most linear accelerators [LINACs]) acquiring 2-dimensional (2D) projections of implanted fiducial markers. Three-dimensional (3D) positions are then reconstructed by maximum likelihood estimation (MLE) of a 3D probability density function. In this study, we report the first use of KIM in a prospective clinical study with prostate cancer patients undergoing intensity modulated arc therapy (IMAT).
Methods and Materials
Overview of KIM
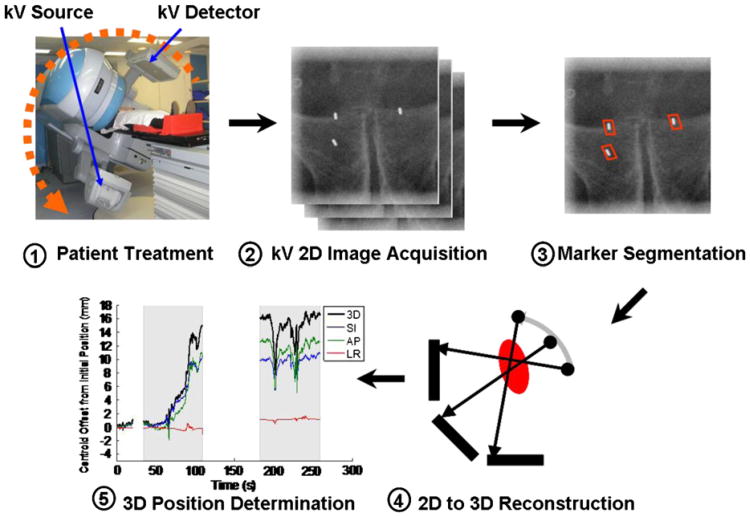
Figure 1 shows the workflow of the clinical study. As the gantry rotates around the patient during treatment (Fig. 1, 1), the kV imager acquires 2D projections of the prostate (Fig. 1, 2). The fiducial markers are segmented using software developed in-house (Fig. 1, 3). Three-dimensional positions are determined via MLE of a 3D probability density function (pdf) (Fig. 1, 4) (9). The 3D trajectory of the prostate is then plotted as a function of time (Fig. 1, 5).
Fig. 1.
Workflow of the KIM clinical study.
This clinical study was observational only. Trajectories were computed after the patient treatments, and therefore no intervention, such as patient realignment, was performed based on the results of the intrafraction monitoring information.
Patient details
Ten patients with localized prostate cancer with implanted fiducial markers undergoing conventionally fractionated double-arc IMAT (RapidArc) were enrolled in an ethics-approved study of KIM. All patients gave informed consent. A dose of 80 Gy was delivered over 40 fractions at 2 Gy/fraction. Fraction duration was approximately 2.5 min for all patients. Three cylindrical gold fiducial markers (1-mm diameter × 3-mm length) were implanted using transrectal ultrasound guidance. Planning computed tomography (CT) scans were performed while patients' rectums were empty and bladders were comfortably full in the supine position with ankle stocks (10). Before the CT scan, patients were given written information advising them to follow a low-residue diet to reduce gas production and a daily bladder-filling protocol. The rectal diameter objective during the CT scan was aimed at less than 3.5 cm. Prior to treatment, patient position was aligned based on markers using either daily cone beam CT or kV/kV matching. Table 1 shows the treatment and imaging parameters of the 10 patients.
Table 1. Patient and treatment imaging parameters that influence image quality.
| Patient | No. of fractions acquired (of 40) | Stage | Prostate PTV vol (cm3) | Nodal PTV vol (cm3) | Jaw field size (cm × cm)* | Patient lateral width (cm)† | Patient vertical thickness (cm)† | Imaging frequency (Hz) |
|---|---|---|---|---|---|---|---|---|
| 1 | 26 | T3B | 134 | 507 | 13.9 × 18.0 | 35.9 | 21.1 | 10 |
| 2 | 23 | T1C | 117 | 356 | 13.9 × 16.2 | 38.0 | 22.0 | 10 |
| 3 | 29 | T3A | 159 | 246 | 14.0 × 14.0 | 34.2 | 19.9 | 5 |
| 4 | 25 | T1C | 248 | 0 | 12.0 × 10.1 | 41.2 | 26.7 | 10 |
| 5 | 24 | T1C | 327 | 0 | 14.0 × 12.7 | 33.8 | 19.5 | 5 |
| 6 | 30 | T2A | 170 | 0 | 12.8 × 10.6 | 36.5 | 21.8 | 10 |
| 7 | 25 | T2B | 108 | 590 | 14.0 × 16.8 | 37.9 | 22.9 | 10 |
| 8 | 30 | T1C | 218 | 0 | 11.1 × 10.6 | 36.0 | 21.4 | 10 |
| 9 | 28 | T1C | 138 | 0 | 10.3 × 10.0 | 34.5 | 23.1 | 5 |
| 10 | 28 | T3A | 126 | 333 | 14.0 × 16.1 | 33.5 | 20.4 | 5 |
All images were acquired with 125 kVp, 80 mA, 13 ms with the detector at 180 cm from the source.
Averaged over 2 treatment arcs.
As measured in the isocenter axial plane.
Image acquisition during treatment
Image acquisition during treatment used a gantry-mounted kV x-ray imager mounted perpendicularly to the radiation treatment beam source (OBI; Varian). kV x-ray images were acquired as the gantry rotated around the patient during IMAT Exposure parameters used were 125 kVp, 80 mA, 13 ms (which is a standard pelvic cone beam computed tomography scan setting), with a 6 × 6 cm2 field size. The field size was chosen as the minimum (to reduce patient dose) that covered the marker positions with a margin from all angles based on a previous study (11). No filter was used during KIM acquisition. Imaging frequency was 5 or 10 Hz depending on image quality. The kV detector source-to-detector distance (SDD) was set to 180 cm (compare, 150 cm for CBCT) to reduce scatter from the MV source. The gantry, kV source, and kV detector sag during rotation, and the magnitude of this sag varies with gantry angle. Gantry sag correction was performed in the calculation of 3D position following the method outlined in the study by Cho et al (6).
Marker segmentation and 3D position determination
Images were input into software developed in-house for automated determination of the 2D positions of each marker in each projection (12). The software reconstructs 3D positions of the markers from 2D positions using the method developed by Poulsen et al (9). In brief, a 3D Gaussian pdf is assumed for each marker. As the gantry rotates around the patient, each 2D projection acquired contributes information to build the 3D pdf. MLE is applied to determine the pdf that best matches the observed 2D marker positions. The 3D position for each image is then determined from the 3D pdf. For prospective applications, such as gating or tracking, an initial pdf needs to be determined prior to treatment. Hence, a 120° pretreatment arc was acquired for 120° of gantry rotation.
With the 3D positions obtained, the trajectories of the centroid of all 3 markers (offset from their initial position) were then determined for the left-right (LR), superior-inferior (SI), anterior-posterior (AP), and 3D directions. Three sections of each the trajectory were recorded: pretreatment and first and second arcs. Also, for the entire patient cohort, the following statistics were calculated: displacement as a function of treatment time; standard deviations of systematic and random errors; and PTV margins based on the van Herk formula (13), under the ideal assumption that no other errors contribute significantly to the margin.
A total of 268 fractions were acquired from 400 patient fractions with KIM. Not all fractions were acquired, because KIM acquisitions were cancelled during periods with high patient loads or LINAC malfunctions. No LINAC malfunctions were caused by this study. All 268 fractions were successfully segmented.
Verification of KIM clinical dynamic localization accuracy using kV/MV triangulation
KIM method accuracy has been validated in experimental phantom settings in previous studies. The most relevant study quantified the geometric accuracy from a motion phantom programmed with 5 Calypso-measured patient trajectories treated with arc therapy (14). The resultant accuracy averaged over all patients using 5-Hz imaging was 0.6 mm. However, there are no validations of its accuracy in a clinical prostate cancer patient setting. In order to evaluate the clinical accuracy of the KIM method, we compared 3D positions determined by KIM to kV/MV triangulation measured during 7 fractions from 6 patients. kV/MV images were acquired for only a subset of fractions because the intratreatment MV image acquisition requires additional setup that adds time to the clinical workflow.
The MV imager was deployed to an SDD of 150 cm, and images were acquired at a rate of 7.5-8.0 Hz. Visual inspection was used to obtain the positions for markers that could be positively identified. Most of the images were not usable for triangulation. They either did not contain markers, as they were obscured by the dynamic multileaf collimator leaves, or the markers had poor contrast and did not allow for clear marker position determination. Combined with kV images acquired simultaneously, the kV/MV pairs allowed triangulation of the position of each marker. The vector difference between the kV/MV triangulation and the KIM trajectory was then computed.
Results
Prostate trajectory types
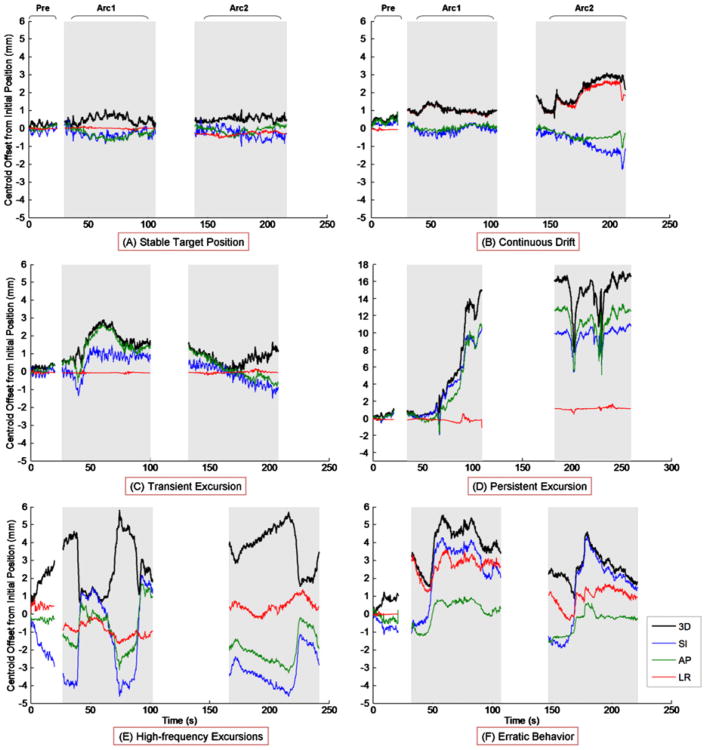
Examples of prostate trajectories observed using KIM are shown in Figure 2. These trajectory types are similar to those observed using the Calypso EM guidance modality for prostate tumor position localization (7). Of particular note is Figure 2D, representing persistent excursion, in which >12-mm displacement was observed for most of the treatment, indicating a large uncorrected geometric miss.
Fig. 2.
Trajectory types determined by KIM include (A) stable target position, (B) continuous drift, (C) transient excursion, (D) persistent excursion, (E) high-frequency excursions, and (F) erratic behavior. Each trajectory is divided into three sections, pretreatment and first and second arcs. The 3D (black), SI (blue), AP (green), and LR (red) trajectories are displayed. The first and second arcs are in gray to show that the MV treatment beam is switched on. Gaps between the arcs represent the time for setup between arcs.
Patient motion statistics
Table 2 lists the motion statistics for all patients. It is evident that LR motion is nearly negligible. Total 3D motion exceeds 3 mm only 4.7% of the time. However, in one instance, 3D displacements >15 mm were observed for patient 8 (Table 2). Cases like these would benefit from radiation beam gating or real-time tumor tracking, particularly for hypofractionated treatment regimes.
Table 2. Percentage of time that the prostate was displaced by magnitude and direction for all patients.
| % of displacement | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Motion direction | ≤1 mm | >1 mm | >3 mm | >5 mm | >7 mm | >10 mm |
| Left-right | 95.0 | 5.0 | 0.5 | 0.0 | 0.0 | 0.0 |
| Superior-inferior | 82.4 | 17.6 | 1.8 | 0.6 | 0.2 | 0.1 |
| Anterior-posterior | 79.8 | 20.2 | 1.4 | 0.6 | 0.3 | 0.2 |
| 3-dimensional | 62.4 | 37.6 | 4.7 | 1.7 | 0.4 | 0.2 |
For all patients, the LR, SI, AP, and 3D standard deviations of systematic error, Σ, were 0.23, 0.32, 0.26, and 0.36 mm, respectively. The LR, SI, AP, and 3D standard deviations of random error, σ, were 0.53, 0.98, 1.04, and 1.10 mm, respectively. The LR, SI, and AP PTV margins computed using the van Herk equation (13) assuming (unrealistically) no other sources of error were 0.94, 1.48, and 1.37 mm, respectively.
Verification of KIM clinical dynamic localization accuracy using kV/MV triangulation
Table 3 lists the difference in positions computed between kV/MV triangulation and the KIM method. Poulsen et al (15) reported a dynamic localization accuracy of 0.23 mm (at 1-Hz imaging) in a phantom simulation study assuming perfect marker segmentation. However, as shown in Table 3, the KIM dynamic localization accuracy (0.46 mm) was lower in a clinical setting, potentially due to increased uncertainty in the marker segmentation process in patients as well as imager sag.
Table 3. 3D position discrepancy between KIM and kV/MV triangulation.
| Patient | No. of fractions | No. of images acquired | No. of images used for triangulation | Accuracy ± SD (mm)* |
|---|---|---|---|---|
| 5 | 39 | 872 | 50 | 0.25 ± 0.25 |
| 6 | 40 | 543 | 40 | 0.34 ± 0.18 |
| 7 | 38 | 735 | 40 | 0.86 ± 0.48 |
| 8 | 37 | 332 | 34 | 0.67 ± 0.44 |
| 9 | 20 | 766 | 270 | 0.36 ± 0.20 |
| 9 | 36 | 561 | 90 | 0.32 ± 0.17 |
| 10 | 39 | 994 | 30 | 0.39 ± 0.58 |
| Total | 4803 | 728 | 0.46 ± 0.58 |
Standard deviation (SD) provides a measure of the variability of the accuracy.
Discussion
A new method of prostate intrafraction motion monitoring using kilovoltage imaging was successfully clinically implemented in a cohort of 10 patients undergoing conventionally fractionated IMAT. The measured motion information could be used with dose reconstruction tools to give an estimate of the dose delivered to the prostate. Real-time implementation of the KIM method (14) could be used with gating or tracking motion management. Although prostate cancer was the focus of the current study, the KIM method could be used for other cancer sites, where there are implanted markers or radioopaque internal surrogates such as the lung (16). Furthermore, although the current study used IMAT, the KIM method is applicable to conformal and IMRT treatments (17).
Table 4 compares characteristics of the KIM method with those of 2 clinically used methods, Calypso and Navotek. All 3 methods have submillimeter dynamic localization accuracy: KIM, Calypso, and Navotek have accuracies of 0.46 mm, (Table 3), 0.54 mm (18), and 0.89 mm (8), respectively. Using KIM, the additional imaging dose delivered for an entire conventionally fractionated (40 fractions) regimen was determined to be 61 mSv at 10 Hz. In comparison, Calypso EM guidance delivers no dose and the Navotek radioactive fiducial delivers a reported 64 mSv of lifetime dose (19). The use of gold fiducial markers (1-mm diameter × 3-mm length) required for KIM means there are no distortions in follow-up magnetic resonance imaging (MRI) scans. In comparison, Calypso EM guidance requires (1.85-mm diameter × 8-mm length) iron core transponders which produce artifacts in MRI scans (20). Navotek radioactive fiducials are the smallest and cause no distortions in MRI scans.
Table 4. Comparison of KIM with other real-time prostate tumor localization modalities.
| Localization method | Kilovoltage intrafraction monitoring | Calypso electromagnetic guidance | Navotek radioactive fiducial |
|---|---|---|---|
| Accuracy | 0.46 mm | 0.54 mm (18) | 0.89 mm (8) |
| Imaging dose | 61 mSv at 10 Hz | None | 64 mSv (19) |
| MRI distortion | No | Yes | No |
| Information type | Image plus 3 points | 3 points | 1 point |
| Marker size | 1-mm diameter × 3-mm length | 1.85-mm diameter × 8-mm length | 240-μm diameter × 2-cm length coil that crumples into a smaller size |
| Rotation information potential | Yes | Yes | No |
| Intratreatment validation potential | Yes (kV/MV triangulation) | No | No |
| Image reconstruction potential | Yes | No | No |
| Accessibility potential | Widespread | Limited | Limited |
KIM can potentially provide rotation information for the tumor because it has 3 markers. Calypso EM guidance can also provide rotation information, whereas Navotek radioactive fiducials cannot because they only have a single marker. KIM provides anatomical information because it is an image-based method. Calypso EM guidance and Navotek radioactive fiducials provide only the location information for the prostate. Hence, no associations with surrounding anatomical structures can be made. As a result of acquiring anatomical images during gantry rotation, KIM can potentially be used to reconstruct intrafraction CBCTs, although at reduced image quality due to MV scatter. KIM has the potential to be widely available as most LINACs have a gantry-mounted kV X-ray imager. In comparison, Calypso EM and Navotek systems require the purchase, storage, maintenance, commissioning, and ongoing quality assurance of a separate system.
Patient motion statistics
The motion observed in this study using KIM for conventionally fractionated IMAT was small. Prostate 3D displacements of >3 mm were observed 4.7% of the time. This compares well with the motion observed in the Langen study (21), in which the shorter treatment time of the cohort (2.5 min vs 10 min, respectively) was taken into account (cf. ref. [21], Fig. 4, 2-3 min). Langen et al (21) also found that for treatment times lasting as much as 12 minutes, the 3D displacement of the prostate tended to hover between 3 and 5 mm (22). While prostate motion was generally small for the current study of patients during conventionally fractionated treatment times, this may not be the case when treatment times are increased, such as with hypofractionated stereotactic body radiation therapy regimens. These regimens would lend themselves to real-time beam adaptation (ie, radiation beam gating or real-time tumor tracking.
Technical challenges and possible improvements
One of the challenges with KIM is that the MV beam contributes scatter to the kV images. To attempt to reduce the impact of the MV scatter on the kV imaging panel the kV detector SDD was increased to 180 cm. Nevertheless, MV scatter on the kV panel was still significant and prohibited lower frame rates (eg, 1 Hz), due to either detector saturation or increased noise, making marker segmentation unreliable. The patients in this study were imaged at 5 or 10 Hz. Even at the higher frame rate, the MV scatter and patient anatomy caused the automated marker segmentation to be challenging, typically in the lateral projections. Where necessary, frame averaging was used. Marker segmentation was successful in all fractions where kV images were acquired.
Even though submillimeter accuracy was observed in this study, an important point to note with the current KIM results is that the method was implemented within the framework and tools available with the clinical LINAC used (Clinac; Varian) and therefore represents lower performance and higher dose than would be achievable in a specifically developed KIM implementation. Performance and dose-limiting issues in the current study that could be improved include the following.
Improve software
In-house researcher-written codes were used for marker segmentation and 2D-to-3D trajectory reconstruction, which could be further developed and improved with larger datasets.
Reduce MV scatter and kV frame rate
The high frame rate used (typically 10 Hz) and associated imaging dose (61 mSv) were necessary because the MV scatter accumulates between image acquisitions. Reading out the imaging panel immediately prior to acquiring a kV image would reduce the MV scatter signal and the need to image at high frequencies. Also, if the MV scatter was reduced, the mAs for each image could also be reduced while still achieving robust marker segmentation. Alternatively, as proposed by Ling et al (23), the MV beam could be halted (or the dose rate reduced) during kV acquisition.
Use patient and gantry angle-specific kV field sizes
In the current study, a fixed 6 × 6 cm2 field size was used for KIM for all patients. However, Crocker et al (11), in a 22-patient study, found that by varying the field size for patients and imaging angles, the median field size would be 3.2 × 2.7 cm2, including a 15-mm margin, for prostate KIM IMAT. The field size reduction alone would drop the dose by a factor of 4.
Vary dose rate with gantry angle
Image acquisition settings are independent of the gantry angle, which means that a higher-than-necessary imaging dose is given for the AP imaging directions to ensure sufficient signal-to-noise ratio to detect the markers in the lateral projections. Using the CT analogy of automatic brightness control to vary the dose with angle would further reduce the imaging dose.
Conclusions
KIM for prostate IMAT was successfully implemented clinically for the first time, and its geometric accuracy was demonstrated. KIM is a clinically viable and objective method for monitoring prostate motion during treatment. Key advantages of this method are (1) submillimeter accuracy, (2) widespread applicability. and (3) a low barrier to clinical implementation. A disadvantage is that KIM delivers additional imaging dose to the patient. Several strategies to reduce the patient imaging dose are proposed.
Acknowledgments
Julie Baz, University of Sydney, improved the clarity and readability of the manuscript.
Funding support was received from the Australian National Health and Medical Research Council Australia Fellowship and US National Institutes of Health/National Cancer Institute grant CI R01CA93626. Drs Keall and Poulsen are inventors of the kilovoltage intrafraction motion monitoring method investigated clinically in this study. Stanford University has filed a US patent application (no. 20100172469) and has licensed the method to Varian Medical Systems. No commercial support was received for this study.
Footnotes
Conflict of interest: none.
References
- 1.de Crevoisier R, Tucker SL, Dong L, et al. Increased risk of biochemical and local failure in patients with distended rectum on the planning CT for prostate cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:965–973. doi: 10.1016/j.ijrobp.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 2.Kupelian PA, Willoughby TR, Reddy CA, et al. Impact of image guidance on outcomes after external beam radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:1146–1150. doi: 10.1016/j.ijrobp.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Sandler HM, Liu PY, Dunn RL, et al. Reduction in patient-reported acute morbidity in prostate cancer patients treated with 81-Gy Intensity-modulated radiotherapy using reduced planning target volume margins and electromagnetic tracking: assessing the impact of margin reduction study. Urology. 2010;75:1004–1008. doi: 10.1016/j.urology.2009.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlosser J, Salisbury K, Hristov D. Telerobotic system concept for real-time soft-tissue imaging during radiotherapy beam delivery. Med Phys. 2010;37:6357–6367. doi: 10.1118/1.3515457. [DOI] [PubMed] [Google Scholar]
- 5.Keall PJ, Todor AD, Vedam SS, et al. On the use of EPID-based implanted marker tracking for 4D radiotherapy. Med Phys. 2004;31:3492–3499. doi: 10.1118/1.1812608. [DOI] [PubMed] [Google Scholar]
- 6.Cho B, Poulsen PR, Sloutsky A, et al. First demonstration of combined kV/MV image-guided real-time dynamic multileaf-collimator target tracking. Int J Radiat Oncol Biol Phys. 2009;74:859–867. doi: 10.1016/j.ijrobp.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kupelian P, Willoughby T, Mahadevan A, et al. Multi-institutional clinical experience with the Calypso System in localization and continuous, real-time monitoring of the prostate gland during external radiotherapy. Int J Radiat Oncol Biol Phys. 2007;67:1088–1098. doi: 10.1016/j.ijrobp.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 8.Shchory T, Schifter D, Lichtman R, et al. Tracking accuracy of a real-time fiducial tracking system for patient positioning and monitoring in radiation therapy. Int J Radiat Oncol Biol Phys. 2010;78:1227–1234. doi: 10.1016/j.ijrobp.2010.01.067. [DOI] [PubMed] [Google Scholar]
- 9.Poulsen PR, Cho B, Langen K, et al. Three-dimensional prostate position estimation with a single x-ray imager utilizing the spatial probability density. Phys Med Biol. 2008;53:4331–4353. doi: 10.1088/0031-9155/53/16/008. [DOI] [PubMed] [Google Scholar]
- 10.Eade TN, Guo L, Forde E, et al. Image-guided dose-escalated intensity-modulated radiation therapy for prostate cancer: treating to doses beyond 78 Gy. BJU Int. 2011:1–6. doi: 10.1111/j.1464-410X.2011.10668.x. [DOI] [PubMed] [Google Scholar]
- 11.Crocker JK, Ng JA, Keall PJ, et al. Measurement of patient imaging dose for real-time kilovoltage x-ray intrafraction tumour position monitoring in prostate patients. Phys Med Biol. 2012;57:2969. doi: 10.1088/0031-9155/57/10/2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fledelius W, Worm E, Elstrom UV, et al. Robust automatic segmentation of multiple implanted cylindrical gold fiducial markers in cone-beam CT projections. Med Phys. 2011;38:6351–6361. doi: 10.1118/1.3658566. [DOI] [PubMed] [Google Scholar]
- 13.van Herk M. Errors and margins in radiotherapy. Semin Radiat Oncol. 2004;14:52–64. doi: 10.1053/j.semradonc.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Poulsen PR, Cho B, Sawant A, et al. Implementation of a new method for dynamic multileaf collimator tracking of prostate motion in arc radiotherapy using a single kV imager. Int J Radiat Oncol Biol Phys. 2010;76:914–923. doi: 10.1016/j.ijrobp.2009.06.073. [DOI] [PubMed] [Google Scholar]
- 15.Poulsen PR, Cho B, Keall PJ. Real-time prostate trajectory estimation with a single imager in arc radiotherapy: a simulation study. Phys Med Biol. 2009;54:4019. doi: 10.1088/0031-9155/54/13/005. [DOI] [PubMed] [Google Scholar]
- 16.Poulsen PR, Cho B, Ruan D, et al. Dynamic multileaf collimator tracking of respiratory target motion based on a single kilovoltage imager during arc radiotherapy. Int J Radiat Oncol Biol Phys. 2010;77:600–607. doi: 10.1016/j.ijrobp.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 17.Poulsen PR, Cho B, Sawant A, et al. Dynamic MLC tracking of moving targets with a single kV imager for 3D conformal and IMRT treatments. Acta Oncol. 2010;49:1092–1100. doi: 10.3109/0284186X.2010.498438. [DOI] [PubMed] [Google Scholar]
- 18.Balter JM, Wright JN, Newell LJ, et al. Accuracy of a wireless localization system for radiotherapy. Int J Radiat Oncol Biol Phys. 2005;61:933–937. doi: 10.1016/j.ijrobp.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Neustadter D, Barnea G, Stokar S, et al. Analysis of dose to patient, spouse/caretaker, and staff, from an implanted trackable radioactive fiducial for use in the radiation treatment of prostate cancer. Med Phys. 2010;37:1220–1224. doi: 10.1118/1.3317436. [DOI] [PubMed] [Google Scholar]
- 20.Willoughby TR, Kupelian PA, Pouliot J, et al. Target localization and real-time tracking using the Calypso 4D localization system in patients with localized prostate cancer. Int J Radiat Oncol Biol Phys. 2006;65:528–534. doi: 10.1016/j.ijrobp.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 21.Langen KM, Willoughby TR, Meeks SL, et al. Observations on realtime prostate gland motion using electromagnetic tracking. Int J Radiat Oncol Biol Phys. 2008;71:1084–1090. doi: 10.1016/j.ijrobp.2007.11.054. [DOI] [PubMed] [Google Scholar]
- 22.Su Z, Zhang L, Murphy M, et al. Analysis of prostate patient setup and tracking data: potential intervention strategies. Int J Radiat Oncol Biol Phys. 2011;81:880–887. doi: 10.1016/j.ijrobp.2010.07.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ling C, Zhang P, Etmektzoglou T, et al. Acquisition of MV-scatterfree kilovoltage CBCT images during RapidArc or VMAT. Radiother Oncol. 2011;100:145–149. doi: 10.1016/j.radonc.2011.07.010. [DOI] [PubMed] [Google Scholar]