Abstract
Acriflavine, a fluorescent drug previously used for bacterial and trypanosomal infections, reduces hypoxia-inducible factor-1 (HIF-1) and HIF-2 transcriptional activity. In mice with oxygen-induced ischemic retinopathy, intraocular or intraperitoneal injections of acriflavine caused dose-dependent suppression of retinal neovascularization (NV) and significantly reduced expression of HIF-1-responsive genes. Intraocular injection of 100 ng caused inner retina fluorescence within 1 hour that was seen throughout the entire retina between 1 and 5 days, and at 7 days after injection, strongly suppressed choroidal NV at Bruch’s membrane rupture sites. After suprachoroidal injection of 300 ng in rats, there was retinal fluorescence in the quadrant of the injection at 1 hour that spread throughout the entire retina and choroid by 1 day, was detectable for 5 days, and dramatically reduced choroidal NV 14 days after rupture of Bruch’s membrane. After topical administration of acriflavine in mice, fluorescence was seen in the retina and retinal pigmented epithelium within 5 minutes and was detectable for 6–12 hours. Administration of 0.5% drops to the cornea twice a day, significantly reduced choroidal NV in mice. Electroretinographic b-wave amplitudes were normal 7 days after intravitreous injection of 100 ng of acriflavine in mice, showed mild threshold reductions at highest stimulus intensities after injection of 250 ng, and more extensive changes after injection of 500 ng. These data provide additional evidence for an important role for HIF-1 in retinal and choroidal NV and suggest that acriflavine can target HIF-1 through a variety of modes of administration and has good potential to provide a novel therapy for retinal and choroidal vascular diseases.
Keywords: age-related macular degeneration, diabetic retinopathy, ischemia, suprachoroidal injection, vascular endothelial growth factor
Introduction
Neovascularization (NV) and/or excessive vascular leakage occur in several retinal/choroidal vascular diseases. Choroidal NV occurs in age-related macular degeneration (AMD), the most common cause of moderate and severe vision loss in patients over the age of 60 [1]. Diabetic retinopathy is a common cause of vision loss in working age patients; vision loss can occur from retinal NV leading to vitreous hemorrhage and retinal detachment, or from excess vascular leakage resulting in macular edema [2]. Retinal NV and macular edema also cause vision loss in patients with retinal vein occlusion. This group of highly prevalent diseases share molecular mechanisms because in each, stabilization of HIF-1α plays an important role [3–7]. Stabilization of HIF-1α causes increased production of several hypoxia-regulated vasoactive proteins that contribute to NV and vascular leakage [8]. VEGF plays a particularly important role and intraocular injections of VEGF-neutralizing proteins provide substantial benefits in neovascular AMD [9, 10], diabetic macular edema [11, 12], macular edema due to retinal vein occlusions [13–15], and background diabetic retinopathy [16].
While most patients with these retinal/choroidal vascular diseases are benefited by intraocular injections of anti-VEGF injections, some have a suboptimal response, because HIF-1 increases levels of other vasoactive proteins in addition to VEGF that may contribute including platelet-derived growth factor-B (PDGF-B) [17, 18], angiopoietin-2 (Angpt2) [19–22], and stromal-derived factor-1 (SDF-1) [23]. Combining anti-VEGF agents with new agents targeting some of these other factors or their receptors is an ongoing strategy to improve outcomes in retinal/choroidal vascular diseases [24–26]. An alternative strategy is to target HIF-1. To achieve this goal, a cell-based reporter assay was developed to screen for drugs that inhibit HIF-1 transcriptional activity. Screening of a library of approved drugs identified digoxin and other cardiac glycosides and the anthracycline chemotherapeutic agents doxorubicin (DXR) and daunorubicin (DNR) as potent inhibitors of HIF-1-mediated gene transcription [27, 28]. Digoxin acts by reducing HIF-1 levels, while DXR and DNR have no effect on levels and exert their effect by blocking the binding of HIF-1 to DNA. Digoxin, DXR, and DNR strongly suppress ocular NV [6, 7].
Lee et al. [29] used an assay in which dimerization of HIF-1α and HIF-1β provided complementation of split Renilla luciferase thereby providing a tool to screen for drugs that block formation of HIF-1 from its subunits. Using this tool, acriflavine, a mixture of trypaflavin (3,6-diamino-10-methylacridinium chloride) and proflavine (3,6-diaminoacridine), was found to bind to HIF-1α and HIF-2α and prevent their dimerization with HIF-1β to form HIF-1 and HIF-2. In a xenograft tumor model, acriflavine reduced expression of angiogenic cytokines and decreased tumor vascularization and growth. In this study, we investigated the effects of acriflavine in models of ocular neovascularization (NV).
Methods
Mice
Pathogen-free C57BL/6 mice (Charles River, Wilmington, MA, USA) were treated in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals and the guidelines of the Johns Hopkins University Animal Care and Use Committee.
Mouse model of oxygen-induced ischemic retinopathy (OIR)
At postnatal day (P) 7, litters of C57BL/6 mice were placed in an incubator containing 75%±3% oxygen continuously monitored with a Pro:Ox model 110 oxygen sensor (Reming Bioinstruments Co., Redfield, NY). At P12, the mice were returned to room air and dosed with acriflavine by the following routes: (1) an intravitreous (intraocular) injection of 1 μl of PBS containing 50 or 100 ng of acriflavine in one eye and 1 μl of PBS in other eye, (2) daily intraperitoneal injections of 0.1, 1, or 5 mg/kg of acriflavine, or (3) topical eye drops containing 0.1% or 0.5% acriflavine three times a day. At various time points after initiation of dosing, mice were euthanized and retinas were dissected and used for quantitative real-time RT-PCR, ELISA, or measurement of area of retinal NV.
Quantitative real-time RT-PCR (qrtPCR)
At P17, mice with OIR or control mice were euthanized and retinas were dissected. Retinal RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and treated with DNase (Ambion, Austin, TX, USA) according to the manufacturer’s instructions. Oligonucleotide primers (Table 1) were designed usingPrimer3 input (version 0.4.0), and cDNA was prepared using Superscript III First Strand Synthesis kit (Invitrogen, USA). Quantitative RT-PCR was done to measure target gene expression using Lightcycler real-time PCR detection system (Roche) using Cyclophilin-A for normalization as previously described [30].
Table 1.
Primers for qrtPCR
| Name | Forward | Reverse |
|---|---|---|
| Erythropoietin | CGAGTTCTGGAGAGGTACATC | TGACTTTGGTATCTGGGACTG |
| VEGF A | CTTGCCTTGCTGCTCTACC | CACACAGGATGGCTTGAAG |
| PDGF B | CGGAGTCGGCATGAATCG | AAGGAGCGGATGGAGTGG |
| Angiopoietin 2 | CTGTGCGGAAATCTTCAATGC | TGCCATCTTTCGGTGTTG |
| Placental growth factor | GGATGTGTGCTCTGAATGC | CCTCTGCGTGGCTGGTTAC |
Measurement of VEGF levels by ELISA
At P12, mice with OIR had injection of 100 ng of acriflavine in one eye and PBS in the other eye. At P15, mice were euthanized and retinas were dissected from each eye, frozen on dry ice, and stored at −80°C. Specimens were extracted using 0.1% Triton X-100 in PBS with protease inhibitors (Roche, Mannheim, Germany) for 2 hours at 4°C, and microfuged. Total protein was measured in supernatants using the Bio-Rad Protein Assay (Bio-Rad, Hercules, CA), and VEGF was measured using a single antibody sandwich ELISA in 96-well immunoplates (R&D Systems, Inc., Minneapolis, MN) according to the manufacturer’s instructions.
Measurement of the area of retinal NV in OIR mice
Mice were euthanized at P17 and eyes were fixed in 10% formalin for 2 hours at room temperature. Retinas were dissected, washed, and incubated for 1 hour in 1:500 Alexa-594 labeled Griffonia Simplicifolia Lectin I (GSA, Invitrogen, USA) and flat mounted. With this short incubation time, there is selective staining of retinal NV and hyaloid vessels but no staining of pre-existent retinal vessels. Retina images were obtained using an Axioskop fluorescent microscope (Zeiss, Thornwood, NY) and video camera and frame grabber (IKTU40A, Toshiba, Tokyo, Japan). The area of retinal NV per retina was measured by image analysis using Image-Pro Plus software (Media Cybernetics, Silver Spring, MD) with the observer masked with regard to treatment group.
Mouse model of laser-induced choroidal NV
Choroidal NV was generated as previously described [31]. Briefly, 6 week old C57BL/6 mice had rupture of Bruch’s membrane in 3 locations in each eye by laser photocoagulation and were treated with acriflavine or vehicle by various modes of administration: intraperitoneal injections, intravitreous injections, or topical administration. Seven days after rupture of Bruch’s membrane, mice were euthanized, eyecups were stained with FITC-labeled GSA (Vector Laboratories, Burlingame, CA), and flat mounted. The area of choroidal NV at each Bruch’s membrane rupture site was measured by image analysis by an observer masked with respect to treatment group. The area of choroidal NV at the 3 rupture sites in one eye were averaged to give one experimental value.
Rat model of laser-induced choroidal NV
Male Brown Norway rats at 7–8 weeks of age had laser-induced rupture of Bruch’s membrane in 5 locations four disc areas from the optic nerve in each eye using a 532-nm diode laser photocoagulation (100 μm spot size, 100 ms duration, 150 mW power). Immediately after the laser, suprachoroidal injections of 30 ng or 300 ng of acriflavine were done in one eye and PBS in the fellow eye. The sclera was partially penetrated 2mm from the limbus with a 27-gauge needle and then a 33-gauge Hamilton syringe with a blunt end was inserted and using blunt dissection, the tip was advanced into the suprachoroidal space and 3 μl of acriflavine was injected. Fourteen days after injection, rats were euthanized, eyes were removed, retinas were removed, and eyecups were stained with 1:500 Alexa-594 labeled GSA. The area of choroidal NV at Bruch’s membrane rupture sites were measured by image analysis as described above. The mean of the 5 values in each eye was used as a single experimental value.
Visualization of acriflavine in the retina
At several time points after intraocular injection or topical administration of acriflavine in mice or suprachoroidal injection of acriflavine in rats, animals were euthanized, eyes were removed and frozen in OCT and 10 μm frozen ocular sections were cut. The frozen sections were post-fixed in 4% paraformaldehyde for 15 minutes, washed with PBS, mounted, and examined by fluorescence microscopy. Images were photographed with the same exposure time for each section.
Measurement of acriflavine in retina and eyecup after topical administration
Mice were given 5 μl of 1% acriflavine in one eye taking care to blot any excess acriflavine that came onto the lids to avoid cross-contamination. The other eye was left untreated. Another group of mice did not receive any acriflavine. After two hours, the mice were euthanized, eyes removed, and retinas and eyecups were dissected and homogenized in 300 μl of PBS. The homogenates were centrifuged at 18,400 × g for 5 minutes. The spectral properties of acriflavine vary depending upon the proportion of trypaflavin and proflavine, and whether it is in solution or bound to macromolecules in solution or tissue [32, 33]. The acriflavine used in our study in PBS had peak excitation and emission wave lengths of 416 nm and 514 nm. The protein concentration of supernatants was measured, each sample was diluted to 1μg/μl, and a plate reader was used to measure fluorescence at 514 nm after stimulation at 416 nm. Supernatants from retinal and eyecup homogenates from eyes of mice that were not treated with acriflavine were used to measure fluorescence from endogenous fluorophores and this was subtracted from the values obtained from supernatants of acriflavine-treated and fellow eyes. We made several concentrations of acriflavine in PBS, stimulated with 416 nm light and recorded fluorescence at 514 nm to generate a standard curve The standard curve was used to convert these values to acriflavine levels.
Electroretinography (ERG)
Mice had intravitreous injection of 100 ng, 250 ng, or 500 ng of acriflavine in one eye and PBS in the fellow eye. Seven days after injection, ERGs were recorded with an Espion ERG Diagnosys machine (Diagnosys, Littleton, MA) as previously described.[34] Mice were dark-adapted, anesthetized, placed on a pad heated to 39°C, pupils were dilated, and platinum loop electrodes were placed on each cornea after application of Gonioscopic prism solution (Alcon Labs, Fort Worth, TX). A reference electrode was placed subcutaneous in the anterior scalp between the eyes, and a ground electrode was inserted into the tail. The head of the mouse was held in a standardized position in a ganzfeld bowl illuminator that ensured equal illumination to each eye. Recordings for both eyes were made simultaneously with electrical impedance balanced. Sixty scotopic measurements were taken and the average value was recorded. Mice were adapted for 12h to a background of white light of 30 cd/m2, and photopic ERGs were performed with a background intensity of 10 cd/m2.
Statistical analyses
Shapiro-Wilk and Shapiro-Francia tests for normality were used for examining the normality of each variable. Paired t-tests or ANOVA with Dunnett’s correction for multiple comparisons were used for normally distributed variables and Wilcoxon matched-pairs signed-ranks tests were used for variables with skewed distributions.
Results
Intravitreous or intraperitoneal injections of acriflavine suppress ischemia-induced retinal NV and choroidal NV
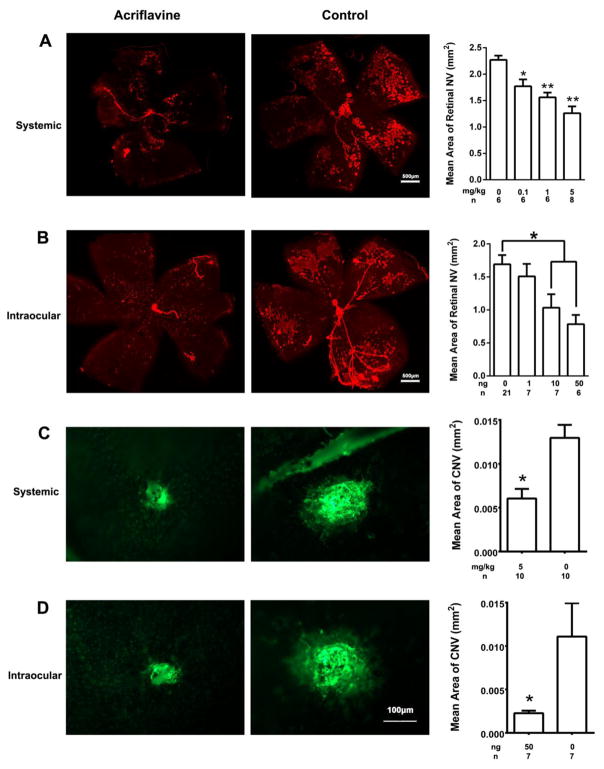
Retinal ischemia plays a critical role in the development of retinal NV in proliferative diabetic retinopathy, retinopathy of prematurity, and other ischemic retinopathies. Oxygen-induced ischemic retinopathy (OIR) is a model that is relevant to these diseases and provides predictive information for therapeutic interventions [35, 36]. In this model, neonatal mice are placed in 70% oxygen at postnatal day (P) 7 which causes down-regulation of VEGF resulting in regression of newly developed retinal vessels [37]. The mice are returned to room air at P12 and HIF-1 and VEGF rapidly increase in portions of retina lacking blood vessels [3]. Therefore, the effect of acriflavine on retinal NV was tested in mice with OIR. At P12, mice with OIR were given daily intraperitoneal (systemic) injections of vehicle or 0.1, 1, or 5 mg/kg of acriflavine and at P17 mice were euthanized, retinas were dissected and stained with GSA lectin using an incubation time that selectively stains retinal NV and hyaloid vessels but not pre-existent retinal vessels. A representive retinal flat mount from a mouse treated with 5 mg/kg of acriflavine shows markedly less retinal NV than that seen in a control retina (Figure 1A). Compared with control retinas, the mean area of NV per retina was significantly less in mice injected with 0.1, 1, or 5 mg/kg of acriflavine. Another group of mice with ischemic retinopathy had an intravitreous injection of 0, 1, 10, or 50 ng of acriflavine at P12 and measurement of retinal NV at P17. A representative retinal flat mount from an eye injected with 50 ng of acriflavine shows less retinal NV than that in a control eye (Figure 1B). Compared with controls, the mean area of retinal NV per retina was significantly less in eyes injected with 10 or 50 ng, but not 1ng of acriflavine.
Fig 1. Acriflavine suppresses retinal and choroidal NV.
At P12, mice with oxygen-induced ischemic retinopathy began receiving daily intraperitoneal (systemic) injections of 0, 0.1, 1, or 5 mg/kg of acriflavine or they received a single intravitreous (intraocular) injection of 0, 1, 10, or 50 ng of acriflavine. At P17, mice were euthanized, retinas were dissected and stained with FITC-labeled Griffonia Simplicifolia lectin, and flat mounted. With our staining protocol, there is selective staining of retinal NV and hyaloid vessels but no staining of pre-existent retinal vessels. (A) Systemically administered acriflavine suppressed retinal NV: the retina from a mouse treated with 5mg/kg/day of acriflavine (left) shows little retinal NV compared with a retina from a mouse treated with PBS injections (middle) and compared with controls, the mean (±SEM) area of NV per retina was significantly less than control in retinas from mice treated with 0.1, 1 or 5 mg/kg (*p<0.05 for comparison with control, **p<0.01 for comparison with control). (B) Intraocular injection of acriflavine suppressed retinal NV: the retina from an eye injected with 50ng of acriflavine (left) shows little NV compared with the retina from an eye injected with PBS (middle) and the mean (±SEM) area of NV per retina was significantly less than that in control retinas for eyes injected with 10 or 50 ng, but not 1 ng (right, *p<0.01 by ANOVA with Dunnett’s correction). (C) Systemically administered acriflavine suppressed choroidal NV: the mean (±SEM) area of choroidal NV was significantly less (right, p=0.018 by Wilcoxon matched-pairs signed-ranks test) in eyes from mice treated with 5 mg/kg acriflavine (left) compared with eyes from control PBS-injected mice (middle). (D) Intraocular injection of acriflavine suppressed choroidal NV: the mean (±SEM) area of choroidal NV was significantly less (right, p=0.01 for difference from control by unpaired t-test) in eyes injected with 50 ng of acriflavine (left) compared with those injected with PBS (middle).
Choroidal NV occurs in diseases of Bruch’s membrane and the retinal pigmented epithelium (RPE), the most prevalent of which is AMD. Mice with laser-induced rupture of Bruch’s membrane [31] provide a model of choroidal NV that is predictive of therapeutic effects in patients with neovascular AMD [10, 38, 39]. After laser-induced rupture of Bruch’s membrane, daily intraperitoneal injections of 5 mg/kg of acriflavine (Figure 1C) or intravitreous injection of 50 ng of acriflavine (Figure 1D) significantly reduced the mean area of choroidal NV compared with vehicle controls. Thus, acriflavine suppresses retinal and choroidal NV.
Intraocular injection of acriflavine reduces expression of HIF-1-responsive genes in ischemic retina
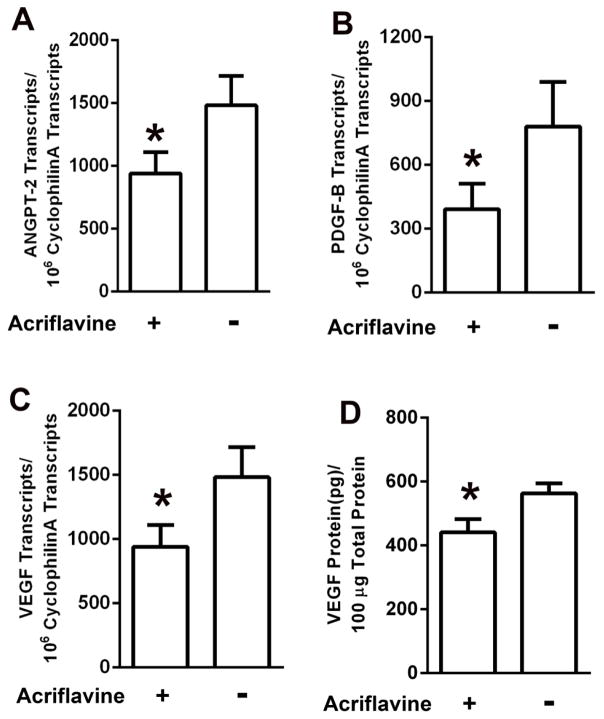
Mice with ischemic retinopathy had intraocular injection of 100 ng acriflavine in one eye and vehicle in the fellow eye at the onset of retinal ischemia at P12. At P15, compared to ischemic retinas in fellow eye controls, those from eyes injected with acriflavine showed significant reduction in mRNA for Angpt-2, PDGF-B, and VEGF-A, (Figure 2A–C). Acriflavine also caused a significant reduction in VEGF protein in ischemic retina (Figure 2D).
Fig 2. Acriflavine suppresses expression of hypoxia-regulated genes in ischemic retina.
At P12, mice with ischemic retinopathy were given an intraocular injection of 100ng of acriflavine in one eye and PBS in the other eye. Mice were euthanized at P15, eyes (n=5 for each panel) were removed, and total retinal RNA was isolated or the retina was homogenized in ELISA buffer. The mean (±SEM) number of transcripts per 106 cyclophilin A transcripts measured by qRT-PCR for Angiopoietin 2 (Angpt-2, A), platelet-derived growth factor-B (PDGF-B, B), and vascular endothelial growth factor-A (VEGF, C) was significantly less in ischemic retinas from acriflavine-injected eyes compared with PBS-injected fellow eyes (*p<0.05 by unpaired t-test). The mean (±SEM) level of VEGF protein measured by ELISA was significantly less in ischemic retinas from acriflavine-injected eyes compared with PBS-injected eyes (*p<0.05 by unpaired t-test).
Time course of acriflavine visualization in retina after intravitreous injection
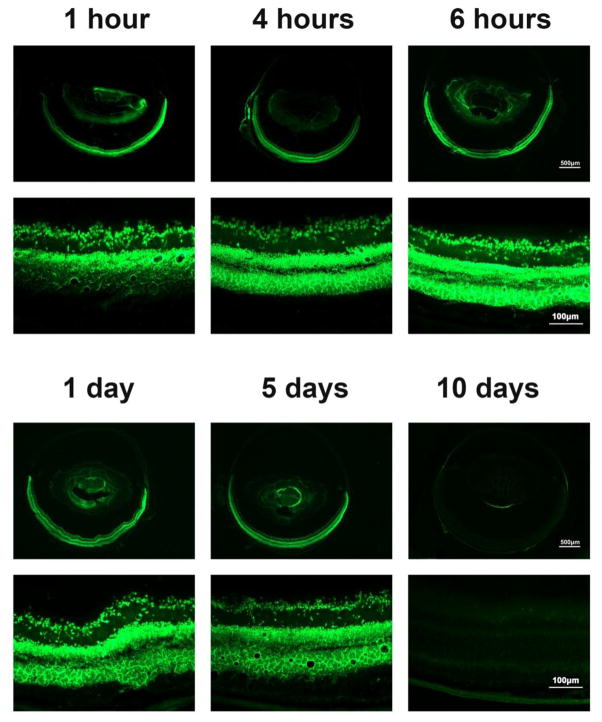
Acriflavine is fluorescent with excitation and emission wavelenghs similar to fluorescein allowing its visualization with the same filters used for FITC-labeled molecules. This allowed us to visualize acriflavine in ocular sections at various time points after intravitreous injection of acriflavine in normal adult mice. One hour after intravitreous injection of 100 ng of acriflavine, bright fluorecence was seen throughout the ganglion cell and inner nuclear layers (Figure 3, upper left). By 4 hours after injection, there was strong fluorescence throughout the entire retina (upper middle) that appeared quite similar at 6 hours (upper right), 1 day (lower left), and 5 days (lower middle) after injection. By 10 days after injection, there was little remaining fluorescence (Figure 3, lower right).
Fig 3. Visualization of acriflavine in the retina after intraocular injection.
Adult C57BL/6 mice were given an intraocular injection of 100ng of acriflavine and euthanized at 1 hour, 4 hours, 6 hours, 1 day, 5 days, or 10 days. Frozen ocular sections were examined by fluorescence microscopy. Acriflavine was visualized in the inner retina at 1 hour after injection and throughout the entire retina through 5 days. It became undetectable in the retina between 5 and 10 days.
Suprachoroidal injection of acriflavine strongly suppresses rat choroidal NV
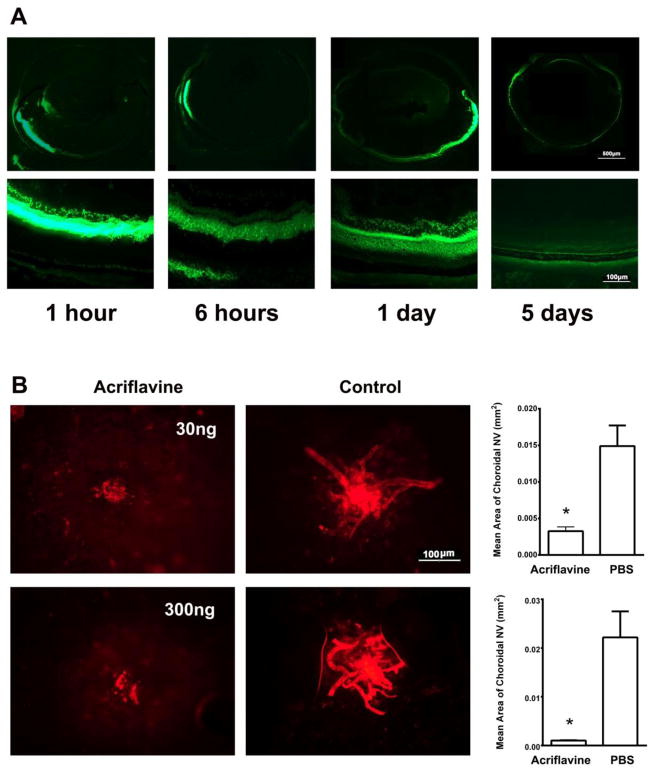
Suprachoroidal injection is a relatively new route of delivery that may have some advantages over intravitreous injection because drug is confined in a space posterior to the retina and does not spread as much throughout the entire eye. This type of injection is not feasible in mice, but is feasible in rats. Therefore, suprachoroidal injections of acriflavine were tested in Brown Norway rats. One hour after suprachoroidal injection of 300 ng of acriflavine in rats, there was fluorescence in the retina primarily in the quandrant of the eye that the injection had been given (Figure 4A, column 1, top). High magnification showed intense fluorescence in the outer nuclear layer of the retina, some light staining in the sclera with an overlying hypofluorescent space probably due to fluid in the suprachoroidal space (column 1, bottom). The pattern of fluorescence was very similar 6 hours after injection (Figure 4A, column 2), but by one day the fluorescent staining extended throughout the entire retina and even anterior to the retina 180° from the site of injection (Figure 4A, column 3, top). High magnification showed faint staining in the sclera with reduction in the thickness of the overlying hypofluorescent band suggesting resorption of much of the fluid in the suprachoroidal space (Figure 4A, column 3, bottom). Five days after injection there was faint staining in the sclera, choroid, RPE, and outer retina around the circumference of the globe (Figure 4A, column 4). A cohort of rats had laser-induced rupture of Bruch’s membrane followed by suprachoroidal injection of 30 or 300 ng of acriflavine or PBS and after 14 days, eyes injected with either dose of acriflavine showed marked reduction in the mean area of choroidal NV compared with vehicle controls (Figure 4B).
Fig 4. Acriflavine injected into the suprachoroidal is visualized throughout the entire retina and suppresses choroidal neovascularization (NV) in rats.
(A) Pigmented rats were given a suprachoroidal injection of 300 ng of acriflavine and euthanized 1 hour, 6 hours, 1 day, or 5 days after injection. Frozen ocular sections were examined by fluorescence microscopy and showed acriflavine in the anterior part of the choroid and retina on the side of the injection at 1 and 6 hours after injection. At 1 day after injection acriflavine fluorescence was greatest in the retina on the side of the injection, but could be seen throughout the posterior retina and in the retina on the side opposite the injection. At 5 days after injection, there was only faint fluorescence remaining.
(B) Pigmented rats had laser-induced rupture of Bruch’s membrane in 5 locations in each eye followed by a suprachoroidal injection of 30 or 300 ng of acriflavine in one eye and PBS in the fellow eye (n=6 for each dose). After 14 days, the rats were euthanized and choroidal flat mounts were stained with FITC-labeled Griffonia Simplicifolia lectin. A representative choroidal flat mount from an eye injected with acriflavine shows a small area of choroidal NV (left panel) compared to that from the fellow eye injected with PBS (middle panel). The mean area of choroidal NV was significantly less in eyes injected with 30 or 300 ng acriflavine compared to corresponding controls.
*p=0.0014 (30 ng); p=0.0012 (300 ng) for difference between acriflavine and corresponding PBS controls by unpaired t-test.
Acriflavine is visualized in the retina for several hours after administration of a single eye drop in mice
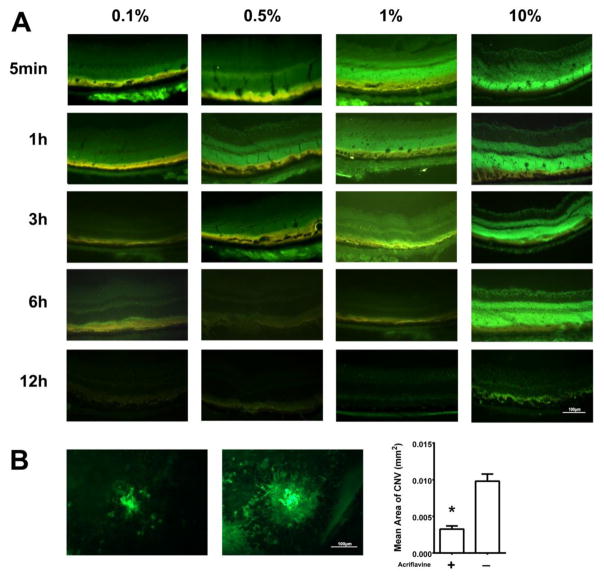
Next we sought to visualize and track the entry of acriflavine into the eye after its topical application to the ocular surface. Five minutes after topical administration of 5 μl of a 0.1% solution of acriflavine (5 μg), there was fluorescence in the RPE and outer retina (Figure 5A, column 1, top row). By 1 hour after administration, there was faint fluorescence throughout the entire retina that faded over the next 5 hours and was gone by 12 hours after administration (Figure 5A, column 1). After administration of 5 μl of a 0.5% solution of acriflavine (25 μg), the pattern and time course of fluorescence were similar to that seen after a 0.1% drop, but the intensity was greater (Figure 5A, column 2). There was intense fluorescence throughout the entire retina for at least 3 hours after administration of 5 μl of 1% (50 μg) acriflavine (Figure 5A, column 3) and for at least 6 hours after administration of 10% (500 μg) acriflavine (Figure 5A, column 4).
Fig 5. Topically administered acriflavine is visualized in the retina and suppresses choroidal neovascularization (NV).
(A) C57BL/6 mice had 5 μl of 0.1% (5 μg), 0.5% (25 μg) 1.0% (50 μg) or 10.0% (500 μg) acriflavine placed on the cornea of one eye of mice and 5 μl of PBS was placed in the other eye. Mice were euthanized at various times after administration and ocular frozen sections were examined by fluorescence microscopy. Fluorescence was visible in the retinal pigmented epithelium (RPE) and outer retina 5 minutes after administration of 0.1% acriflavine and was seen throughout the retina 5 minutes after administration of 0.5%, 1%, or 10%. The fluorescence became more intense and then faded with residence time in the retina greater for higher doses.
(B) Six mice had laser induced rupture of Bruch’s membrane in three locations in each eye and then had topical administration three times a day of 5 μl of 0.5% acriflavine in one eye and PBS in the other eye. After 7 days, choroidal flat mounts were stained with FITC-labelled GSA-Lectin. The mean area of choroidal NV was significantly smaller in acriflavine-treated eyes compared with PBS-treated eyes (*p=0.028 by Wilcoxon matched-pairs signed-ranks test.
Topical administration of acriflavine suppresses choroidal NV
After observing that topically applied acriflavine penetratedi into the eye and was detectable in the retina and choroid, we sought to determine the effect of topically applied acriflavine could suppress choroidal NV. Bruch’s membrane was ruptured in both eyes of mice and then one eye was treated by topical administration of 5 μl of 0.5% acriflavine (25μg) three times and the fellow eye was treated with 5 μl of PBS three time a day. Representative choroidal flat mounts stained with FITC-GSA showed small areas of choroidal NV in eyes treated with acriflavine (Figure 5B, left panel) compared to the corresponding control eye (middle panel). The mean area of choroidal NV was significantly less in acriflavine-treated eyes compared with fellow eye controls (Figure 5B, right panel).
Acriflavine levels are greater in RPE/choroid than in retina 2 hours after topical delivery
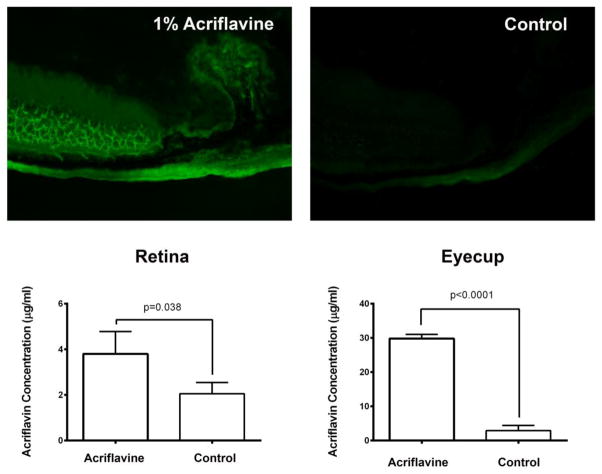
Based upon the change in fluorescence over time in ocular sections after topical administration of acriflavine, it appeared that acriflavine penetrated through sclera to the choroid and then the retina. To quantitively test this, we sought to measure levels of acriflavine in RPE/choroid and retina at an early time point. Two hours after administration of 5 μl of 1% acriflavine in one eye there was intense fluorescence in the choroid, outer retina, and ciliary body of the treated eye and faint fluorescence in the fellow eye, which primarily represents autofluorescence, because it appears the same as that seen in a negative control eye of a mouse never exposed to acriflavine (Figure 6, top row). Using a standard curve, the amount of acriflavine in supernatants of retinal and eyecup (RPE, choroid, and sclera) homogenates was about 3 and 28 μg/mg protein and significantly greater than measurements in the control fellow eye (Figure 6, bottom row). These data suggest that after a drop of acriflavine is placed on the eye, it passes through the conjunctiva and sclera to enter the choroid and then the retina.
Figure 6. Two hours after topical application of acriflavine, levels are higher in RPE/choroid than in retina and far higher in dosed eye than in fellow eye.
Mice (n=5) were given a single 5 μl drop of 0.5% acriflavine in one eye and after 2 hours fluorescence was seen in the sclera, choroid, RPE, and outer retina of treated eyes (top row, left), but fellow eyes showed only mild fluorescence (top row, middle) that is likely predominantly autofluorescence because it appears identical to that seen on ocular sections from eyes of negative control mice that never received acriflavine (top row, right). Fluorescence was extracted from retinal and eyecup (RPE/choroid/sclera) homogenates, normalized based upon protein concentration, and measured on a plate reader (excitation 416 nm, emission 514 nm) along with several concentrations of acriflavine to generate a standard curve. Mean autofluorescence obtained from negative control eyes (n=5) was subtracted from each sample reading and the standard curve was used to calculate the concentration of acriflavine in each sample. The mean level of acriflavine was significantly higher in the retinas and eyecups of treated eyes compared to fellow eyes and substantially higher in eyecups than retinas (bottom row).
Effect of intraocular injection of acriflavine on retinal function
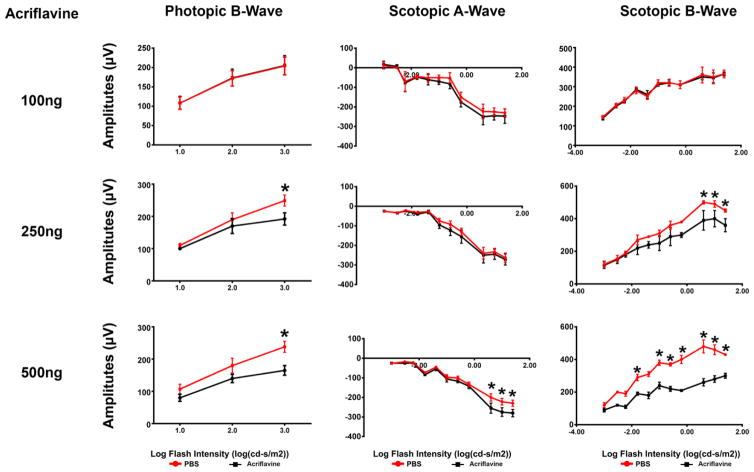
The electrical activity of the retina is measured by ERGs. Scotopic ERGs are done with dim background illumination after dark adaptation and test rod photoreceptor function; scotopic a-wave amplitudes provide a direct measure of rod function, while scotopic b-wave amplitudes are generated by the inner retina and provide an assessment of the function of rods and second order neurons. Photopic ERGs are done after light adaptation with bright background illumination and photopic ERG b-waves provide an assessment of cone photoreceptor function. Mice were given an intravitreous injection of 100, 250, or 500 ng of acriflavine in one eye and PBS in the fellow eye. After 7 days, there was no difference in scotopic a- or b-wave amplitudes or photopic b-wave amplitudes in eyes injected with 100 ng acriflavine compared to fellow eye controls (Figure 7, left column). Seven days after injection of 250 ng of acriflavine, there was a small, but significant reduction in photopic and scotopic b-wave amplitudes for the highest stimulus intensities. (Figure 7, middle column). After injection of 500 ng acriflavine there were larger reductions in ERG function (Figure 7, right column). These data suggest that there is normal retinal function after intravitreous injection of 100 ng of acriflavine, mild dysfunction after injection of 250 ng, and substantial dysfunction after injection of 500 ng.
Fig 7. Retinal electroretinogram (ERG) function after intraocular injection of acriflavine.
C57BL6 mice had an intravitreous injection of 100 ng, 250 ng, or 500 ng of acriflavine in one eye and PBS in the other eye (n=5 for each). After 7 days, eyes injected with 100 ng acriflavine had photopic b-wave amplitudes and scotopic a- and b-wave amplitudes that were identical to those seen in PBS-injected fellow-eyes (left column). Eyes injected with 250 ng acriflavine had normal scotopic a-wave amplitudes but scotopic and photopic b-wave amplitudes were mildly, but significantly reduced at the highest stimulus intensities (middle column). Eyes injected with 500 ng acriflavine had greater reduction in ERG amplitudes than that seen with 250 ng (right column).
*p<0.05 compared with corresponding fellow-eye PBS control by unpaired t-test
Discussion
Acriflavine has been known to have trypanocidal and anti-bacterial activity for nearly a century [40]. Its first clinical use was for treatment of gonorrhea, for which 40–80 mg was given intravenously at 2–3 day intervals [41]. Daily oral doses of 90–120 mg for 2 weeks in 2500 men with gonorrhea were reported to be effective and well-tolerated with one case of jaundice. It was replaced by penicillin and there is no clinical experience with its use in modern medicine. However, interest in acriflavine has been rekindled by demonstration that it binds HIF-1α and HIF-2 α, and prevents their dimerization with HIF-1β, thereby blocking HIF-1- and HIF-2-mediated transcriptional activation [29]. In this study, we found that intraocular injection of acriflavine reduced the expression of multiple HIF-1-regulated genes in ischemic retina. Daily intraperitoneal injections or a single intravitreous injection of acriflavine showed dose-dependent suppression of ischemia-induced retinal NV, which is relevant to proliferative diabetic retinopathy. Acriflavine was potent with suppression of retinal NV seen with systemic doses as low as 0.1 mg/kg or a single intraocular injection of 10 ng. Intraperitoneal injections or a single intravitreous injection of acriflavine also strongly inhibited choroidal NV, which is relevant to neovascular AMD.
These data add to the growing body of evidence suggesting that HIF-1 is an important target for treatment of retinal and choroidal vascular diseases. Other drugs that decrease HIF-1 transcriptional activity by other mechanisms also suppress retinal and choroidal NV, including digoxin which reduces HIF-1 levels by preventing the accumulation of HIF-1α in hypoxic cells [6, 27] and anthracyclines which inhibit HIF-1 binding to DNA [7, 28]. Each of these drugs has other activities, but they each reduce transcription of HIF-1 target genes in ischemic retina and strongly suppress retinal and choroidal NV indicating the critical role of HIF-1 in these disease processes.
In addition to providing another tool to explore the role of HIF-1 in ocular disease processes, acriflavine is fluorescent making it possible track its penetration into the retina and its exit over time. With any new therapeutic, it is useful to explore efficacy after different routes of administration to consider which might be most useful in the clinic. With acriflavine it was possible to also track entry and exit from the retina after different routes of administration. It penetrated through the inner retina by 1 hour after intravitreous injection of 100 ng and throughout the entire retina within 4 hours. The fluorescence intensity decreased slightly between 4 hours and 5 days and was eliminated from the retina by 10 days. Since it was possible to track acriflavine entry into and exit from the retina after intravitreous injection, we sought to assess entry and exit after other modes of administration. Within 5 minutes of placement of 5 μl of acriflavine on the eye, fluorescence was seen in the RPE and outer retina and penetrated into the inner retina at later time points. Measurement of acriflavine levels 2 hours after topical administration confirmed that levels were higher in RPE/choroid than retina. This suggests that acriflavine penetrated through the sclera and choroid into the retina, which is consistent with previous observations with other topical agents [42]. There was strong fluorescence throughout the retina 6 hours after administration of 10% acriflavine and it was still detectable at 12 hours, suggesting that with a 10% solution twice a day dosing would be sufficient to maintain some acriflavine within the retina. Fluorescence disappeared from the retina between 3 and 6 hours after administration of 5 μl of 0.5% acriflavine suggesting that three times a day dosing would allow retinal levels of acriflavine to drop to undetectable levels between doses, but this was sufficient to strongly suppress choroidal NV in dosed eyes compared to fellow eyes (Figure 5B). The comparison with fellow eyes indicates that the suppression occurred from local entry into the dosed eye and not entry into the systemic circulation.
Suprachoroidal injection provides a relatively new route of drug delivery to the retina [43, 44]. For at least 6 hours after suprachoroidal injection, acriflavine remained localized in the quadrant of the injection, but by 1 day after injection it was seen throughout the entire retina even in the quadrant 180° from the injection site and was detectable for at least 5 days after injection. Suprachoroidal injection of 30 ng or 300 ng of acriflavine dramatically reduced choroidal NV at Bruch’s membrane rupture sites in rats suggesting that this mode of administration, particularly if combined with sustained delivery, is very promising.
We previously observed that intraocular injection of high doses of two other HIF-1 inhibitors, digoxin and doxorubicin, caused reduction in ERG b-wave amplitudes. We therefore investigated the effect of intraocular injection of acriflavine on ERG function. One week after intravitreous injection of 250 ng of acriflavine in mice there were mild reduction in scotopic and photopic b-wave amplitudes at highest stimulus intensities and greater ERG deficits occurred after intravitreous injection of 500 ng. Efficacy is seen in the 10–50 ng range providing an approximate margin of 10 to 50-fold between efficacious and toxic intravitreous doses. The level of suppression of ocular NV by acriflavine after systemic or intraocular delivery is comparable to that of other HIF-1 inhibitors digoxin, DNR, or DXR, but the therapeutic window after intravitreous injection appears somewhat wider for acriflavine compared to digoxin, DNR, or DXR [6, 7]. Sustained release of low levels of acriflavine may be the best strategy to target the efficacious range of intraocular concentrations with the greatest safeguard against exceeding the lower threshold of the toxic range. In addition, it will be important to determine if the margin between efficacy and toxicity is greater with different modes of administration.
HIF-1 is also an important target for treatment of tumors. Like acriflavine, a cyclic peptide, cyclo-CLLFVY, that inhibits HIF-1 dimerization and transcriptional activity was identified by high-throughput screening and was demonstrated to reduce HIF-1 signaling in several cancer cell lines [45]. Photodynamic therapy (PDT) uses light exposure to activate a tumor-localized photosensitizer causing production of reactive oxygen species that damage tumor vasculature and promotes tumor cell death; however it is accompanied by HIF-1 stabilization which promotes tumor cell survival. In A431 tumor cells, inhibition of HIF-1 with acriflavine increased PDT-induced tumor cell death [46]. Thus acriflavine may be useful for treatment of some tumors.
In conclusion, we have demonstrated that the HIF-1 inhibitor acriflavine strongly suppresses retinal NV which is relevant to proliferative diabetic retinopathy and other ischemic retinopathies and choroidal NV which is relevant to neovascular AMD. This provides additional evidence of the important role of HIF-1 in these disease processes and suggests that acriflavine may provide a novel therapeutic agent that could have advantages over currently used agents because it targets multiple participating vasoactive proteins. The fluorescence of acriflavine allowed tracking its entrance and exit from the retina after several different routes of administration. Efficacy was demonstrated after topical administration in mice and after suprachoroidal injection in rats. Additional studies are needed to further explore these relatively noninvasive delivery approaches in other animal models.
Key Messages.
Acriflavine, an inhibitor of HIF-1, suppresses retinal and choroidal neovascularization.
HIF-1 plays a critical role in ocular neovascularization.
Acriflavine’s fluorescence provides a mean to track its entry and exit from the retina.
Acriflavine has therapeutic potential for the treatment of ocular neovascularization.
Acknowledgments
Supported by EY012609 from the National Eye Institute
Footnotes
Disclosure Statement: None of the authors have a conflict of interest
References
- 1.Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992;99:933–943. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- 2.Klein R, Klein B. Group NDD, editor. Diabetes in America. National Institutes of Health; Washington, D.C: 1995. Vision disorders in diabetes; pp. 293–330. [Google Scholar]
- 3.Ozaki H, Yu A, Della N, Ozaki K, Luna JD, Yamada H, Hackett SF, Okamoto N, Zack DJ, Semenza GL, et al. Hypoxia inducible factor-1a is increased in ischemic retina: temporal and spatial correlation with VEGF expression. Invest Ophthalmol Vis Sci. 1999;40:182–189. [PubMed] [Google Scholar]
- 4.Kelly BD, Hackett SF, Hirota K, Oshima Y, Cai Z, Berg-Dixon S, Rowan A, Yan Z, Campochiaro PA, Semenza GL. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res. 2003;93:1074–1081. doi: 10.1161/01.RES.0000102937.50486.1B. [DOI] [PubMed] [Google Scholar]
- 5.Vinores SA, Xiao WH, Aslam S, Shen J, Oshima Y, Nambu H, Liu H, Carmeliet P, Campochiaro PA. Implication of the hypoxia response element of the VEGF promoter in mouse models of retinal and choroidal neovascularization, but not retinal vascular development. J Cell Physiol. 2006;206:749–758. doi: 10.1002/jcp.20525. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida T, Zhang H, Iwase T, Shen J, Semenza G, Campochiaro PA. Digoxin inhibits retinal ischemia-induced HIF-1alpha expression and ocular neovascularization. FASEB J. 2010;24:1759–1767. doi: 10.1096/fj.09-145664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwase T, Fu J, Yoshida T, Muramatusu D, Miki A, Hashida N, Lu L, Oveson B, Lime e Silva R, Seidel C, et al. Sustained delivery of a HIF-1 antagonist for ocular neovascularization. J Control Release. 2013;172:625–633. doi: 10.1016/j.jconrel.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campochiaro PA. Molecular pathogenesis of retinal and choroidal vascular diseases. Prog Retin Eye Res. 2015;49:67–81. doi: 10.1016/j.preteyeres.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY, Group MS. Ranibizumab for neovascular age-related macular degeneration. N Eng J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 10.Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, Kirchhof B, Ho A, Ogura Y, Yancopoulos GD, et al. Intravitreal Aflibercept (VEGF Trap-Eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen QD, Tatlipinar S, Shah SM, Haller JA, Quinlan E, Sung J, Zimmer-Galler I, Do DV, Campochiaro PA. Vascular endothelial growth factor is a critical stimulus for diabetic macular edema. Am J Ophthalmol. 2006;142:961–969. doi: 10.1016/j.ajo.2006.06.068. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, Gibson A, Sy J, Rundle AC, Hopkins JJ, et al. Ranibizumab for Diabetic Macular Edema. Results from 2 Phase III Randomized Trials: RISE and RIDE. Ophthalmology. 2012;119:789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 13.Campochiaro PA, Hafiz G, Shah SM, Nguyen QD, Ying H, Do DV, Quinlan E, Zimmer-Galler I, Haller JA, Solomon S, et al. Ranibizumab for macular edema due to retinal vein occlusions; implication of VEGF as a critical stimulator. Molec Ther. 2008;16:791–799. doi: 10.1038/mt.2008.10. [DOI] [PubMed] [Google Scholar]
- 14.Campochiaro PA, Heier JS, Feiner L, Gray S, Saroj N, Rundle AC, Murahashi WY, Rubio RG, Group BS. Ranibizumab for macular edema following branch retinal vein occlusion: 6-month primary endpoint results of a phase III study. Ophthalmology. 2010;117:1102–1112. doi: 10.1016/j.ophtha.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 15.Brown DM, Campochiaro PA, Singh RP, Gray S, Rundle AC, Li Z, Rubio RG, Murahashi WY, Group CS. Efficacy and safety of ranibizumab in the treatment of macular edema secondary to central retinal vein occlusion:6-month results of the phase III CRUISE study. Ophthalmology. 2010;117:1124–1133. doi: 10.1016/j.ophtha.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 16.Ip MS, Domalpally A, Hopkins JJ, Wong P, Ehrlich JS. Long-term effects of ranibizumab on diabetic retinopathy severity and progression. Arch Ophthalmol. 2012;130:1145–1152. doi: 10.1001/archophthalmol.2012.1043. [DOI] [PubMed] [Google Scholar]
- 17.Jo N, Mailhos C, Ju M, Cheung E, Bradley J, Nishijima K, Robinson GS, Adamis AP, Shima DT. Inhibition of platelet-derived growth factor B signaling enhances the efficacy of anti-vascular endothelial growth factor therapy in multiple models of ocular neovascularization. Am J Pathol. 2006;168:2036–2053. doi: 10.2353/ajpath.2006.050588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong A, Seidel C, Snell D, Ekawardhani S, Ahlskog JK, Baumann M, Shen J, Iwase T, Tian J, Stevens R, et al. Antagonism of PDGF-BB suppresses subretinal neovascularization and enhances the effects of blocking VEGF-A. Angiogenesis. 2014;17:553–562. doi: 10.1007/s10456-013-9402-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hackett SF, Ozaki H, Strauss RW, Wahlin K, Suri C, Maisonpierre P, Yancopoulos G, Campochiaro PA. Angiopoietin 2 expression in the retina: upregulation during physiologic and pathologic neovascularization. J Cell Physiol. 2000;184:275–284. doi: 10.1002/1097-4652(200009)184:3<275::AID-JCP1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 20.Hackett SF, Wiegand SJ, Yancopoulos G, Campochiaro P. Angiopoietin-2 plays an important role in retinal angiogenesis. J Cell Physiol. 2002;192:182–187. doi: 10.1002/jcp.10128. [DOI] [PubMed] [Google Scholar]
- 21.Gale NW, Thurston G, Hackett SF, Renard R, Wang Q, McClain J, Martin C, Witte C, Witte M, Jackson D, et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by angiopoietin-1. Devel Cell. 2002;3:411–423. doi: 10.1016/s1534-5807(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 22.Oshima Y, Oshima S, Nambu H, Kachi S, Takahashi K, Umeda N, Shen J, Dong A, Apte RS, Duh E, et al. Different effects of angiopoietin 2 in different vascular beds in the eye; new vessels are most sensitive. FASEB J. 2005;19:963–965. doi: 10.1096/fj.04-2209fje. [DOI] [PubMed] [Google Scholar]
- 23.Lima e Silva R, Shen J, Hackett SF, Kachi S, Akiyama H, Kiuchi K, Yokoi K, Hatara C, McLauer T, Aslam S, et al. The SDF-1/CXCR4 ligand/receptor pair is an important contributor to several types of ocular neovascularization. FASEB J. 2007;21:3219–3230. doi: 10.1096/fj.06-7359com. [DOI] [PubMed] [Google Scholar]
- 24.Jaffe GJ, Eliott D, Well JA, Prenner JL, Papp A, Patel S. A Phase 1 Study of Intravitreous E10030 in Combination with Ranibizumab in Neovascular Age-Related Macular Degeneration. Ophthalmology. 2016;123:78–85. doi: 10.1016/j.ophtha.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Campochiaro PA, Sophie R, Tolentino M, Miller DM, Browning D, Boyer DS, Heier JS, Gambino L, Withers B, Brigell M, et al. Treatment of diabetic macular edema with an inhibitor of vascular endothelial-protein tyrosine phosphatase that activates Tie2. Ophthalmology. 2015;122:545–554. doi: 10.1016/j.ophtha.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 26.Campochiaro PA, Khanani A, Singer M, Patel S, Boyer D, Dugel P, Kherani S, Withers B, Gambino L, Peters K, et al. Enhanced benefit in diabetic macular edema from AKB-9778 Tie2 activation combined with vascular endothelial growth factor suppression. Ophthalmology. 2016;123:1722–1730. doi: 10.1016/j.ophtha.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 27.Zhang H, Qian DZ, Tan YS, Lee K, Gao P, Ren YR, Rey S, Hammers H, Chang D, Pili R, et al. Digoxin and other cardiac glycosides inhibit HIF-1alpha sythesis and block tumor growth. Proc Natl Acad Sci USA. 2008;105:19579–19586. doi: 10.1073/pnas.0809763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee K, Qian DZ, Rey S, Wei H, Liu JO, Semenza GL. Antracycline chemotherpy inhibits HIF-1 transcriptional activity and tumor induced mobilizatio of circulating angiogenic cells. Proc Natl Acad Sci USA. 2009;106:2353–2358. doi: 10.1073/pnas.0812801106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Lee K, Zhang H, Qlan DZ, Rey S, Liu JO, Semenza GL. Acriflavine inhibits HIF-1 dimerization, tumor growth, and vascularization. Proc Natl Acad Sci USA. 2009;106:17910–17915. doi: 10.1073/pnas.0909353106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Shen J, Yang XR, Xiao WH, Hackett SF, Sato Y, Campochiaro PA. Vasohibin is up-regulated by VEGF in the retina and suppresses VEGF receptor 2 and retinal neovascularization. FASEB J. 2006;20:723–725. doi: 10.1096/fj.05-5046fje. [DOI] [PubMed] [Google Scholar]
- 31.Tobe T, Ortega S, Luna JD, Ozaki H, Okamoto N, Derevjanik NL, Vinores SA, Basilico C, Campochiaro PA. Targeted disruption of the FGF2 gene does not prevent choroidal neovascularization in a murine model. Am J Pathol. 1998;153:1641–1646. doi: 10.1016/S0002-9440(10)65753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levinson JW, Desostoa A, Liebes LF, McCormick JJ. Fluorescent labeling of DNa in solution with covalently bound acriflavin. Biochim Biophys Acta. 1976;447:260–273. doi: 10.1016/0005-2787(76)90049-6. [DOI] [PubMed] [Google Scholar]
- 33.Levinson JW, Maher vM, McCormick JJ. Purification of commercil acriflavine by sephadex LH-20 column chromatography. J Histochem Cytochem. 1977;25:1275–1277. doi: 10.1177/25.11.597361. [DOI] [PubMed] [Google Scholar]
- 34.Komeima K, Rogers BS, Lu L, Campochiaro PA. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc Natl Acad Sci USA. 2006;103:11300–11305. doi: 10.1073/pnas.0604056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith LEH, Wesolowski E, McLellan A, Kostyk SK, D’Amato R, Sullivan R, D’Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- 36.Aiello LP, Pierce EA, Foley ED, Takagi H, Chen H, Riddle L, Ferrara N, King GL, Smith LEH. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci USA. 1995;92:10457–10461. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LEH. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci USA. 1995;92:905–909. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwak N, Okamoto N, Wood JM, Campochiaro PA. VEGF is an important stimulator in a model of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2000;41:3158–3164. [PubMed] [Google Scholar]
- 39.Saishin Y, Saishin Y, Takahashi K, Lima Silva R, Hylton D, Rudge JJ, WS, Campochiaro PA. VEGF-TRAPR1R2 suppresses choroidal neovascularization and VEGF-induced breakdown of the blood-retinal barrier. J Cell Physiol. 2003;195:241–248. doi: 10.1002/jcp.10246. [DOI] [PubMed] [Google Scholar]
- 40.Browning CH, Cohen JB, Gaunt R, Gulbransen R. Relationships between antiseptic action and chemical constitution with special reference to compounds of the pyridine, quinoline, acridine, and phenazine series. Proc Royal Soc. 1922;93:329–366. [Google Scholar]
- 41.Assinder EW. Acriflavine as a urinary antiseptic. Lancet. 1936:227. [Google Scholar]
- 42.Doukas J, Mahesh S, Umeda N, Kachi S, Akiyama H, Yokoi K, Cao J, Chen Z, Dellamary L, Tam B, et al. Topical administration of a multi-targeted kinase inhibitor suppresses choroidal neovasculaization and retinal edema. J Cell Physiol. 2008;216:29–37. doi: 10.1002/jcp.21426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel SRP, LAS, Edelhauser HF, Prausnitz MR. Suprachoroidal drug delivery to the back of the eye using hollow needles. Pharm Res. 2011;28:166–176. doi: 10.1007/s11095-010-0271-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patel SR, Berezovsky DE, McCarey BE, Zarnitsyn V, Edelhauser HF, Prausnitz MR. Targeted administration into the suprachoroidal space using a microneedle for drug delivery to the posterior segment of the eye. Invest Ophthalmol Vis Sci. 2012;53:4433–4441. doi: 10.1167/iovs.12-9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miranda E, Nordgren IK, Male AL, Lawrence CE, Hoakwie F, Cuda F, Court W, Fox KR, Townsend PA, Packham GK, et al. A cyclic peptide inhibitor of HIF-1 heterodimerization that inhibits hypoxia signaling in cancer cells. J Am Chem Soc. 2013;135:10418–10425. doi: 10.1021/ja402993u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Broekgaarden M, Weijer R, Krekorian M, van den IJssel B, Kos M, Alles LK, van Wijk AC, Hazai E, van Gulik TM, Heger M. Inhibition of hypoxia-inducible factor 1 with acriflavine sensitizes hypoxic tumor cells to photodynamic therapy with zinc phthalocyanine-encapsulating cationic liposomes. Nano Research. 2016;9:1639–1662. [Google Scholar]