Abstract
Background
Idiopathic normal pressure hydrocephalus (iNPH) is ventriculomegaly syndrome characterized by dementia, urinary incontinence, and gait disturbance, which is potentially reversible following ventriculoperitoneal shunting (VPS). Magnetic resonance elastography (MRE) is an evolving imaging technology that noninvasively measures tissue viscoelasticity.
Objective
We studied iNPH patients using MRE prior to shunting, compared them to normal controls, and analyzed associations between MRE findings and clinical features, as a pilot assessment of MRE in iNPH.
Methods
Stiffness values were measured on preoperative MRE in 10 iNPH patients scheduled for VPS, and compared with those in 20 age- and sex-matched controls. Stiffness results were correlated with clinical iNPH symptoms.
Results
MRE demonstrated significantly increased stiffness in iNPH in cerebrum (p=0.04), occipital (p=0.002), and parietal (p=0.01) regions-of-interest (ROI), and significantly decreased stiffness in periventricular ROI (p<0.0001). Stiffness was not significant different in frontal (p=0.1) and deep grey ROI (p=0.4). Univariate analysis showed associations between preoperative iNPH symptoms and abnormally increased stiffness, including urinary incontinence with cerebrum (p=0.005), frontal (p=0.04), and cerebellum (p=0.03), and Parkinsonism with occipital (p=0.04). Postoperative improvement was associated with increased deep grey stiffness (p=0.01); failure was associated with increased temporal (p=0.0002) stiffness.
Conclusion
Based on the preliminary results of this small, limited analysis, brain stiffness may be altered in iNPH, and these alterations in parenchymal viscoelastic properties may be correlated with clinical symptoms. Increased temporal stiffness may predict surgical failure, and potentially suggest an alternative dementing pathology underlying the iNPH-like symptoms. These findings highlight the potential future utility of MRE in iNPH management.
Keywords: Magnetic resonance elastography, idiopathic normal pressure hydrocephalus, periventricular white matter
INTRODUCTION
Idiopathic normal pressure hydrocephalus (iNPH) is a potentially reversible disturbance of cerebrospinal fluid (CSF) dynamics that classically presents with ventriculomegaly and the triad of gait disturbance, urinary incontinence, and dementia.1,2 Although these symptoms are characteristic, the clinical reality is far more heterogeneous and often obscured by comorbid neurodegenerative pathology.3,4 CSF shunting is an effective treatment for iNPH, yet patient selection is often challenging, and attempts to establish reliable predictors of clinical improvement after shunt placement have had mixed results.4–7 Currently the best indicator is an invasive trial of CSF diversion, either via a high volume lumbar puncture (LP) or indwelling lumbar drain.
Magnetic resonance elastography (MRE) is an evolving imaging technique that non-invasively evaluates mechanical tissue properties by subjecting the brain to propagating acoustic strain waves and quantitatively mapping physical responses.8–10 Preceding authors have hypothesized that microstructural changes in the brain parenchyma may underlie iNPH, a finding that is indirectly supported by diffusion tensor imaging changes to the corticospinal tract that have been observed in iNPH.9,11,12 These and related abnormalities may be and may be better detected by MRE than conventional modalities, suggesting that its may be of particular utility in diagnosing iNPH and predicting responsiveness to CSF diversion. Correspondingly, we performed MRE imaging studies on iNPH patients prior to ventriculoperitoneal shunt placement, compared them to normal age-matched controls, and evaluated associations between MRE abnormalities and clinical findings.
METHODS
Ten consecutive patients with iNPH who were scheduled for VPS placement underwent preoperative MRE. Inclusion criteria—which for the purposes of the present study were also considered sufficient to substantiate a diagnosis of iNPH—were radiographic ventriculomegaly and symptomatic gait disturbance that improved following drainage of 30cc of CSF via LP, as demonstrated by gait analysis on pre- and post-LP video recordings. Patients with a history of neurologic disease associated with non-idiopathic hydrocephalus including meningitis, subarachnoid hemorrhage, and traumatic brain injury were excluded. All patients were implanted with a Delta 1.0 valve VPS.
Follow-up included plain x-ray shunt series and head CT in-hospital, followed by clinical evaluation and MRI at approximately 30 days. Postoperative MRE was not performed within this study protocol. Postoperative success was defined as improvement in gait, as demonstrated from pre- to postoperative video recordings, as well as patient-reported subjective improvement in their presenting iNPH symptoms.
A two-to-one age- and sex-matched control population of 20 individuals was generated using data from a preceding study of patients who were known to be free of neurologic disease conducted at our institution using the same imaging protocol, the results of which we have described previously.13–15
MRE studies were performed on a 3.0T scanner (GE Healthcare, Milwaukee, WI, USA) using a single-shot, spin-echo EPI pulse sequence. A vibration source was positioned beneath the head within an eight-channel receive-only head coil, and mechanical waves were introduced at a single frequency of 60Hz. MRE sequence parameters were TR/TE=3600/62 ms; field-of-view=24 cm; BW=±250kHz; 72×72 imaging matrix reconstructed to 80×80; 3x ASSET acceleration, frequency encoding in the right-left direction; 48 contiguous 3mm thick axial slices; one 4G/cm 18.2-ms zeroth- and first-order moment nulled motion encoding gradient on each side of the refocusing RF pulse synchronized to the motion; motion encoding in the positive and negative X, Y, and Z directions; and eight phase offsets sampled over one period of the 60 Hz cycle. Total acquisition time was within seven minutes. The final isotropic resolution of acquired images was 3mm.
For post-processing, we employed a previously published protocol that has been demonstrated to have less than 1% variation for global brain stiffness and less than 2% for the cerebellum and discrete cerebral lobes in reproducibility studies.16 Stiffness was defined as the square of wave speed multiplied by density. An elastogram, or stiffness map, was calculated by applying the inverse Helmholtz equation to the smoothed curled wave field. Regions-of-interest (ROI) were defined using a brain mask that was derived from mapping a brain atlas to the anatomical T1-weighted volume of the patient and then registering it to the MRE imaging domain. ROIs defined for this study were cerebrum, cerebellum, frontal, temporal, parietal, occipital, deep grey, and periventricular, as described previously.16 The median stiffness in each ROI volume was reported (Supplemental Table 1). Voxels with significant CSF contributions were masked to further ensure that recorded stiffness values accurately reflected changes in parenchymal tissue, rather than propagation of waveforms in spinal fluid spaces.8,15–18 MRE image acquisition and processing protocols have been previously described.16,17,19, which we have previously demonstrated decreases variation in measurements of brain stiffness.
Stiffness values for each ROI were tested for statistical significance between iNPH patients and controls. Among iNPH patients, associations were tested between stiffness and clinical outcomes including cognitive decline, urinary incontinence, gait disturbance, falls, Parkinsonism, Mini-Mental score, CSF aqueductal flow rate, extent of ventriculomegaly, duration-of-symptoms, LP opening pressure, clinical improvement after LP, and postoperative clinical improvement. Statistical tests included Student’s t, linear regression, multivariate analysis, and derived receiver operating characteristic curves. All statistical testing was performed using JMP 10.0.0 (SAS Institute Inc., Cary, NC, 1989–2007). The Mayo Clinic Institutional Review Board approved the study protocol, and all patients provided written informed consent prior to enrollment and imaging (#13-002812).
RESULTS
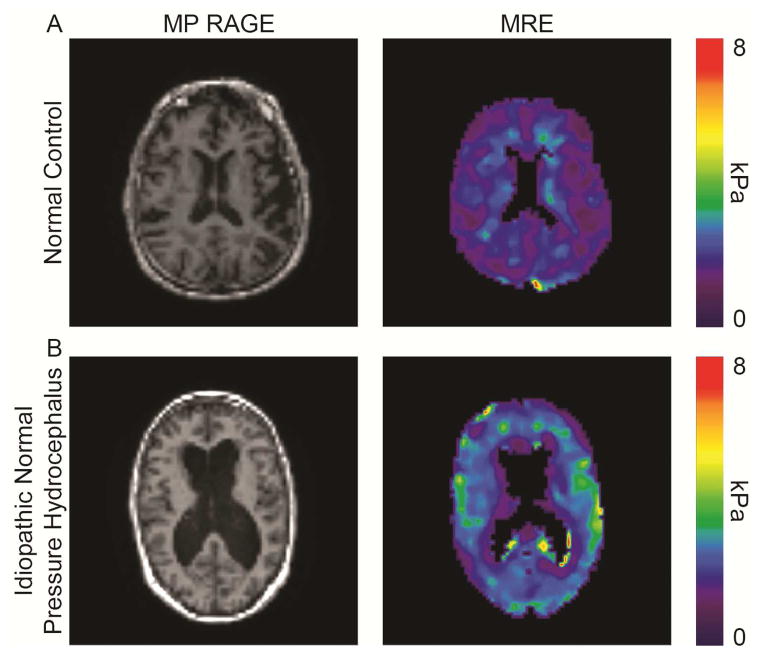
There were no significant demographic differences between the groups: median ages were 72 and 76 years among iNPH patients and controls, mean ages were 72 (67–79) and 75 (67–80) respectively, and a slight female predominance was noted in both (60% vs. 55%, p=1.0). Corrected stiffness values by ROI comparing iNPH patients and control subjects are reported as mean values with standard error (Table 2). MRE demonstrated significantly increased parenchymal stiffness in iNPH over multiple ROIs including the cerebrum (p=0.04), occipital lobe (p=0.002), and parietal lobes (p=0.01). Mean corrected stiffness was decreased in iNPH in the periventricular ROI (p<0.0001). Characteristic MRE abnormalities, including decreased periventricular stiffness, and increased cerebral, parietal, and occipital stiffness are presented alongside anatomic MP RAGE images, for both a normal control and an iNPH patient (Figure 1).
Table 2.
Brain stiffness in iNPH patients and healthy controls
| NPH (n=10) | Control (n=20) | p-value | |
|---|---|---|---|
| Region-of-Interest | |||
| Cerebrum | 2.64 (±0.11) | 2.55 (±0.11) | 0.04 |
| Frontal | 2.65 (±0.05) | 2.74 (±0.03) | 0.1 |
| Occipital | 2.97 (±0.15) | 2.75 (±0.16) | 0.002 |
| Parietal | 2.63 (±0.18) | 2.45 (±0.12) | 0.01 |
| Temporal | 2.79 (±0.48) | 2.73 (±0.03) | 0.3 |
| Deep grey | 2.91 (±0.09) | 3.00 (±0.06) | 0.4 |
| Cerebellum | 2.20 (±0.04) | 2.23 (±0.03) | 0.6 |
| Periventricular | 1.74 (±0.24) | 2.26 (±0.29) | <0.0001 |
Corrected stiffness reported as mean (±standard error)
Figure 1.
Axial MP RAGE images and MRE elastograms from a normal age-matched control (A) and a patient with clinical iNPH (B), demonstrating decreased periventricular stiffness, with increased cerebral, parietal, and occipital stiffness
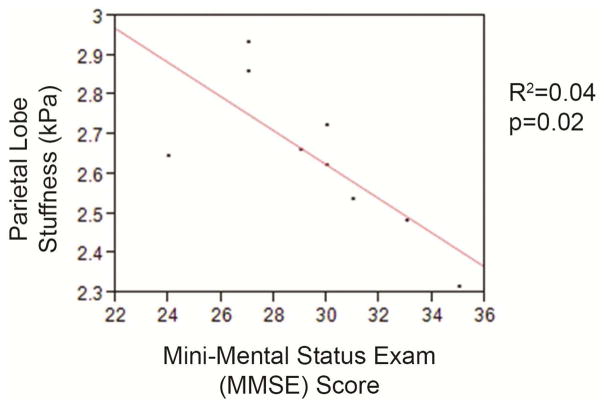
Significant associations and relationships between clinical changes and MRE abnormalities in iNPH patients are described by symptom and ROI (Table 3). Univariate analysis showed associations including urinary incontinence with increased stiffness in cerebrum (p=0.005), frontal (p=0.04), and cerebellum (p=0.03), and with decreased periventricular stiffness (p=0.05). Parkinsonism was associated with increased occipital lobe stiffness (p=0.04). Mini-Mental score was analyzed using a linear regression model, which suggested an inverse relationship with parietal stiffness, but a weak association, likely attributable to the small number of data points (Figure 2).
Table 3.
Clinical symptoms and brain stiffness in iNPH
| Symptom Present | Symptom Absent | p-value | |
|---|---|---|---|
| Urinary Incontinence | |||
| Cerebrum | 2.67 (±0.10) | 2.53 (±0.01) | 0.005 |
| Frontal | 2.68 (±0.16) | 2.53 (±0.02) | 0.04 |
| Cerebellum | 2.23 (±0.09) | 2.11 (±0.03) | 0.03 |
| Periventricular | 1.67 (±0.07) | 2.04 (±0.14) | 0.05 |
| Parkinsonism Occipital | 3.10 (±0.08) | 2.92 (±0.16) | 0.04 |
|
| |||
| Linear Fit | Root Mean Sq Error | p-value | |
|
| |||
| Mini-Mental Score Parietal | R2=0.04 | 0.13 | 0.02 |
Corrected stiffness reported as mean (±standard error)
Figure 2.
Scatter plot with superimposed best-fit linear regression analysis of Mini-Mental State Examination score and brain stiffness in the parietal region-of-interest
The mean stiffness in each ROI is compared between iNPH patients who improved postoperatively with those who did not (Table 4). Postoperative failure was associated with decreased deep grey stiffness, and increased temporal stiffness (p=0.01, p=0.0002).
Table 4.
Postoperative improvement and brain stiffness in iNPH
| Improved (n=8) | Not Improved (n=2) | p-value | |
|---|---|---|---|
| Region-of-Interest | |||
| Cerebrum | 2.61 (±0.04) | 2.73 (±0.07) | 0.2 |
| Frontal | 2.64 (±0.06) | 2.67 (±0.11) | 0.8 |
| Occipital | 2.95 (±0.06) | 3.03 (±0.12) | 0.6 |
| Parietal | 2.63 (±0.07) | 2.66 (±0.13) | 0.9 |
| Temporal | 2.75 (±0.11) | 3.00 (±0.02) | 0.0002 |
| Deep grey | 2.98 (±0.05) | 2.60 (±0.11) | 0.01 |
| Cerebellum | 2.19 (±0.03) | 2.28 (±0.06) | 0.2 |
| Periventricular | 1.74 (±0.09) | 1.78 (±0.18) | 0.9 |
Corrected stiffness reported as mean (±standard error)
DISCUSSION
iNPH is a common yet poorly understood neurosurgical entity, whose definitive diagnosis and optimal management have remained elusive in spite of significant clinical and scientific efforts. A key component in our evolving knowledge of this disease process is the physical transformation of the diseased brain, which for the first time can be evaluated non-invasively using MRE, a tool that empowers quantitative measurement of parenchymal stiffness changes.8,16,18,20 It is possible that MRE may prove to be a useful tool to predict shunting success in iNPH patients.
Our findings suggest that the characteristic iNPH symptoms may be attributable to regional alterations in viscoelasticity. Most prominently, these relationships appear to include cognitive changes in association with parietal stiffness—a rational correlation, given the established connections between neurodegenerative disease and both temporal and parietal abnormalities.21–24 This possibility finds further support in the significant inverse relationship we observed between parietal stiffness and Mini-Mental State Examination (MMSE) score.25
Urinary incontinence is a complex symptom, and predominantly mediated by detrusor hyperactivity in iNPH.26 Mechanistically, this may be due to decreased tonic inhibition attributable to globally increased stiffness, or specific changes within the paracentral lobule—a fronto-parietal structure, which we’ve identified as exhibiting pathologically increased stiffness in iNPH.27 Further, functional MRI studies have demonstrated that detrusor over-activity and poor bladder control are associated with weak orbitofrontal activation, which correlates with decreased frontal stiffness.28
Parkinsonism is common in iNPH, due to nigrostriatal axis disturbance secondary to decreased periventricular perfusion, which accords with our observation of abnormal periventricular softness.29–32 In parallel, nigrostriatal dysfunction has been associated with occipital hypometabolism and hypoperfusion, suggesting a relationship between Parkinsonism and the occipital abnormalities we observed.33
Among patients who did not improve after surgery, preoperative MRE demonstrated increased temporal stiffness, possibly suggesting the presence of an unrelated, comorbid neurodegenerative disease. This is particularly likely given that the characteristic pathologic ultrastructural changes in Alzheimer’s include hippocampal atrophy and fronto-temporal hypometabolism.34,35 Although our previous results demonstrated a global decrease of parenchymal stiffness in Alzheimer’s disease, these studies evaluated the cerebrum ROI in isolation, without lobar analysis.19 Integrating these observations, we recommend that patients with clinically suspected iNPH and abnormal MRE findings including either increased temporal stiffness or globally decreased stiffness undergo comprehensive neuropsychiatric assessment.
The present study reproduces our own previous results and those of most related studies; however, the juxtaposition of our observations with Streitberger’s warrants a nuanced review.15,36 The Streitberger protocol is based on three adjacent 6mm slices, which are subsequently integrated into two regions—global and periventricular—both of which were found to be less stiff in iNPH. By contrast, our protocol is driven by 3mm full-volume data sets segmented into anatomical regions using a warped lobar atlas. Additionally, our technique integrates a pipeline masking voxels with significant CSF contributions, which we have previously demonstrated decreases variation in measurements of brain stiffness.15 It remains unclear how to best resolve several of the incongruities in the respective findings; however, we find reassurance that the parallel findings of periventricular softness indicate true pathologic changes.
The findings of the present study are clearly preliminary, and cannot substantiate a conclusive, unifying theory regarding pathophysiology of iNPH; however, our findings do suggest a number of hypotheses that may warrant further, more rigorous evaluation in future studies. One model departs from the recent discovery of the glymphatic system, and suggests a failure of solute and metabolite clearance—particularly during sleep—as the primary insult in iNPH.37–39 This results in glial dysfunction and cytoskeletal rearrangements as the driving forces behind generalized parenchymal stiffening, with a positive feedback loop that ultimately produces periventricular neuronal dysfunction, and the observed softening within that ROI.40–45 In a parallel scenario, the periventricular softness may reflect injury and dysfunction in axonal white matter tracts, resulting in conduction disruption that produces the characteristic ultimately iNPH symptoms.
Interpretation of the results of our study is complicated by several major limitations. Patients were recruited prospectively, but clinical data were collected retrospectively. MRE was performed after patients had undergone LP. The metrics used to analyze both presenting symptoms and postoperative improvements were heterogeneous and subjective. Finally, no postoperative MRE studies have been performed, to document any reversal of pathologic stiffness after shunting.
Statistical analyses were performed on very small samples in which a normal distribution could not be assumed, potentially invalidating their results, and many of the comparisons were underpowered, requiring a universally cautions and qualified interpretation. The impact of these compromises is readily apparent in several inconsistencies within the study dataset—for example, abnormal deep grey ROI stiffness was noted to be a significant marked of iNPH patients who improved, yet it was not significantly different between iNPH patients and controls. Although this most likely reflects sampling variation in a study of small populations, it may also represent statistical noise, and mandates further study in a large, prospective cohort. Additionally, we did not perform statistical corrections for multiple comparisons, given the preliminary nature of the data being collected, which allowed us to make only the most guarded interpretations of the study findings, regardless of any such adjustment.
Notwithstanding, our results suggest the possibility that MRE may become a useful new tool in the clinical evaluation of iNPH. If our results are confirmed in subsequent analyses, MRE may be able to effectively distinguish between three clinically significant patient groups: unconfounded iNPH that will most likely benefit from VPS placement, possible iNPH confounded by other neurodegenerative pathologies that may benefit, and unrelated etiologies of hydrocephalus or dementia that are unlikely to benefit.
Perhaps most importantly, the preliminary findings of this pilot study will hopefully light the way for more a more rigorous, standardized, and fully powered investigation of the viscoelastic parenchymal changes in iNPH, and their implications for diagnostic and treatment algorithms in this common but poorly understood neurosurgical disease. By intention, the present analysis was built to provide proof-of-concept data using limited resources prior to the initiation of a more rigorous and far-reaching study. To that end, our center is actively enrolling patients in a prospective trial, which is designed to address all the limitations of the present pilot project, including preoperative and postoperative MRE assessments in a large number of patients with suspected iNPH, as well as standardized neuropsychiatric metrics for the clinical diagnosis and follow-up of iNPH.
CONCLUSION
Our findings of potentially abnormal brain stiffness among iNPH patients may also inform a more evolved perspective on iNPH, potentially enhancing our understanding of this elusive disease and its confounding pathophysiology, particularly as prospective results become available. Significant new research must be done to reproduce our findings in a more statistically valid cohort, as well as to further evaluate and characterize the underlying mechanism responsible for the possible parenchymal changes; however, we hope that our initial results highlight an important area for future research, and early step towards true insight into iNPH and its optimal diagnosis, management, and treatment.
Supplementary Material
Supplemental Table 1. Median corrected stiffness values by ROI
Table 1.
Patient demographics
| NPH (n=10) | Control (n=20) | p-value | |
|---|---|---|---|
| Mean age | 72 | 76 | 0.1 |
| Median age (range) | 72 (67–79) | 75 (67–80) | 0.1 |
| Female sex | 6 (60%) | 11 (55%) | 1.0 |
HIGHLIGHTS.
MRE is an imaging technology that noninvasively measures tissue viscoelasticity
Brain stiffness may be significantly altered in iNPH, in a symptom-correlated fashion
Increased temporal stiffness may predict VPS failure
Deep grey matter stiffness may predict VPS success
Decreased periventricular stiffness may be associated with iNPH
ABBREVIATIONS
- iNPH
Idiopathic normal pressure hydrocephalus
- CSF
Cerebrospinal fluid
- LP
Lumbar puncture
- MRE
Magnetic resonance elastography
- MRI
Magnetic resonance imaging
- CT
Computed tomography
- ROI
Regions-of-interest
Footnotes
PREVIOUS PRESENTATIONS: Components of this work were presented as abstracts at the Congress of Neurological Surgeons 2015 and Minnesota Neurosurgical Society 2015
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jaraj D, Rabiei K, Marlow T, Jensen C, Skoog I, Wikkelso C. Prevalence of idiopathic normal-pressure hydrocephalus. Neurology. 2014;82(16):1449–1454. doi: 10.1212/WNL.0000000000000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams RD, Fisher CM, Hakim S, Ojemann RG, Sweet WH. Symptomatic Occult Hydrocephalus with “Normal” Cerebrospinal-Fluid Pressure. A Treatable Syndrome. N Engl J Med. 1965;273:117–126. doi: 10.1056/NEJM196507152730301. [DOI] [PubMed] [Google Scholar]
- 3.Savolainen S, Paljarvi L, Vapalahti M. Prevalence of Alzheimer’s disease in patients investigated for presumed normal pressure hydrocephalus: a clinical and neuropathological study. Acta Neurochir (Wien) 1999;141(8):849–853. doi: 10.1007/s007010050386. [DOI] [PubMed] [Google Scholar]
- 4.Golomb J, Wisoff J, Miller DC, et al. Alzheimer’s disease comorbidity in normal pressure hydrocephalus: prevalence and shunt response. Journal of neurology, neurosurgery, and psychiatry. 2000;68(6):778–781. doi: 10.1136/jnnp.68.6.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boon AJ, Tans JT, Delwel EJ, et al. The Dutch normal-pressure hydrocephalus study. How to select patients for shunting? An analysis of four diagnostic criteria. Surg Neurol. 2000;53(3):201–207. doi: 10.1016/s0090-3019(00)00182-8. [DOI] [PubMed] [Google Scholar]
- 6.Boon AJ, Tans JT, Delwel EJ, et al. Dutch normal-pressure hydrocephalus study: prediction of outcome after shunting by resistance to outflow of cerebrospinal fluid. Journal of neurosurgery. 1997;87(5):687–693. doi: 10.3171/jns.1997.87.5.0687. [DOI] [PubMed] [Google Scholar]
- 7.Hebb AO, Cusimano MD. Idiopathic normal pressure hydrocephalus: a systematic review of diagnosis and outcome. Neurosurgery. 2001;49(5):1166–1184. doi: 10.1097/00006123-200111000-00028. discussion 1184–1166. [DOI] [PubMed] [Google Scholar]
- 8.Kruse SA, Rose GH, Glaser KJ, et al. Magnetic resonance elastography of the brain. NeuroImage. 2008;39(1):231–237. doi: 10.1016/j.neuroimage.2007.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muthupillai R, Lomas DJ, Rossman PJ, Greenleaf JF, Manduca A, Ehman RL. Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science. 1995;269(5232):1854–1857. doi: 10.1126/science.7569924. [DOI] [PubMed] [Google Scholar]
- 10.Green MA, Bilston LE, Sinkus R. In vivo brain viscoelastic properties measured by magnetic resonance elastography. NMR Biomed. 2008;21(7):755–764. doi: 10.1002/nbm.1254. [DOI] [PubMed] [Google Scholar]
- 11.Freimann FB, Streitberger KJ, Klatt D, et al. Alteration of brain viscoelasticity after shunt treatment in normal pressure hydrocephalus. Neuroradiology. 2012;54(3):189–196. doi: 10.1007/s00234-011-0871-1. [DOI] [PubMed] [Google Scholar]
- 12.Hattori T, Yuasa T, Aoki S, et al. Altered microstructure in corticospinal tract in idiopathic normal pressure hydrocephalus: comparison with Alzheimer disease and Parkinson disease with dementia. American Journal of Neuroradiology. 2011;32(9):1681–1687. doi: 10.3174/ajnr.A2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: Design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30(1):58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arani A, Murphy MC, Glaser KJ, et al. Measuring the effects of aging and sex on regional brain stiffness with MR elastography in healthy older adults. NeuroImage. 2015;111:59–64. doi: 10.1016/j.neuroimage.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fattahi N, Arani A, Perry A, et al. MR Elastography Demonstrates Increased Brain Stiffness in Normal Pressure Hydrocephalus. American Journal of Neuroradiology. 2015 doi: 10.3174/ajnr.A4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy MC, Huston J, 3rd, Jack CR, Jr, et al. Measuring the characteristic topography of brain stiffness with magnetic resonance elastography. PloS one. 2013;8(12):e81668. doi: 10.1371/journal.pone.0081668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy MC, Curran GL, Glaser KJ, et al. Magnetic resonance elastography of the brain in a mouse model of Alzheimer’s disease: initial results. Magnetic resonance imaging. 2012;30(4):535–539. doi: 10.1016/j.mri.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu L, Lin Y, Xi ZN, Shen H, Gao PY. Magnetic resonance elastography of the human brain: a preliminary study. Acta Radiol. 2007;48(1):112–115. doi: 10.1080/02841850601026401. [DOI] [PubMed] [Google Scholar]
- 19.Murphy MC, Huston J, 3rd, Jack CR, Jr, et al. Decreased brain stiffness in Alzheimer’s disease determined by magnetic resonance elastography. Journal of magnetic resonance imaging : JMRI. 2011;34(3):494–498. doi: 10.1002/jmri.22707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo J, Hirsch S, Fehlner A, et al. Towards an elastographic atlas of brain anatomy. PloS one. 2013;8(8):e71807. doi: 10.1371/journal.pone.0071807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ackl N, Ising M, Schreiber YA, Atiya M, Sonntag A, Auer DP. Hippocampal metabolic abnormalities in mild cognitive impairment and Alzheimer’s disease. Neuroscience letters. 2005;384(1–2):23–28. doi: 10.1016/j.neulet.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 22.Butterfield DA, Kanski J. Brain protein oxidation in age-related neurodegenerative disorders that are associated with aggregated proteins. Mechanisms of ageing and development. 2001;122(9):945–962. doi: 10.1016/s0047-6374(01)00249-4. [DOI] [PubMed] [Google Scholar]
- 23.Salmon E, Sadzot B, Maquet P, et al. Differential diagnosis of Alzheimer’s disease with PET. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1994;35(3):391–398. [PubMed] [Google Scholar]
- 24.Brun A, Gustafson L. Distribution of cerebral degeneration in Alzheimer’s disease. A clinico-pathological study. Arch Psychiatr Nervenkr (1970) 1976;223(1):15–33. doi: 10.1007/BF00367450. [DOI] [PubMed] [Google Scholar]
- 25.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 26.Sakakibara R, Kanda T, Sekido T, et al. Mechanism of bladder dysfunction in idiopathic normal pressure hydrocephalus. Neurourol Urodyn. 2008;27(6):507–510. doi: 10.1002/nau.20547. [DOI] [PubMed] [Google Scholar]
- 27.Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9(6):453–466. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griffiths D, Derbyshire S, Stenger A, Resnick N. Brain control of normal and overactive bladder. J Urol. 2005;174(5):1862–1867. doi: 10.1097/01.ju.0000177450.34451.97. [DOI] [PubMed] [Google Scholar]
- 29.Jacobs L, Conti D, Kinkel WR, Manning EJ. “Normal-pressure” hydrocephalus. Relationship of clinical and radiographic findings to improvement following shunt surgery. Jama. 1976;235(5):510–512. doi: 10.1001/jama.235.5.510. [DOI] [PubMed] [Google Scholar]
- 30.Krauss JK, Regel JP, Droste DW, Orszagh M, Borremans JJ, Vach W. Movement disorders in adult hydrocephalus. Mov Disord. 1997;12(1):53–60. doi: 10.1002/mds.870120110. [DOI] [PubMed] [Google Scholar]
- 31.Curran T, Lang AE. Parkinsonian syndromes associated with hydrocephalus: case reports, a review of the literature, and pathophysiological hypotheses. Mov Disord. 1994;9(5):508–520. doi: 10.1002/mds.870090503. [DOI] [PubMed] [Google Scholar]
- 32.Meyer JS, Kitagawa Y, Tanahashi N, et al. Pathogenesis of normal-pressure hydrocephalus--preliminary observations. Surg Neurol. 1985;23(2):121–133. doi: 10.1016/0090-3019(85)90329-5. [DOI] [PubMed] [Google Scholar]
- 33.Bohnen NI, Minoshima S, Giordani B, Frey KA, Kuhl DE. Motor correlates of occipital glucose hypometabolism in Parkinson’s disease without dementia. Neurology. 1999;52(3):541–546. doi: 10.1212/wnl.52.3.541. [DOI] [PubMed] [Google Scholar]
- 34.Chan D, Fox NC, Scahill RI, et al. Patterns of temporal lobe atrophy in semantic dementia and Alzheimer’s disease. Ann Neurol. 2001;49(4):433–442. [PubMed] [Google Scholar]
- 35.Jobst KA, Smith AD, Szatmari M, et al. Rapidly progressing atrophy of medial temporal lobe in Alzheimer’s disease. Lancet. 1994;343(8901):829–830. doi: 10.1016/s0140-6736(94)92028-1. [DOI] [PubMed] [Google Scholar]
- 36.Streitberger KJ, Wiener E, Hoffmann J, et al. In vivo viscoelastic properties of the brain in normal pressure hydrocephalus. NMR in biomedicine. 2011;24(4):385–392. doi: 10.1002/nbm.1602. [DOI] [PubMed] [Google Scholar]
- 37.Iliff JJ, Lee H, Yu M, et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest. 2013;123(3):1299–1309. doi: 10.1172/JCI67677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iliff JJ, Nedergaard M. Is there a cerebral lymphatic system? Stroke. 2013;44(6 Suppl 1):S93–95. doi: 10.1161/STROKEAHA.112.678698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stephensen H, Tisell M, Wikkelso C. Intracranial pressure during wakefulness and sleep in 55 adult patients with chronic hydrocephalus. Neurosurgery. 2006;59(2):326–332. doi: 10.1227/01.NEU.0000223513.89586.9A. discussion 326–332. [DOI] [PubMed] [Google Scholar]
- 41.Leinonen V, Koivisto AM, Savolainen S, et al. Post-mortem findings in 10 patients with presumed normal-pressure hydrocephalus and review of the literature. Neuropathol Appl Neurobiol. 2012;38(1):72–86. doi: 10.1111/j.1365-2990.2011.01195.x. [DOI] [PubMed] [Google Scholar]
- 42.Sack I, Beierbach B, Wuerfel J, et al. The impact of aging and gender on brain viscoelasticity. Neuroimage. 2009;46(3):652–657. doi: 10.1016/j.neuroimage.2009.02.040. [DOI] [PubMed] [Google Scholar]
- 43.Schregel K, Wuerfel E, Garteiser P, et al. Demyelination reduces brain parenchymal stiffness quantified in vivo by magnetic resonance elastography. Proc Natl Acad Sci U S A. 2012;109(17):6650–6655. doi: 10.1073/pnas.1200151109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levine DN. The pathogenesis of normal pressure hydrocephalus: a theoretical analysis. Bull Math Biol. 1999;61(5):875–916. doi: 10.1006/bulm.1999.0116. [DOI] [PubMed] [Google Scholar]
- 45.Ertl-Wagner BB, Lienemann A, Reith W, Reiser MF. Demonstration of periventricular brain motion during a Valsalva maneuver: description of technique, evaluation in healthy volunteers and first results in hydrocephalic patients. Eur Radiol. 2001;11(10):1998–2003. doi: 10.1007/s003300100941. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Median corrected stiffness values by ROI