Abstract
Human tissues and cells from pre- and postnatal life were cultivated and studied for plasminogen activator activity. Cultures were obtained from kidney, renal blood vessels, ureter, bladder, lung, and heart. Local activator activity of cells was demonstrated by histochemical techniques. Activator released by cells into the supernatant culture media was assayed by fibrin plate techniques and was investigated for immunological identity using specific antisera to an activator of human origin, urokinase (UK).
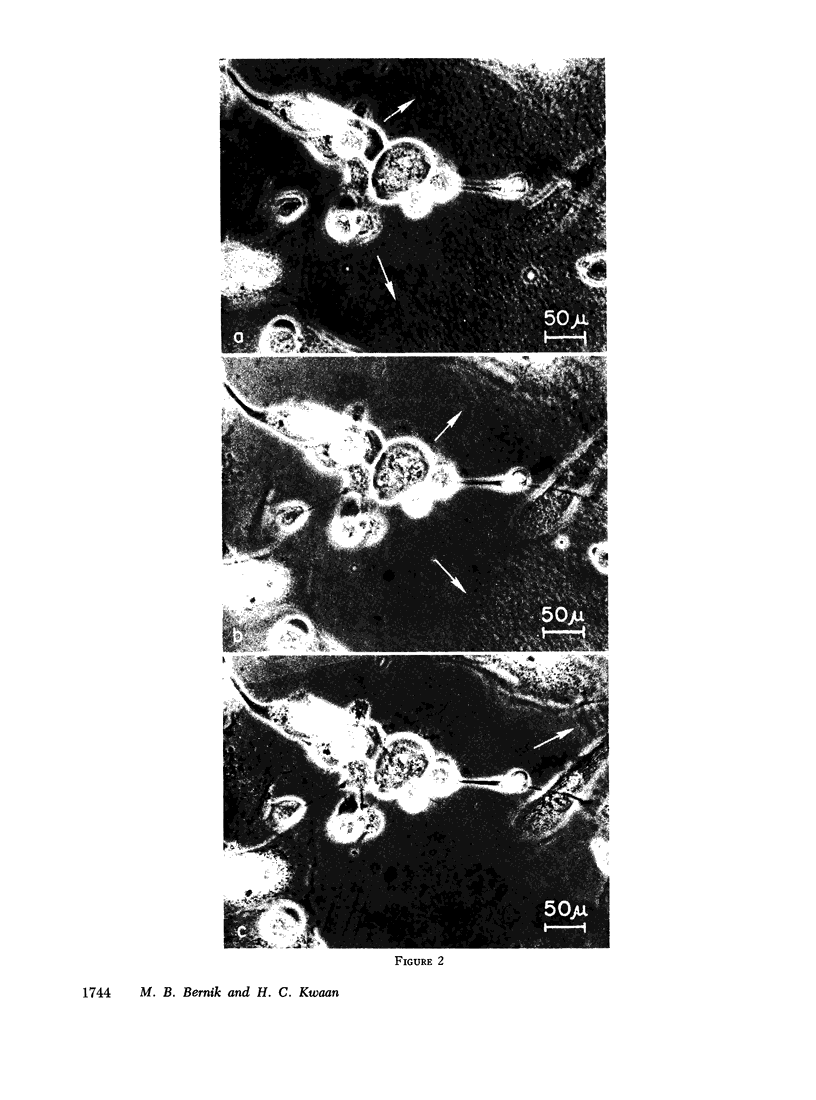
Plasminogen activator was produced in primary cultures where cells retain specific functions and generally reflect the enzyme pattern of the tissues of origin. Cells from fetal and adult sources were found to yield activator antigenically identical to UK, as well as activator activity which differed from that of UK in immunoassays and which may represent tissue type activator. Such activity was released after injury or death of cells while UK was produced in cultures containing live, metabolizing cells.
Primary cultures of kidney confirmed that this organ is a rich source of UK and demonstrated, in addition, that UK is produced from the early stages of gestation and in increasing amounts thereafter. However, primary cultures also demonstrated that the ability to produce UK is not limited to the kidney but is a function of cells which are distributed widely in body tissues. Thus, activator antigenically identical to UK accumulated progressively after many refeedings in culture supernates of fetal lung and ureter, as well as in supernates of renal blood vessels of adults. These findings indicate continuous formation of UK by the cultured cells and, furthermore, provide evidence of UK production in blood vessels. In cultures from other tissues, particularly those from fetal heart and adult lung and bladder, investigation of activator was hindered by inhibitory activity which accumulated in the supernates. Such activity was derived from cells in culture and was directed selectively against UK, indicating that inhibitor as well as UK are produced by cells of various organs of the body.
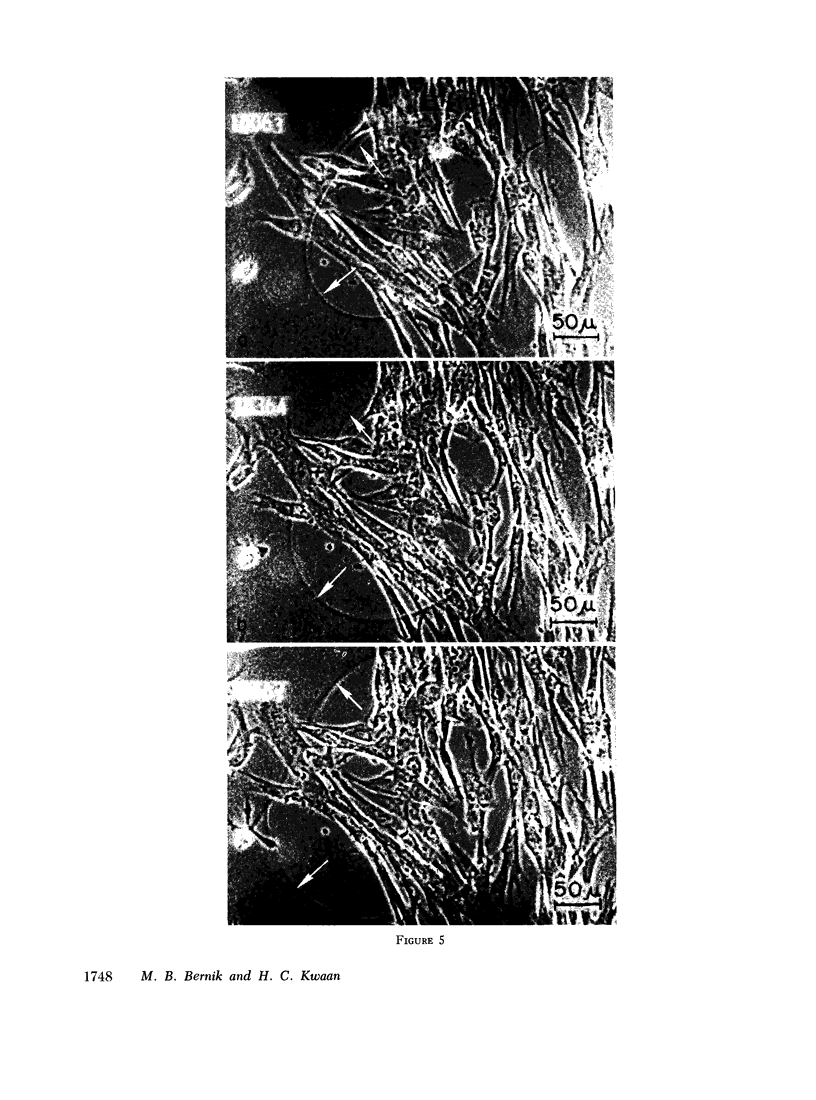
Plasminogen activator also was produced by serially propagated cells, diploid and heteroploid. However, only diploid cell lines retained activator activity of the original tissues and continued to produce activator antigenically identical to UK. In contrast, heteroploid line appeared to have lost the ability to form UK by yielded activator activity that differed from that of UK in immunoassays. Serially propagated cells thus provide an additional tool for in vitro study of plasminogen activator and may facilitate investigation of the fibrinolytic system in man.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBRUS C. M., WEINTRAUB D. H., NISWANDER K. R., AMBRUS J. L. STUDIES ON HYALINE MEMBRANE DISEASE. II. THE ONTOGENY OF THE FIBRINOLYSIN SYSTEM. Pediatrics. 1965 Jan;35:91–96. [PubMed] [Google Scholar]
- ASTRUP T., MULLERTZ S. The fibrin plate method for estimating fibrinolytic activity. Arch Biochem Biophys. 1952 Oct;40(2):346–351. doi: 10.1016/0003-9861(52)90121-5. [DOI] [PubMed] [Google Scholar]
- Astrup T. Tissue activators of plasminogen. Fed Proc. 1966 Jan-Feb;25(1):42–51. [PubMed] [Google Scholar]
- BACHMANN F., FLETCHER A. P., ALKJAERSIG N., SHERRY S. PARTIAL PURIFICATION AND PROPERTIES OF THE PLASMINOGEN ACTIVATOR FROM PIG HEART. Biochemistry. 1964 Oct;3:1578–1585. doi: 10.1021/bi00898a033. [DOI] [PubMed] [Google Scholar]
- BARNETT E. V., BARON S. An activator of plasminogen produced by cell culture. Proc Soc Exp Biol Med. 1959 Nov;102:308–311. doi: 10.3181/00379727-102-25229. [DOI] [PubMed] [Google Scholar]
- BRAKMAN P., ASTRUP T. SELECTIVE INHIBITION IN HUMAN PREGNANCY BLOOD OF UROKINASE INDUCED FIBRINOLYSIS. Scand J Clin Lab Invest. 1963;15:603–609. doi: 10.3109/00365516309051342. [DOI] [PubMed] [Google Scholar]
- BRAKMAN P. BOVINE FIBRINOGEN WITHOUT DETECTABLE PLASMINOGEN. Anal Biochem. 1965 Apr;11:149–152. doi: 10.1016/0003-2697(65)90055-2. [DOI] [PubMed] [Google Scholar]
- Bernik M. B., Kwaan H. C. Origin of fibrinolytic activity in cultures of the human kidney. J Lab Clin Med. 1967 Oct;70(4):650–661. [PubMed] [Google Scholar]
- CELANDER D. R., GUEST M. M. The biochemistry and physiology of urokinase. Am J Cardiol. 1960 Aug;6:409–419. doi: 10.1016/0002-9149(60)90333-7. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Nutrition needs of mammalian cells in tissue culture. Science. 1955 Sep 16;122(3168):501–514. doi: 10.1126/science.122.3168.501. [DOI] [PubMed] [Google Scholar]
- FEARNLEY G. R., REVILL R., TWEED J. M. Observations on the inactivation of fibrinolytic activity in shed blood. Clin Sci. 1952 Aug;11(3):309–314. [PubMed] [Google Scholar]
- HAYFLICK L., MOORHEAD P. S. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961 Dec;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- HOLEMANS R. ENHANCEMENT OF FIBRINOLYSIS IN THE DOG BY INJECTION OF VASOACTIVE DRUGS. Am J Physiol. 1965 Mar;208:511–520. doi: 10.1152/ajplegacy.1965.208.3.511. [DOI] [PubMed] [Google Scholar]
- Holemans R., Johnston J. G., Reddick R. L. Release of plasminogen activator by the isolated perfused dog kidney. Nature. 1965 Oct 16;208(5007):291–292. doi: 10.1038/208291a0. [DOI] [PubMed] [Google Scholar]
- KWAAN H. C., LO R., MCFADZEAN A. J. On the production of plasma fibrinolytic activity within veins. Clin Sci. 1957 May;16(2):241–253. [PubMed] [Google Scholar]
- Kawano T., Morimoto K., Uemura Y. Urokinase inhibitor in human placenta. Nature. 1968 Jan 20;217(5125):253–254. doi: 10.1038/217253a0. [DOI] [PubMed] [Google Scholar]
- Kok P., Astrup T. Isolation and purification of a tissue plasminogen activator and its comparison with urokinase. Biochemistry. 1969 Jan;8(1):79–86. doi: 10.1021/bi00829a013. [DOI] [PubMed] [Google Scholar]
- Kucinski C. S., Fletcher A. P., Sherry S. Effect of urokinase antiserum on plasminogen activators: demonstration of immunologic dissimilarity between plasma plasminogen activator and urokinase. J Clin Invest. 1968 Jun;47(6):1238–1253. doi: 10.1172/JCI105816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwaan H. C., Astrup T. Demonstration of cellular fibrinolytic activity by the histochemical fibrin slide technique. Lab Invest. 1967 Aug;17(2):140–145. [PubMed] [Google Scholar]
- LASSEN M. Heat denaturation of plasminogen in the fibrin plate method. Acta Physiol Scand. 1953 Feb 28;27(4):371–376. doi: 10.1111/j.1748-1716.1953.tb00951.x. [DOI] [PubMed] [Google Scholar]
- LASSEN M. The estimation of fibrinolytic components by means of the lysis time method. Scand J Clin Lab Invest. 1958;10(4):384–389. doi: 10.3109/00365515809051241. [DOI] [PubMed] [Google Scholar]
- MULLERTZ S. Activation of plasminogen. Ann N Y Acad Sci. 1957 Aug 30;68(1):38–51. doi: 10.1111/j.1749-6632.1957.tb42611.x. [DOI] [PubMed] [Google Scholar]
- Norman P. S. Antiplasmins. Fed Proc. 1966 Jan-Feb;25(1):63–67. [PubMed] [Google Scholar]
- PAINTER R. H., CHARLES A. F. Characterization of a soluble plasminogen activator from kidney cell cultures. Am J Physiol. 1962 Jun;202:1125–1130. doi: 10.1152/ajplegacy.1962.202.6.1125. [DOI] [PubMed] [Google Scholar]
- ROSE G. G., POMERAT C. M., SHINDLER T. O., TRUNNELL J. B. A cellophane-strip technique for culturing tissue in multipurpose culture chambers. J Biophys Biochem Cytol. 1958 Nov 25;4(6):761–764. doi: 10.1083/jcb.4.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SGOURIS J. T., INMAN J. K., McCALL K. B., HYNDMAN L. A., ANDERSON H. D. The preparation of human fibrinolysin (plasmin). Vox Sang. 1960 Jul;5:357–376. doi: 10.1111/j.1423-0410.1960.tb03750.x. [DOI] [PubMed] [Google Scholar]
- SHULMAN N. R. Studies on the inhibition of proteolytic enzymes by serum. II. Demonstration that separate proteolytic inhibitors exist in serum; their distinctive properties and the specificity of their action. J Exp Med. 1952 Jun;95(6):593–603. doi: 10.1084/jem.95.6.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White W. F., Barlow G. H., Mozen M. M. The isolation and characterization of plasminogen activators (urokinase) from human urine. Biochemistry. 1966 Jul;5(7):2160–2169. doi: 10.1021/bi00871a003. [DOI] [PubMed] [Google Scholar]