Abstract
The first clinical trial aimed at targeting fundamental processes of aging will soon be launched (TAME: Targeting Aging with Metformin). In its wake is a robust pipeline of therapeutic interventions that have been demonstrated to extend lifespan or healthspan of preclinical models, including rapalogs, antioxidants, anti-inflammatory agents, and senolytics. This ensures that if the TAME trial is successful, numerous additional clinical trials are apt to follow. But a significant impediment to these trials remains the question of what endpoints should be measured? The design of the TAME trial very cleverly skirts around this based on the fact that there are decades of data on metformin in humans, providing unequaled clarity of what endpoints are most likely to yield a positive outcome. But for a new chemical entity, knowing what endpoints to measure remains a formidable challenge. For economy's sake, and to achieve results in a reasonable time frame, surrogate markers of lifespan and healthy aging are desperately needed. This review provides a comprehensive analysis of molecular endpoints that are currently being used as indices of age-related phenomena (e.g., morbidity, frailty, mortality) and proposes an approach for validating and prioritizing these endpoints.
INTRODUCTION
Molecular pathology can be defined as the study and diagnosis of disease through the examination of molecules within organs, tissues, or bodily fluids. The Geropathology Research Network established a Molecular Pathology Working Group to help define sets of sentinel biomarkers that could be incorporated into preclinical and clinical aging intervention studies to increase the relevance, productivity, efficiency, and economy of these studies. The group recently met at the Geropathology Research Network Symposium in Seattle, Washington with the goals of i) developing a plan to increase the consistency of communicating molecular pathology endpoints in preclinical animal models of aging (mice) and ii) to identify surrogate markers of lifespan, healthspan, and frailty, which could significantly shorten interventional studies and make the translation of preclinical interventions targeting fundamental aging processes into humans feasible. The group will interact closely with two other working groups of the Geropathology Research Network. First, Molecular Pathology will work with the Anatomical Pathology Working Group in order to identify molecular endpoints that correspond with the histological grade of age-related lesions. Second, working with the Translational Working Group, it will be important to ensure that the molecular endpoints selected are translatable (e.g., measured in a tissue or fluid readily accessed in humans).
There are numerous challenges to implementing the goals of the Molecular Pathology Working Group. First, there is currently no consensus about what is the best outcome to measure when trying to evaluate a new intervention that targets basic aging mechanisms. Options include measuring the impact of a therapeutic on lifespan, frailty, age-at onset, or severity of an age-related disease or handful of diseases, or healthspan, defined as the period of overall health and function in old age. Second, molecular endpoints are by their nature almost exclusively specific to the study design. For example, very different molecular endpoints would be selected to inform about metabolic changes linked to end-of-life, senescent cell burden linked to frailty, diagnosis or staging of an age-related disease, or validating the mechanism of a particular intervention. Thus, until the first challenge is met, achieving consensus on molecular endpoints for aging studies is impossible. Third, there is no consensus about what causes aging. Should we be measuring changes in reactive oxygen species, DNA damage, mitochondrial function, or autophagic flux? Fourth, there is a lack of data about “translatability”. For example, if a therapeutic that targets aging processes reduces the burden of senescent cells in mice, will it do so in humans? And if so, how will we measure that? There is no lack of molecular endpoints that could potentially be tested and implemented, but lacking a unifying mechanism of aging and study design for intervention testing, the real challenge comes in prioritizing which molecular endpoints to pursue and validate.
Nevertheless, the overwhelming value of molecular endpoints for aging studies should drive progress despite the lack of a clear path forward. Molecular endpoints have the potential to: 1) reveal or validate the mechanism of action by which an intervention extends lifespan. For example, detecting reduced pS6K1 implicates reduced mTORC1 activity. This has the potential to inform about mechanisms of aging; 2) reveal a healthspan extension when no lifespan extension is seen. For example, if a therapeutic intervention is tested to see if it extends the lifespan of mice but fails, the inclusion of molecular endpoints might reveal improved health, for example using calcein labeling to measure bone mineralization. In this case, molecular endpoints can expand the range of outcomes measured in interventional testing; 3) help identify some other valuable phenotype or outcome that points to a clinical application. For example, measuring fasting glucose might reveal preservation of functional insulin-producing cells, indicating that a particular intervention, while having no effect on lifespan, might have applications in diabetes; 4) contribute to defining mechanisms of age-related diseases. For example, if an intervention attenuates senescence-associated secretory phenotype (SASP) factors in the blood and delays cognitive decline, it suggests that senescent cells are a driving force in age-related disease.
In all likelihood, multiple molecular endpoints will be needed for any one clinical design. Pharmacokinetic biomarkers are required to demonstrate that a particular therapeutic intervention is measurable at the desired site of action. For example, a therapeutic aimed at improving mild cognitive impairment should be detected in the cerebral spinal fluid proving penetrance of the blood brain barrier. Pharmacodynamic biomarkers are required to provide direct evidence that a drug elicits a specific pharmacological effect. For example, reduced phosphorylation of S6K1 supports inhibition of mTOR. Aging biomarkers are required to determine if fundamental mechanisms of aging are being targeted. For example, reduced p16 expression supports the notion that the burden of senescent cells is reduced. Finally, and most challenging, surrogate aging biomarkers offer the potential to predict a subject's outcome (improved function, extended survival, or arrest of age-related disease). For example, could expression of factors identified in heterochronic parabiosis as being anti-geronic predict these extended outcomes? Below is a summary of molecular endpoints currently being used or considered in preclinical aging studies.
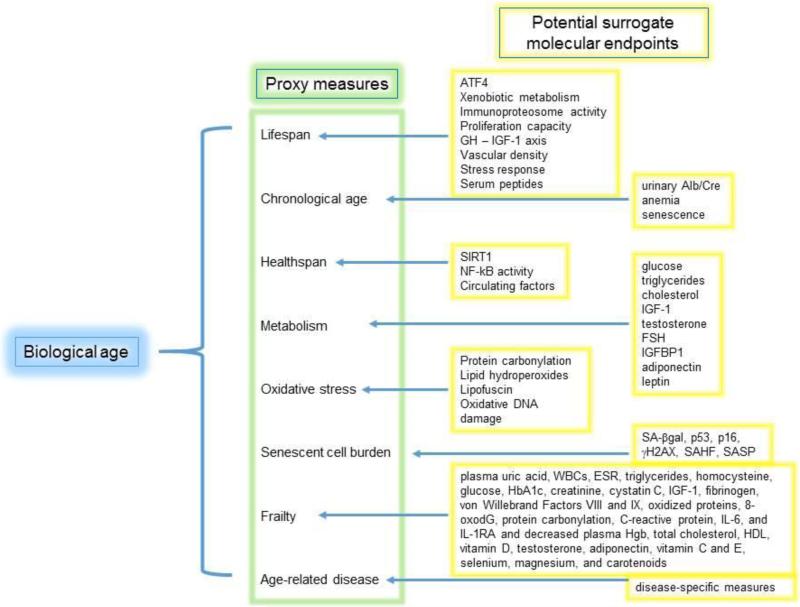
It is important to emphasize that the ultimate goal is to identify measures of biological age. Currently, we have no means to measure biological age. Thus proxy measures of biological age are used such as chronological age, lifespan, frailty, or senescent cell burden (Figure 1). We organized this review into sections based on these proxy measures. We summarize surrogate molecular measures of these proxies that if proven to be a good surrogate would afford economy in terms of cost and time. Not all molecular endpoints inform about all of the proxy measures. But perhaps a subset will be surrogate markers for multiple proxies, and these collectively will help measure biological age.
Figure 1.
Schematic diagram of the hierarchy of measures of aging. The ultimate goal is to measure biological age. Since we currently have no methods to measure this, proxy measures of biological age are used in preclinical work. The goal of the Molecular Pathology Group in the Geropathology Network is to identify molecular endpoints that are surrogate markers of the proxy measures, and ultimately biological aging, in order to accelerate and economize preclinical research in aging.
MOLECULAR ENDPOINTS THAT CORRELATE WITH LIFESPAN
The following biomarkers are increased in multiple examples of lifespan extension. They offer the potential that, if measured in a setting of intervention testing, they could identify therapeutics that will (eventually) extend lifespan. The strength of these biomarkers is that they correlate with lifespan regardless of the method used to elicit lifespan extension. Furthermore, extension of lifespan is more rigorous an outcome than lifespan shortening. In each heading, it is indicated how changes in expression were detected.
Increased expression of ATF4 & downstream effectors (mRNA and protein)
ATF4 is a transcription factor that senses deficits in protein translation typically caused by ER stress or amino acid starvation, and responds by activating a group of genes that promote amino acid transport and combating oxidative stress (Harding et al., 2003; Su et al., 2009). ATF4 was first implicated in aging by the observation that lifespan extension in yeast, caused by nutrient deprivation, reduced TOR activity or mutations in the 60S ribosome, requires the yeast homologue of ATF4, Gcn4 (Steffen et al., 2008). Expression of ATF4 and several of its target genes are increased in the liver of two long-lived strains of mice, Snell dwarf and PAPP-A mice (Li and Miller, 2015). Likewise, expression of ATF4 and downstream effectors is increased in mice in which lifespan has been extended by several interventions including acarbose, rapamycin, caloric restriction, methionine restriction, or litter crowding (Li et al., 2014). This provides strong evidence that the stress response transcription factor ATF4 is upregulated when mammalian lifespan is extended by genetic, dietary manipulation, or even drug interventions.
Increased expression of enzymes involved in xenobiotic metabolism (mRNA)
Gene expression profiling in liver of several long-lived strains of mice (Ames and Snell dwarf, Ghr−/−, and GhrhrLit/Lit) revealed strong up-regulation of genes involved in xenobiotic metabolism (Amador-Noguez et al., 2004; Swindell, 2007). Similarly, xenobiotic metabolizing enzymes are up-regulated in multiple tissues when murine lifespan is extended by caloric restriction (Swindell, 2008). Numerous xenobiotic metabolizing enzymes are also upregulated in response to litter crowding or rapamycin treatment (Steinbaugh et al., 2012). Thus, like ATF4, increased expression of these xenobiotic metabolizing enzymes corresponds with lifespan extension whether elicited by genes, diet, or therapeutic intervention.
Indices of increased immunoproteosome activity (mRNA and protein)
The immunoproteosome is a system of protein degradation that is critical for the turnover of oxidized, aggregated, or misfolded proteins and regulation of the T cell response to antigens. Catalytic activity of the proteosome is 50% lower in skeletal muscle of aged compared to young rats (Ferrington et al., 2005). Across 14 primate species, expression of immunoproteosome components in dermal fibroblasts significantly correlates with lifespan (Pickering et al., 2015). Upregulation of immunoproteosome components also occurs in the liver of long-lived Snell dwarf mice and normal mice in which lifespan extension is induced by chronic treatment with 17-α–estradiol, nordihydroguaiaretic acid, and rapamycin (Pickering et al., 2015). Thus, in multiple species, expression of immunoproteosome components correlates with lifespan extension, including that induced by drug intervention.
Markers of proliferation
Proliferation of cells in vivo declines significantly with age in multiple tissues of mice, including liver, kidney, and pancreas (Wolf and Pendergrass, 1999). This leads to a decline in regenerative capacity and the ability to maintain tissue homeostasis (Yun, 2015). Prolonged caloric restriction attenuates this age-related decline in cell proliferation (Li et al., 1997; Wolf et al., 1995). Ex vivo, proliferation of fibroblasts isolated from birds correlates with species lifespan (Harper et al., 2011). Similarly, fibroblasts from long-lived Snell dwarf mice maintain their proliferative capacity ex vivo longer than fibroblasts from normal mice (Maynard and Miller, 2006). Collectively, these studies suggest that the capacity of cells to proliferate correlates with longevity or lifespan extension. This may be at least in part why inhibition of mTOR extends lifespan, by blocking mitogen signaling and thereby preserving cell proliferation capacity (Perl, 2015).
Insulin-like growth factor 1 (IGF1) and growth hormone (GH)
Circulating levels of IGF-1 and GH decline with age in humans and rodents (reviewed in (Sonntag et al., 1999)). Caloric restriction, which extends lifespan in numerous species, increases GH secretion, but causes a decline in plasma IGF-1 (Sonntag et al., 1999). Genetic depletion of the growth hormone receptor or IGF-1 extends the lifespan of numerous species (Guevara-Aguirre et al., 2011; Laron, 2008) and decreases senescent cell burden (Stout et al., 2016), while IGF-1 can induce cellular senescence (Tran et al., 2014). Thus suppression of the GH-IGF-1 axis is thought to be protective in terms of staving off some age-related pathologies, in particular cancer, but comes at a price contributing to, for example, age-related loss of skeletal muscle mass and memory, as well as increased risk of cardiovascular disease (Carlzon et al., 2014; Sharples et al., 2015; Sonntag et al., 2000; Sonntag et al., 1999). Nevertheless, monitoring plasma IGF-1 levels in individuals before and after therapeutic intervention may be useful for identifying dietary restriction mimetics that extend lifespan.
Vascular density
Cerebral blood flow and vascular density decline with age in mammals (Lynch et al., 1999). In rats and mice, caloric restriction, which extends lifespan, improves vascular function in the brain (Guo et al., 2015; Lin et al., 2015; Lynch et al., 1999). This is accompanied by improved long-term memory (Guo et al., 2015). Thus immunostaining for endothelial cell markers that highlight preservation of vascular density might be used as a surrogate marker to identify drugs that extend lifespan.
Stress response genes
Genetic studies have revealed numerous genes that when overexpressed extend lifespan of a variety of species. These gene products are therefore potential surrogate markers of lifespan. For example, sirtuin protein activity is strongly linked to replicative lifespan and age-related diseases (Longo and Kennedy, 2006). Overexpression of SIRT1 deacetylase in neurons extends the lifespan of mice (Conti et al., 2015; Longo and Kennedy, 2006), while systemic overexpression of SIRT6 extends the lifespan of male mice (Kanfi et al., 2012). Overexpression of the anti-oxidant catalase extends the lifespan of mice (Schriner et al., 2005). Overexpression of klotho extends life- and healthspan, while genetic depletion causes accelerated aging (Dubal et al., 2014). Thus increased expression or sustained expression of any of these proteins might be used to predict lifespan extension.
Serum peptides (proteins)
Serum peptides make ideal potential surrogate aging biomarkers due to ready access and low risk of sample acquisition. Measurement of 15 peptides in the serum of humans revealed an association between levels of four of them and healthy aging (centenarians vs. healthy 70-80 year olds) (Sanchis-Gomar et al., 2015). Chemerin (an adipokine), FGF21 (a regulator of glucose homeostasis), PEDF (angiogenesis), and fetuin-A (calcium phosphate homeostasis) were significantly lower in serum of centenarians, whereas FGF19 (a regulator of bile acids), sFlt1 (angiogenesis), and osteoprotegerin (bone metabolism) were significantly higher (Sanchis-Gomar et al., 2015). In particular, chemerin, FGF19, FGF21, and fetuin-A all associated with successful aging regardless of sex.
MOLECULAR ENDPOINTS THAT CORRELATE WITH CHRONOLOGICAL AGE
The following biomarkers are associated with advanced age. They offer the potential that, if measured in a setting of intervention testing, they could identify therapeutics that slow aging processes or extend youthfulness.
Urinary albumin/creatinine ratio
Biomarkers that can be measured in urine are highly translatable. The urinary albumin/creatinine ratio (ACR) is an indicator of kidney damage, which can arise as a consequence of numerous age-related diseases, including cardiovascular disease and diabetes (Levey and Coresh, 2012). ACR is a diagnostic criterion for chronic kidney disease, for which aging is a key risk factor. ACR increases with age in mice (Tsaih et al., 2010). Thus ACR could be used as a biomarker to determine if an intervention slows aging processes or the progression of multiple age-related diseases.
Indices of anemia
Anemia is common in aged humans and is associated with increased morbidity and mortality. Hemoglobin, hematocrit, and red blood cell count are significantly lower in aged C57Bl/6 mice compared to adult mice (28 vs. 6 mth) (Guo et al., 2014). Erythropoietin (epo) is significantly elevated, illustrating resistance to this hormone (Guo et al., 2014). Measuring Hgb, Hct, RBC, and/or epo could be used as a biomarker to determine if an intervention slows or prevents an age-related condition of uncertain etiology but significant functional impact.
Markers of cellular senescence
p16INK4a is one of several molecular markers of senescent cells (see below). Genetic or pharmacological depletion of p16+ cells in mice delays age-related dysfunction (Baker et al., 2016; Baker et al., 2011; Roos et al., 2016; Xu et al., 2015; Zhu et al., 2015a), but this needs to be verified in a mouse colony in which the control mice do not have short lifespans. mRNA levels of p16INK4a in peripheral blood T lymphocytes increases exponentially with chronological age in humans (Liu et al., 2009). Expression of p16 mRNA also increases in multiple tissues of mice with age (uterus > cecum > kidney > ovary = liver > duodenum) and this is attenuated with caloric restriction (Krishnamurthy et al., 2004). A second marker of cellular senescence, enzymatic activity of senescence-associated β-galactosidase, increases with age in human skin (Dimri et al., 1995) and fat (Tchkonia et al., 2010), rat kidney (Melk et al., 2003), and mouse kidney (Berkenkamp et al., 2014), heart (Baker et al., 2016), and fat (Xu et al., 2015) and frequently correlates with p16INK4a expression. Markers of senescence are well-established endpoints for testing anti-aging strategies in murine models (Baker et al., 2016; Baker et al., 2011; Roos et al., 2016; Xu et al., 2015; Zhu et al., 2015a).
MOLECULAR ENDPOINTS THAT CORRELATE WITH HEALTHSPAN
The following biomarkers are associated with a health dividend. They offer the potential that, if measured in a setting of intervention testing, could identify therapeutics that extend the period of health or compress the period of morbidity in old age.
SIRT1 or NF-κB
Genetic studies have revealed several genes that can extend the healthspan of mice while not affecting median or maximal lifespan. For example, overexpression of SIRT1 improves glucose tolerance, osteoporosis, wound healing, and gait and delays cancer (Herranz et al., 2010). SIRT1 overexpression only in adipose tissue delays onset of diabetes (Xu et al., 2013). Genetic depletion of NF-κB delays hepatic steatosis, glomerulosclerosis, neurodegeneration, and osteoporosis in a murine model of progeria (Tilstra et al., 2012). Measuring expression of these or other known molecular targets of healthy aging interventions (like mTOR) can be used to identify pathways targeted by novel interventions as well as support claims that healthspan has been extended.
Circulating factors identified by parabiosis
Heterochronic parabiosis experiments have revealed a number of circulating factors that impact health of aged mice. Activators of Wnt signaling in serum of old animals impair muscle regeneration (Brack et al., 2007). GDF-11 was identified as a circulating factor that declines with age (Loffredo et al., 2013). Restoring levels of GDF11 to youthful levels reversed cardiac myopathy in old mice. However, these data have been questioned (Kaiser, 2015). Circulating levels of oxytocin also decline with age, while administration of the hormone improves muscle regeneration following cardiotoxin injury (Elabd et al., 2014). CCL11 was identified as a serum chemokine that increases with aging (Villeda et al., 2011). Suppression of circulating levels of CCL11 through heterochronic parabiosis or administration of plasma from young mice improved learning and memory, while administration of CCL11 caused functional loss. Measuring expression of any of these factors in serum could potentially provide information about an organism's health or function.
MOLECULAR ENDPOINTS THAT INFORM ABOUT METABOLISM
There are a number of standard serological tests that are used clinically to evaluate the metabolic health of patients. These include glucose, triglycerides, cholesterol, IGF-1, testosterone, follicular stimulating hormone, IGFBP1, adiponectin, and leptin. With age-related metabolic changes, levels of these molecules change as well (Adamczak et al., 2005; Rutanen et al., 1993; Sanchez-Rodriguez et al., 2000). Thus measuring a panel of these endpoints could help identify interventions that maintain a youthful metabolism, for example caloric restriction (Mattison et al., 2012).
MOLECULAR ENDPOINTS THAT REVEAL OXIDATIVE STRESS
Oxidative stress is implicated in contributing to aging (Ziegler et al., 2015). There are numerous biomarkers that can be used to detect oxidative stress. Reactive oxygen species (ROS) can modify proteins, lipids, or nucleic acids. Thus quantifying protein carbonylation, lipid hydroperoxides, lipofuscin, and oxidative DNA lesions such as 8-oxodG and cyclopurine adducts can be used to draw conclusions about the relative level of oxidative stress. Other indirect measures of oxidative stress include cellular senescence, apoptosis, antioxidant levels, or expression of stress response genes (HIF1α, NRF2, p53, NF-κB). These, in combination with direct measures of oxidative damage, can be used to emphasize the pathological consequences of increased ROS.
MOLECULAR ENDPOINTS THAT DETECT SENESCENT CELLS
Senescence is a cell fate triggered by DNA damage, oncogenic stress, telomere shortening, and altered mitochondrial function (van Deursen, 2014; Ziegler et al., 2015). Senescent cells accumulate with age in a variety of mouse (Zhu et al., 2015a), primate (Jeyapalan et al., 2007), and human (Dimri et al., 1995; Krishnamurthy et al., 2004) tissues. Senescent cell abundance correlates with lifespan in mice (Stout et al., 2016). Selective elimination of senescent cells increases the health and perhaps median lifespan of mice (Baker et al., 2016; Baker et al., 2011; Roos et al., 2016; Xu et al., 2015; Zhu et al., 2015a), implicating senescent cells as a driver of aging. Thus there is a huge impetus to measure the burden of senescent cells in an organism to determine biological (vs. chronological age) and to quantify the impact of anti-aging therapeutics. Unfortunately, there is no marker unique to senescent cells. Typically two or more of the following are measured to infer a state of senescence.
Senescence-associated β-galactosidase
β-galactosidase is an enzyme that hydrolyzes β-galactosides into monosaccharides. Senescence-associated β-galactosidase (SA-βgal) is galactosidase activity detected in the lysosomal fraction of cells at a low pH. SA-βgal activity increases with aging in the lysosomes and is detected by a colorometric assay using the synthetic substrate X-gal (Dimri et al., 1995).
p53
p53 is a transcription factor activated in response to genotoxic stress. p53 arrests the cell cycle at the G1/S transition, activates DNA repair, and determines cell fate (cellular senescence or apoptosis) if damage is sufficiently excessive or irreparable to cause persistent DNA damage signaling (Qian and Chen, 2013). The p53 protein is stabilized under conditions of stress. Thus the best way to measure it is by immunoblot or immunofluorescence.
p16
Like p53, p16INK4a is required for oncogene-induced senescence (Lin et al., 1998). p16 is a cyclin-dependent kinase inhibitor that prevents progression of the cell cycle from G1 to S phase. p16INK4a expression increases in multiple tissues of mice as they age and this is attenuated by caloric restriction (Krishnamurthy et al., 2004). Similarly, expression of p16INK4a in peripheral blood mononuclear cells correlates with chronologic age (Liu et al., 2009). Ablation of p16+ cells in mice extends healthspan, strongly implicating p16 in driving senescence and aging (Baker et al., 2016; Baker et al., 2011; Roos et al., 2016; Xu et al., 2015; Zhu et al., 2015a). Importantly, in human samples, p16 can be measured by immunodetection methods. Currently, however, antibodies against murine p16 are not specific. Thus qRT-PCR or p16 FISH must be used to detect p16INK4a mRNA.
p21
p21 protein is a cyclin-dependent kinase inhibitor that also regulates the G1 to S transition of the cell cycle. It is a downstream effector of the p53 transcription factor. It is required for senescence of murine fibroblasts (Romanov et al., 2010). p21 has been implicated in both pro- and anti-senescence or aging activities (Bedelbaeva et al., 2010; Dulic et al., 2000). Thus measuring p21 expression can only support information about other senescence biomarkers. p21 levels can be measured accurately by qRT-PCR or immunodetection.
Gamma-H2AX
γ-H2AX refers to the X isoform of histone H2A that is phosphorylated at K139. Histone H2AX resides in close contact with DNA as part of the nucleosome and is phosphorylated by ATM early in the response to detection of double-strand breaks or replication stress, in order to mark the area of damage in the chromatin. The phosphorylated form of H2AX can be detected with antibodies as nuclear foci. Accumulation of γ-H2AX foci is seen in nuclei of late passage cells in culture, in cells taken from aged donors, and in tissues of aged mice (Sedelnikova et al., 2004; Wang et al., 2009). γ-H2AX appears to play a role in the maintenance of senescence, as genetic depletion of H2AX suppresses DNA damage-induced growth arrest and secretion of the SASP factor IL-6 (Turinetto and Giachino, 2015) (see below).
Senescence-associated heterochromatin foci (SAHF)
SAHF are domains of condensed chromatin that suppress expression of genes that drive cell proliferation (Narita et al., 2003). These foci appear in human fibroblasts driven to senescence via serial passaging or expression of an oncogene (Narita et al., 2003). SAHF are thought to be important for maintaining the irreversible cell cycle arrest characteristic of senescent but not quiescent cells (Zhang et al., 2007). SAHF formation occurs spontaneously with aging. SAHF are detected by a combination of DAPI to fluorescently label nucleic acid and immunofluorescence detection of several heterochromatin markers, including HP1 and di- or tri-methylated histone H3 (Aird and Zhang, 2013).
Senescence-associated secretory phenotype (SASP)
Senescent cells have a complex secretory phenotype (Coppe et al., 2008). The SASP includes many pro-inflammatory cytokines, chemokines, growth factors, and proteases that have the potential to cause or exacerbate age-related pathology, both degenerative and hyperplastic. Senescence-associated secretory phenotype (SASP) components can include IL-6, IL-8, PAI-1, IL-1α, IL-1β, TNF-α, IGF, IGFBP3, 5 & 7, TGF-β, VEGF, MMPs (Kuilman and Peeper, 2009), and HMGB1 (Davalos et al., 2013). The SASP may be the main driver of age-related inflammation, at least in adipose tissue (Zhu et al., 2015b). SASP components can be measured by qRT-PCR or ELISA (Tchkonia et al., 2013). CXCR2 is the receptor for IL-8. It is implicated in maintenance of senescence and the SASP (Acosta et al., 2008), thus might act as a surrogate marker for the SASP.
MOLECULAR ENDPOINTS THAT CORRELATE WITH FRAILTY
IL-6 is a cytokine that can be both pro- and anti-inflammatory, impacting lipid metabolism, insulin resistance, mitochondrial function, vascular health, and the neuroendocrine system (Hunter and Jones, 2015). It is expressed at low levels but is induced by infection, trauma, or stress. IL-6 levels in tissue and serum increase with age and are linked to numerous age-related diseases, including osteoporosis, Alzheimer's disease, lymphoproliferative disorders, sarcopenia, anemia, and frailty (Ershler and Keller, 2000). Older individuals with high titers of CMV IgG and plasma IL-6 are at greater risk of frailty (Wang and Casolaro, 2014). Functional decline in humans is associated with increased plasma uric acid, WBCs, ESR, triglycerides, homocysteine, glucose, HbA1c, creatinine, cystatin C, IGF-1, fibrinogen, von Willebrand Factors VIII and IX, oxidized proteins, 8-oxodG, protein carbonylation, C-reactive protein, IL-6, and IL-1RA and decreased plasma Hgb, total cholesterol, HDL, vitamin D, testosterone, adiponectin, vitamin C and E, selenium, magnesium, and carotenoids (Lippi et al., 2014). Many of these factors increase with age in mice as well (Spaulding et al., 1997; Yamato et al., 2014).
MOLECULAR ENDPOINTS THAT CORRELATE WITH AGE-RELATED DISEASE
Fasting serum glucose levels are used to diagnose diabetes. Likewise, a standard clinical chemistry panel reveals information about pancreatic, liver, kidney, and heart function. Urine albumin/creatinine ratio is used to diagnose chronic kidney disease. Heparin-binding EGF is produced in cartilage, drives chondrocyte catabolic activities, and has potential as a biomarker in osteoarthritis (Long et al., 2015). Reduced proteoglycans, aggrecan, and glycosaminoglycans in discs is diagnostic of age-related intervertebral disc degeneration (Vo et al., 2013). Circulating levels of the hormone dehydroepiandosterone (DHEA and its sulfate ester) decline with aging and there is a strong inverse association between DHEA(S) levels and risk of cardiovascular disease (Mannic et al., 2015). Senescent cells (identified as described above) accumulate in the central nervous system with aging and correlate with neurodegeneration (Chinta et al., 2015). Therefore, a combination of several common markers of age-related disease might be used to demonstrate extension of healthspan or effects of a therapeutic intervention targeting fundamental aging mechanisms.
“OMICS” ENDPOINTS FOR REVEALING UNDERLYING MOLECULAR MECHANISMS
As our capacity to produce large data sets inexpensively continues to increase, it is not impossible to imagine using unbiased approaches for identifying new molecular endpoints that correspond with increased health- or lifespan. The secretome, factors released from cells that can be detected in the urine or blood are most translatable. For tissues readily biopsied in humans, like peripheral blood mononuclear cells, skin, or skeletal muscle, it is also possible to measure the transcriptome (changes in expression of mRNA, miRNA, rRNA, small RNA, or non-coding RNAs), the proteome (changes in protein expression or post-translational modification), the metabolome (lipids, signaling molecules or intermediates of respiration, glucose, amino acid, or nucleic acid metabolism), the genome (size, sequence, or organization of the nuclear or mitochondrial genome), or epigenome (changes in or modification of the genome or nucleosome components). Ideally, an unbiased omics approach could ultimately lead to a panel of proteins or metabolites that consistently change whether health- and lifespan is extended by genetics, diet, or therapeutic interventions.
SUMMARY/CONCLUSIONS/HOW TO MOVE FORWARD
The diagnosis of disease often requires the measurement of molecule(s) within organs, tissues, or bodily fluids. More accurate diagnosis is afforded when morphologic changes in tissues (anatomic pathology) are combined with molecular testing. As the FDA is considering treating aging as a condition that can be therapeutically targeted, it becomes imperative to define the morphological and molecular changes that occur with aging more accurately. The objective of the Geropathology Network is to arrive at a refined set of sentinel biomarkers of molecular and anatomic pathology that could be incorporated into preclinical and clinical aging intervention studies to increase the relevance, productivity, efficiency, and economy of these studies. Imperative steps to achieve our objective include the following. First, we need to increase the consistency of communicating molecular and anatomical pathology endpoints in preclinical animal models such as mice. Second, developing standardized protocols for measuring molecular pathology endpoints is absolutely essential for developing an FDA Investigational New Drug application. Last but not least, the most valuable molecular biomarkers will be those that can readily be measured in humans, i.e., in accessible fluids or tissues where risk to the patient is minimized during sampling. To tackle this objective, the Molecular Pathology Working Group will interact with the Anatomical Pathology Working Group to identify molecular endpoints that correspond with the grade of a histological lesion and with the Translational Working Group to ensure that the molecular endpoints selected are translatable. By integrating input from each group, surrogate markers of lifespan and molecular markers of frailty can be identified, which could significantly shorten interventional studies and make translation of preclinical studies to humans possible.
Highlights.
Designing clinical trials against fundamental processes of aging is challenging
There is no measure of biological age, thus proxy measures are used, e.g., lifespan
Surrogate markers are needed to reduce the length and cost of trials targeting aging
ACKNOWLEDGEMENTS
L.J.N. is supported by NIH/NIA P01 AG043376 and a sponsored research agreement from Aldabra Biosciences. J.L.K is supported by NIH AG13925, AG044396, and DK50456, the Connor Group, the Noaber, Ellison, and Glenn Foundations, and the American Federation for Aging Research. W.L. is supported by NIH R24 AG047115.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- Acosta JC, O'Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Fumagalli M, Da Costa M, Brown C, Popov N, Takatsu Y, Melamed J, d'Adda di Fagagna F, Bernard D, Hernando E, Gil J. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–18. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- Adamczak M, Rzepka E, Chudek J, Wiecek A. Ageing and plasma adiponectin concentration in apparently healthy males and females. Clin Endocrinol (Oxf) 2005;62:114–8. doi: 10.1111/j.1365-2265.2004.02182.x. [DOI] [PubMed] [Google Scholar]
- Aird KM, Zhang R. Detection of senescence-associated heterochromatin foci (SAHF). Methods Mol Biol. 2013;965:185–96. doi: 10.1007/978-1-62703-239-1_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amador-Noguez D, Yagi K, Venable S, Darlington G. Gene expression profile of long-lived Ames dwarf mice and Little mice. Aging Cell. 2004;3:423–41. doi: 10.1111/j.1474-9728.2004.00125.x. [DOI] [PubMed] [Google Scholar]
- Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC, Pezeshki A, Khazaie K, Miller JD, van Deursen JM. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530:184–9. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–6. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedelbaeva K, Snyder A, Gourevitch D, Clark L, Zhang XM, Leferovich J, Cheverud JM, Lieberman P, Heber-Katz E. Lack of p21 expression links cell cycle control and appendage regeneration in mice. Proc Natl Acad Sci U S A. 2010;107:5845–50. doi: 10.1073/pnas.1000830107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkenkamp B, Susnik N, Baisantry A, Kuznetsova I, Jacobi C, Sorensen-Zender I, Broecker V, Haller H, Melk A, Schmitt R. In vivo and in vitro analysis of age-associated changes and somatic cellular senescence in renal epithelial cells. PLoS One. 2014;9:e88071. doi: 10.1371/journal.pone.0088071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–10. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- Carlzon D, Svensson J, Petzold M, Karlsson MK, Ljunggren O, Tivesten A, Mellstrom D, Ohlsson C. Both low and high serum IGF-1 levels associate with increased risk of cardiovascular events in elderly men. J Clin Endocrinol Metab. 2014;99:E2308–16. doi: 10.1210/jc.2014-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinta SJ, Woods G, Rane A, Demaria M, Campisi J, Andersen JK. Cellular senescence and the aging brain. Exp Gerontol. 2015;68:3–7. doi: 10.1016/j.exger.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti V, Corbi G, Manzo V, Pelaia G, Filippelli A, Vatrella A. Sirtuin 1 and aging theory for chronic obstructive pulmonary disease. Anal Cell Pathol (Amst) 2015;2015:897327. doi: 10.1155/2015/897327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–68. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos AR, Kawahara M, Malhotra GK, Schaum N, Huang J, Ved U, Beausejour CM, Coppe JP, Rodier F, Campisi J. p53-dependent release of Alarmin HMGB1 is a central mediator of senescent phenotypes. J Cell Biol. 2013;201:613–29. doi: 10.1083/jcb.201206006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–7. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubal DB, Yokoyama JS, Zhu L, Broestl L, Worden K, Wang D, Sturm VE, Kim D, Klein E, Yu GQ, Ho K, Eilertson KE, Yu L, Kuro-o M, De Jager PL, Coppola G, Small GW, Bennett DA, Kramer JH, Abraham CR, Miller BL, Mucke L. Life extension factor klotho enhances cognition. Cell Rep. 2014;7:1065–76. doi: 10.1016/j.celrep.2014.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulic V, Beney GE, Frebourg G, Drullinger LF, Stein GH. Uncoupling between phenotypic senescence and cell cycle arrest in aging p21-deficient fibroblasts. Mol Cell Biol. 2000;20:6741–54. doi: 10.1128/mcb.20.18.6741-6754.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elabd C, Cousin W, Upadhyayula P, Chen RY, Chooljian MS, Li J, Kung S, Jiang KP, Conboy IM. Oxytocin is an age-specific circulating hormone that is necessary for muscle maintenance and regeneration. Nat Commun. 2014;5:4082. doi: 10.1038/ncomms5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–70. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- Ferrington DA, Husom AD, Thompson LV. Altered proteasome structure, function, and oxidation in aged muscle. FASEB J. 2005;19:644–6. doi: 10.1096/fj.04-2578fje. [DOI] [PubMed] [Google Scholar]
- Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng CW, Hwang D, Martin-Montalvo A, Saavedra J, Ingles S, de Cabo R, Cohen P, Longo VD. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med. 2011;3:70ra13. doi: 10.1126/scitranslmed.3001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Bakshi V, Lin AL. Early Shifts of Brain Metabolism by Caloric Restriction Preserve White Matter Integrity and Long-Term Memory in Aging Mice. Front Aging Neurosci. 2015;7:213. doi: 10.3389/fnagi.2015.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Li M, Bhasin S. Testosterone supplementation improves anemia in aging male mice. J Gerontol A Biol Sci Med Sci. 2014;69:505–13. doi: 10.1093/gerona/glt127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–33. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- Harper JM, Wang M, Galecki AT, Ro J, Williams JB, Miller RA. Fibroblasts from long-lived bird species are resistant to multiple forms of stress. J Exp Biol. 2011;214:1902–10. doi: 10.1242/jeb.054643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herranz D, Munoz-Martin M, Canamero M, Mulero F, Martinez-Pastor B, Fernandez-Capetillo O, Serrano M. Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat Commun. 2010;1:3. doi: 10.1038/ncomms1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16:448–57. doi: 10.1038/ni.3153. [DOI] [PubMed] [Google Scholar]
- Jeyapalan JC, Ferreira M, Sedivy JM, Herbig U. Accumulation of senescent cells in mitotic tissue of aging primates. Mech Ageing Dev. 2007;128:36–44. doi: 10.1016/j.mad.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser J. Regenerative medicine. ‘Rejuvenating’ protein doubted. Science. 2015;348:849. doi: 10.1126/science.348.6237.849. [DOI] [PubMed] [Google Scholar]
- Kanfi Y, Naiman S, Amir G, Peshti V, Zinman G, Nahum L, Bar-Joseph Z, Cohen HY. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012;483:218–21. doi: 10.1038/nature10815. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, Sharpless NE. Ink4a/Arf expression is a biomarker of aging. J Clin Invest. 2004;114:1299–307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuilman T, Peeper DS. Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev Cancer. 2009;9:81–94. doi: 10.1038/nrc2560. [DOI] [PubMed] [Google Scholar]
- Laron Z. The GH-IGF1 axis and longevity. The paradigm of IGF1 deficiency. Hormones (Athens) 2008;7:24–7. doi: 10.14310/horm.2002.1111034. [DOI] [PubMed] [Google Scholar]
- Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379:165–80. doi: 10.1016/S0140-6736(11)60178-5. [DOI] [PubMed] [Google Scholar]
- Li W, Li X, Miller RA. ATF4 activity: a common feature shared by many kinds of slow-aging mice. Aging Cell. 2014;13:1012–8. doi: 10.1111/acel.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Miller RA. Elevated ATF4 function in fibroblasts and liver of slow-aging mutant mice. J Gerontol A Biol Sci Med Sci. 2015;70:263–72. doi: 10.1093/gerona/glu040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Yan Q, Wolf NS. Long-term caloric restriction delays age-related decline in proliferation capacity of murine lens epithelial cells in vitro and in vivo. Invest Ophthalmol Vis Sci. 1997;38:100–7. [PubMed] [Google Scholar]
- Lin AL, Zhang W, Gao X, Watts L. Caloric restriction increases ketone bodies metabolism and preserves blood flow in aging brain. Neurobiol Aging. 2015;36:2296–303. doi: 10.1016/j.neurobiolaging.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AW, Barradas M, Stone JC, van Aelst L, Serrano M, Lowe SW. Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes Dev. 1998;12:3008–19. doi: 10.1101/gad.12.19.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi G, Sanchis-Gomar F, Montagnana M. Biological markers in older people at risk of mobility limitations. Curr Pharm Des. 2014;20:3222–44. doi: 10.2174/13816128113196660697. [DOI] [PubMed] [Google Scholar]
- Liu Y, Sanoff HK, Cho H, Burd CE, Torrice C, Ibrahim JG, Thomas NE, Sharpless NE. Expression of p16(INK4a) in peripheral blood T-cells is a biomarker of human aging. Aging Cell. 2009;8:439–48. doi: 10.1111/j.1474-9726.2009.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffredo FS, Steinhauser ML, Jay SM, Gannon J, Pancoast JR, Yalamanchi P, Sinha M, Dall'Osso C, Khong D, Shadrach JL, Miller CM, Singer BS, Stewart A, Psychogios N, Gerszten RE, Hartigan AJ, Kim MJ, Serwold T, Wagers AJ, Lee RT. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153:828–39. doi: 10.1016/j.cell.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long DL, Ulici V, Chubinskaya S, Loeser RF. Heparin-binding epidermal growth factor-like growth factor (HB-EGF) is increased in osteoarthritis and regulates chondrocyte catabolic and anabolic activities. Osteoarthritis Cartilage. 2015;23:1523–31. doi: 10.1016/j.joca.2015.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Kennedy BK. Sirtuins in aging and age-related disease. Cell. 2006;126:257–68. doi: 10.1016/j.cell.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Lynch CD, Cooney PT, Bennett SA, Thornton PL, Khan AS, Ingram RL, Sonntag WE. Effects of moderate caloric restriction on cortical microvascular density and local cerebral blood flow in aged rats. Neurobiol Aging. 1999;20:191–200. doi: 10.1016/s0197-4580(99)00032-9. [DOI] [PubMed] [Google Scholar]
- Mannic T, Viguie J, Rossier MF. In vivo and in vitro evidences of dehydroepiandrosterone protective role on the cardiovascular system. Int J Endocrinol Metab. 2015;13:e24660. doi: 10.5812/ijem.24660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, Barnard D, Ward WF, Qi W, Ingram DK, de Cabo R. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–21. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard SP, Miller RA. Fibroblasts from long-lived Snell dwarf mice are resistant to oxygen-induced in vitro growth arrest. Aging Cell. 2006;5:89–96. doi: 10.1111/j.1474-9726.2006.00187.x. [DOI] [PubMed] [Google Scholar]
- Melk A, Kittikowit W, Sandhu I, Halloran KM, Grimm P, Schmidt BM, Halloran PF. Cell senescence in rat kidneys in vivo increases with growth and age despite lack of telomere shortening. Kidney Int. 2003;63:2134–43. doi: 10.1046/j.1523-1755.2003.00032.x. [DOI] [PubMed] [Google Scholar]
- Narita M, Nunez S, Heard E, Narita M, Lin AW, Hearn SA, Spector DL, Hannon GJ, Lowe SW. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113:703–16. doi: 10.1016/s0092-8674(03)00401-x. [DOI] [PubMed] [Google Scholar]
- Perl A. mTOR activation is a biomarker and a central pathway to autoimmune disorders, cancer, obesity, and aging. Ann N Y Acad Sci. 2015;1346:33–44. doi: 10.1111/nyas.12756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering AM, Lehr M, Miller RA. Lifespan of mice and primates correlates with immunoproteasome expression. J Clin Invest. 2015;125:2059–68. doi: 10.1172/JCI80514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Chen X. Senescence regulation by the p53 protein family. Methods Mol Biol. 2013;965:37–61. doi: 10.1007/978-1-62703-239-1_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanov VS, Abramova MV, Svetlikova SB, Bykova TV, Zubova SG, Aksenov ND, Fornace AJ, Jr., Pospelova TV, Pospelov VA. p21(Waf1) is required for cellular senescence but not for cell cycle arrest induced by the HDAC inhibitor sodium butyrate. Cell Cycle. 2010;9:3945–55. doi: 10.4161/cc.9.19.13160. [DOI] [PubMed] [Google Scholar]
- Roos CM, Zhang B, Palmer AK, Ogrodnik MB, Pirtskhalava T, Thalji NM, Hagler M, Jurk D, Smith LA, Casaclang-Verzosa G, Zhu Y, Schafer MJ, Tchkonia T, Kirkland JL, Miller JD. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell. 2016 doi: 10.1111/acel.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutanen EM, Karkkainen T, Stenman UH, Yki-Jarvinen H. Aging is associated with decreased suppression of insulin-like growth factor binding protein-1 by insulin. J Clin Endocrinol Metab. 1993;77:1152–5. doi: 10.1210/jcem.77.5.7521340. [DOI] [PubMed] [Google Scholar]
- Sanchez-Rodriguez M, Garcia-Sanchez A, Retana-Ugalde R, Mendoza-Nunez VM. Serum leptin levels and blood pressure in the overweight elderly. Arch Med Res. 2000;31:425–8. doi: 10.1016/s0188-4409(99)00079-x. [DOI] [PubMed] [Google Scholar]
- Sanchis-Gomar F, Pareja-Galeano H, Santos-Lozano A, Garatachea N, Fiuza-Luces C, Venturini L, Ricevuti G, Lucia A, Emanuele E. A preliminary candidate approach identifies the combination of chemerin, fetuin-A, and fibroblast growth factors 19 and 21 as a potential biomarker panel of successful aging. Age (Dordr) 2015;37:9776. doi: 10.1007/s11357-015-9776-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–11. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- Sedelnikova OA, Horikawa I, Zimonjic DB, Popescu NC, Bonner WM, Barrett JC. Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nat Cell Biol. 2004;6:168–70. doi: 10.1038/ncb1095. [DOI] [PubMed] [Google Scholar]
- Sharples AP, Hughes DC, Deane CS, Saini A, Selman C, Stewart CE. Longevity and skeletal muscle mass: the role of IGF signalling, the sirtuins, dietary restriction and protein intake. Aging Cell. 2015;14:511–23. doi: 10.1111/acel.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag WE, Lynch C, Thornton P, Khan A, Bennett S, Ingram R. The effects of growth hormone and IGF-1 deficiency on cerebrovascular and brain ageing. J Anat. 197 Pt. 2000;4:575–85. doi: 10.1046/j.1469-7580.2000.19740575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag WE, Lynch CD, Cefalu WT, Ingram RL, Bennett SA, Thornton PL, Khan AS. Pleiotropic effects of growth hormone and insulin-like growth factor (IGF)-1 on biological aging: inferences from moderate caloric-restricted animals. J Gerontol A Biol Sci Med Sci. 1999;54:B521–38. doi: 10.1093/gerona/54.12.b521. [DOI] [PubMed] [Google Scholar]
- Spaulding CC, Walford RL, Effros RB. Calorie restriction inhibits the age-related dysregulation of the cytokines TNF-alpha and IL-6 in C3B10RF1 mice. Mech Ageing Dev. 1997;93:87–94. doi: 10.1016/s0047-6374(96)01824-6. [DOI] [PubMed] [Google Scholar]
- Steffen KK, MacKay VL, Kerr EO, Tsuchiya M, Hu D, Fox LA, Dang N, Johnston ED, Oakes JA, Tchao BN, Pak DN, Fields S, Kennedy BK, Kaeberlein M. Yeast life span extension by depletion of 60s ribosomal subunits is mediated by Gcn4. Cell. 2008;133:292–302. doi: 10.1016/j.cell.2008.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbaugh MJ, Sun LY, Bartke A, Miller RA. Activation of genes involved in xenobiotic metabolism is a shared signature of mouse models with extended lifespan. Am J Physiol Endocrinol Metab. 2012;303:E488–95. doi: 10.1152/ajpendo.00110.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout MB, Steyn FJ, Jurczak MJ, Camporez JG, Zhu Y, Hawse JR, Jurk D, Palmer AK, Xu M, Pirtskhalava T, Evans GL, de Souza Santos R, Frank AP, White TA, Monroe DG, Singh RJ, Casaclang-Verzosa G, Miller JD, Clegg DJ, LeBrasseur NK, von Zglinicki T, Shulman GI, Tchkonia T, Kirkland JL. 17alpha-Estradiol Alleviates Age-related Metabolic and Inflammatory Dysfunction in Male Mice Without Inducing Feminization. J Gerontol A Biol Sci Med Sci. 2016 doi: 10.1093/gerona/glv309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su N, Thiaville MM, Awad K, Gjymishka A, Brant JO, Yang TP, Kilberg MS. Protein or amino acid deprivation differentially regulates the hepatic forkhead box protein A (FOXA) genes through an activating transcription factor-4-independent pathway. Hepatology. 2009;50:282–90. doi: 10.1002/hep.22971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindell WR. Gene expression profiling of long-lived dwarf mice: longevity-associated genes and relationships with diet, gender and aging. BMC Genomics. 2007;8:353. doi: 10.1186/1471-2164-8-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindell WR. Comparative analysis of microarray data identifies common responses to caloric restriction among mouse tissues. Mech Ageing Dev. 2008;129:138–53. doi: 10.1016/j.mad.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H, Khosla S, Jensen MD, Kirkland JL. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9:667–84. doi: 10.1111/j.1474-9726.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123:966–72. doi: 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilstra JS, Robinson AR, Wang J, Gregg SQ, Clauson CL, Reay DP, Nasto LA, St Croix CM, Usas A, Vo N, Huard J, Clemens PR, Stolz DB, Guttridge DC, Watkins SC, Garinis GA, Wang Y, Niedernhofer LJ, Robbins PD. NF-kappaB inhibition delays DNA damage-induced senescence and aging in mice. J Clin Invest. 2012;122:2601–12. doi: 10.1172/JCI45785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran D, Bergholz J, Zhang H, He H, Wang Y, Zhang Y, Li Q, Kirkland JL, Xiao ZX. Insulin-like growth factor-1 regulates the SIRT1-p53 pathway in cellular senescence. Aging Cell. 2014;13:669–78. doi: 10.1111/acel.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsaih SW, Pezzolesi MG, Yuan R, Warram JH, Krolewski AS, Korstanje R. Genetic analysis of albuminuria in aging mice and concordance with loci for human diabetic nephropathy found in a genome-wide association scan. Kidney Int. 2010;77:201–10. doi: 10.1038/ki.2009.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turinetto V, Giachino C. Multiple facets of histone variant H2AX: a DNA double-strand-break marker with several biological functions. Nucleic Acids Res. 2015;43:2489–98. doi: 10.1093/nar/gkv061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509:439–46. doi: 10.1038/nature13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, Lucin KM, Czirr E, Park JS, Couillard-Despres S, Aigner L, Li G, Peskind ER, Kaye JA, Quinn JF, Galasko DR, Xie XS, Rando TA, Wyss-Coray T. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–4. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo N, Niedernhofer LJ, Nasto LA, Jacobs L, Robbins PD, Kang J, Evans CH. An overview of underlying causes and animal models for the study of age-related degenerative disorders of the spine and synovial joints. J Orthop Res. 2013;31:831–7. doi: 10.1002/jor.22204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Jurk D, Maddick M, Nelson G, Martin-Ruiz C, von Zglinicki T. DNA damage response and cellular senescence in tissues of aging mice. Aging Cell. 2009;8:311–23. doi: 10.1111/j.1474-9726.2009.00481.x. [DOI] [PubMed] [Google Scholar]
- Wang GC, Casolaro V. Immunologic changes in frail older adults. Transl Med UniSa. 2014;9:1–6. [PMC free article] [PubMed] [Google Scholar]
- Wolf NS, Pendergrass WR. The relationships of animal age and caloric intake to cellular replication in vivo and in vitro: a review. J Gerontol A Biol Sci Med Sci. 1999;54:B502–17. doi: 10.1093/gerona/54.11.b502. [DOI] [PubMed] [Google Scholar]
- Wolf NS, Penn PE, Jiang D, Fei RG, Pendergrass WR. Caloric restriction: conservation of in vivo cellular replicative capacity accompanies life-span extension in mice. Exp Cell Res. 1995;217:317–23. doi: 10.1006/excr.1995.1092. [DOI] [PubMed] [Google Scholar]
- Xu C, Bai B, Fan P, Cai Y, Huang B, Law IK, Liu L, Xu A, Tung C, Li X, Siu FM, Che CM, Vanhoutte PM, Wang Y. Selective overexpression of human SIRT1 in adipose tissue enhances energy homeostasis and prevents the deterioration of insulin sensitivity with ageing in mice. Am J Transl Res. 2013;5:412–26. [PMC free article] [PubMed] [Google Scholar]
- Xu M, Palmer AK, Ding H, Weivoda MM, Pirtskhalava T, White TA, Sepe A, Johnson KO, Stout MB, Giorgadze N, Jensen MD, LeBrasseur NK, Tchkonia T, Kirkland JL. Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife. 2015;4 doi: 10.7554/eLife.12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamato M, Ishimatsu A, Yamanaka Y, Mine T, Yamada K. Tempol intake improves inflammatory status in aged mice. J Clin Biochem Nutr. 2014;55:11–4. doi: 10.3164/jcbn.14-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun MH. Changes in Regenerative Capacity through Lifespan. Int J Mol Sci. 2015;16:25392–432. doi: 10.3390/ijms161025392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Chen W, Adams PD. Molecular dissection of formation of senescence-associated heterochromatin foci. Mol Cell Biol. 2007;27:2343–58. doi: 10.1128/MCB.02019-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M, O'Hara SP, LaRusso NF, Miller JD, Roos CM, Verzosa GC, LeBrasseur NK, Wren JD, Farr JN, Khosla S, Stout MB, McGowan SJ, Fuhrmann-Stroissnigg H, Gurkar AU, Zhao J, Colangelo D, Dorronsoro A, Ling YY, Barghouthy AS, Navarro DC, Sano T, Robbins PD, Niedernhofer LJ, Kirkland JL. The Achilles' heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015a;14:644–58. doi: 10.1111/acel.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Tchkonia T, Stout MB, Giorgadze N, Wang L, Li PW, Heppelmann CJ, Bouloumie A, Jensen MD, Bergen HR, 3rd, Kirkland JL. Inflammation and the depot-specific secretome of human preadipocytes. Obesity (Silver Spring) 2015b;23:989–99. doi: 10.1002/oby.21053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler DV, Wiley CD, Velarde MC. Mitochondrial effectors of cellular senescence: beyond the free radical theory of aging. Aging Cell. 2015;14:1–7. doi: 10.1111/acel.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]