Abstract
Background
The deleterious effects of dehydration on athletic and cognitive performance have been well documented. As such, dehydrated individuals are advised to consume fluid in volumes equivalent to 1.25 to 1.5 L kg−1 body mass (BM) lost to restore body water content. However, individuals undertaking subsequent activity may have limited time to consume fluid. Within this context, the impact of fluid intake practices is unclear. This systematic review investigated the effect of fluid consumption following a period of dehydration on subsequent athletic and cognitive performance.
Methods
PubMed (MEDLINE), Web of Science (via Thomas Reuters) and Scopus databases were searched for articles reporting on athletic (categorized as: continuous, intermittent, resistance, sport-specific and balance exercise) or cognitive performance following dehydration of participants under control (no fluid) and intervention (fluid intake) conditions. Meta-analytic procedures determined intervention efficacy for continuous exercise performance.
Results
Sixty-four trials (n = 643 participants) derived from 42 publications were reviewed. Dehydration decreased BM by 1.3–4.2%, and fluid intake was equivalent to 0.4–1.55 L kg−1 BM lost. Fluid intake significantly improved continuous exercise performance (22 trials), Hedges’ g = 0.46, 95% CI 0.32, 0.61. Improvement was greatest when exercise was performed in hotter environments and over longer durations. The volume or timing of fluid consumption did not influence the magnitude of this effect. Evidence indicating a benefit of fluid intake on intermittent (10 trials), resistance (9 trials), sport-specific (6 trials) and balance (2 trials) exercise and on cognitive performance (15 trials) was less apparent and requires further elucidation.
Conclusions
Fluid consumption following dehydration may improve continuous exercise performance under heat stress conditions, even when the body water deficit is modest and fluid intake is inadequate for complete rehydration.
Electronic supplementary material
The online version of this article (doi:10.1186/s40798-017-0079-y) contains supplementary material, which is available to authorized users.
Keywords: Athletic, Cognitive, Performance, Mood, Dehydration, Fluid intake
Key Points
Evidence indicating a beneficial effect for fluid intake on athletic performance was strongest when a continuous exercise task was employed; whereas limited research has examined the effect of fluid intake on intermittent, resistance and sport-specific exercise performance.
The magnitude of improvement was greater when the continuous exercise was performed at elevated environmental temperatures and over longer exercise durations.
Whilst the volume of fluid consumed (relative to BM lost) did not appear to influence the size of the treatment effect, fluid intake at levels that comply with current recommendations for restoring body water content (1.25–1.50 L kg BM lost−1) are yet to be thoroughly investigated.
Background
The deleterious effects of dehydration (fluid loss) on athletic and cognitive performance have been extensively researched. Recent meta-analyses detected meaningful decrements in aerobic [1] and anaerobic [2] exercise performance and muscular strength and endurance [2] when subjects commenced activity in an already dehydrated state. Experimental investigations have also demonstrated motor-skill impairments on sport-specific exercise tests (e.g. cricket [3], basketball [4, 5], golf [6], field hockey [7] and surfing [8]) following fluid loss. Whilst evidence indicating a detrimental effect of dehydration on cognitive function is less consistent [9], decline in memory, perceptual discrimination and mood state has been observed in some studies [10]. Dehydration is commonly observed amongst athletes [11–14] and manual workers (e.g. military, fire fighters and labourers) [15], who rely upon physical and mental proficiencies to compete or train at elite levels and remain productive in the workforce. This evidence has provided the rationale for fluid replacement recommendations.
The American College of Sports Medicine (ACSM) Guidelines on Exercise and Fluid Replacement [16] and the Position of the Academy of Nutrition and Dietetics on Nutrition and Athletic Performance [17] recommend dehydrated individuals consume 1.25 to 1.50 L of fluid per kilogram of body mass (BM) lost to replenish body water content, if the fluid deficit is large and recovery time is limited (i.e. <12 h). Whilst the importance of returning to euhydration over a period of a day(s) is not in dispute, many individuals are required to undertake repeated bouts of activity, where limited time between tasks exists or the demands of a subsequent activity (i.e. type, duration and intensity) and/or the environment (e.g. conflict zone) may influence the appropriateness of the aforementioned guidelines. Within this context, consuming fluid has the potential to enhance or inhibit performance. Thus, determining rehydration strategies that counteract the detrimental effects of fluid loss, whilst optimizing performance on subsequent tasks, is important.
Ingesting large volumes of fluid may cause gastrointestinal (GI) discomfort, impeding performance. Particularly if the amount of time available to consume fluid is limited or fluids with higher calorie loads (e.g. milk-based beverages) and hence slower rates of gastric emptying are ingested [18, 19]. The nature of the subsequent activity, e.g. the mechanical ‘bouncing’ action caused by high intensity running, may also impact GI symptomology [20]. Conversely, drinking large fluid volumes promotes rapid initial gastric emptying [19], facilitating fluid absorption, and may convey greater benefit than drinking smaller volumes. To date, the majority of investigations examining the effect of ingested fluid volume on subsequent performance have employed a prolonged (i.e. overnight) rehydration period [21–25], reducing the probability of GI disturbance influencing subsequent performance. Thus, the importance of ingested fluid volume and its impact on subsequent exercise performance outcomes remains unclear. The aim of the present systematic review and meta-analysis was to examine the impact of consuming fluid following a period of dehydrating sweat loss on subsequent athletic and cognitive performance. Understanding how to maximize the benefits of fluid intake under these circumstances will inform the development of future fluid replacement guidelines.
Methods
The following research protocol was devised in accordance with specifications outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocols PRISMA-P 2015 Statement [26]. The methodology of this review is registered at the International Prospective Register for Systematic Reviews, identification code CRD42016036560.
Literature Search
Potential research studies were identified by searching the online databases PubMed (MEDLINE), Web of Science (via Thomas Reuters) and Scopus from inception until April 2016 using the terms exercise, athletic, performance, mood and cognit* (the symbol was used to capture all words beginning with cognit, e.g. cognitive, cognition), each in combination with “fluid replacement” (the enclosed quotation marks were used to search for an exact phrase), “fluid ingestion”, “fluid intake”, “fluid consumption”, “fluid administration”, rehydrat* and euhydrat*. Records that contained irrelevant terms (patient, rat, mouse, aged care, reaction, disease, illness, bacteria, children and elderly) were excluded from the literature search using the Boolean search operator ‘NOT’. Two investigators (D.M. and C.I.) independently screened potential research studies to identify relevant texts. Full details of the screening process are presented in Fig. 1. Initially, all irrelevant titles were discarded. The remaining studies were systematically screened for eligibility by abstract and full text, respectively. The final decision to include or discard research studies was made between two investigators (D.M. and C.I.), with any disagreement resolved in consultation with a third investigator (B.D.). The reference lists of all included studies were then hand searched for missing publications.
Fig. 1.
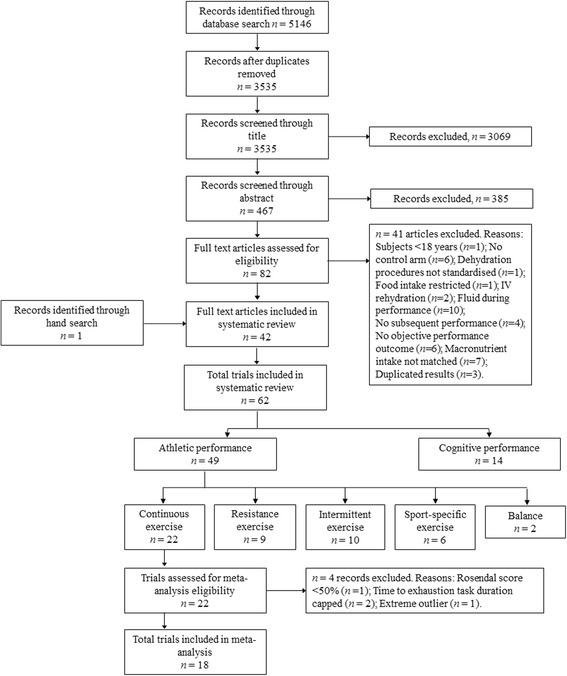
PRISMA Flow Chart (study selection methodology). Where a study contained >1 intervention-arm that was eligible for inclusion (i.e. paired against a suitable control condition), these were treated as separate ‘studies’ termed ‘trials’
Inclusion and Exclusion Criteria
Research studies containing a control-arm and one or more intervention-arms fulfilling the following criteria were eligible for inclusion:
Repeated measures experimental design.
Human studies on adult (≥18 years of age) male or female participants with no known medical conditions or co morbidities.
An athletic or cognitive performance outcome (see “Primary and Secondary Research Outcomes” section below for full description) was measured under control and intervention conditions. The control condition was dehydration with no fluid or negligible fluid intake, where ‘negligible’ fluid intake was accepted as ≤200 mL. This threshold was intended to broaden the inclusion criteria, allowing greater data capture and increased statistical power, since this was the first review to examine the effect of fluid intake on subsequent athletic and cognitive performance. The intervention condition was defined as dehydration with concurrent and/or subsequent fluid intake >200 mL.
The mode of dehydration was standardized, i.e. all participants were subjected to the same dehydration protocol, with or without fluid intake, on intervention and control trials.
Hydration status was manipulated before the performance task commenced, i.e. dehydration and fluid ingestion occurred before, not during, the performance assessment. A schematic representation of the experimental protocol is displayed in Fig. 2.
There is ‘limited’ time to consume fluid, defined as: ≤4 h between completing the dehydration protocol and commencing the subsequent performance test, unless performance followed an overnight fasting period.
An objective measurement of hydration status (e.g. body weight, urine specific gravity, plasma or urine osmolality or plasma volume) was used to indicate the level of dehydration attained.
Accessible full text articles written in English.
Fig. 2.
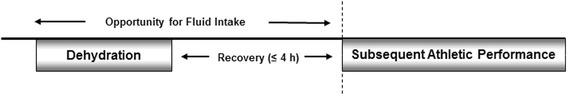
A schematic representation of the experimental protocol employed in studies eligible for inclusion in the present review
Studies were excluded from the review if: (1) dehydration involved restriction of food intake; (2) fluids were not administered orally (e.g. intravenous infusions) or (3) were co-administered with another experimental treatment (e.g. glycerol, l-alanyl-l-glutamine or external cooling); (4) subjects ingested >200 mL of fluid or an unspecified volume of fluid on control trials (e.g. Bardis et al. [27] and Baker et al. [28]); (5) macronutrient intake was not matched on experimental trials or (6) performance data was not adequately reported, i.e. values were not quantified, or descriptive terms were not used.
For the purpose of this systematic review, research studies containing multiple intervention-arms that were eligible for inclusion (each paired against a suitable ‘no fluid’ control group) (e.g. McConell et al. [25] tested athletic performance under two different fluid conditions and Hillman et al. [29] tested athletic performance under different environmental conditions) were treated as separate experimental studies termed ‘trials’. Separate trials derived from a single research study are denoted by additional letters (i.e. a–d) in the citation.
Methodological Quality Assessment
All eligible studies were examined for publication bias using the Rosendal Scale [30]. Excellent methodological quality is indicated by a Rosendal Score ≥60% [31]. Items 7, 8 and 9 of the scale, pertaining to the use of blinding procedures, were omitted from the evaluation as oral fluid ingestion cannot be blinded. Scoring was determined by dividing the number of ‘yes’ responses by the total number of applicable items and reported for all included studies. Studies were excluded from meta-analyses if they received a Rosendal score <50%.
Data Extraction and Synthesis
Data were extracted from relevant publications following the Cochrane Handbook for Systematic Reviews of Interventions Checklist of Items to Consider in Data Collection or Data Extraction [32] and entered into a Microsoft Excel spread sheet.
Primary and Secondary Research Outcomes
The first primary research outcome was (1) objective indicators of athletic performance; subjective measurements of performance (e.g. ratings of perceived exertion) were not examined in this review. The types of athletic performances studied were broadly classified as follows: (a) continuous exercise; (b) intermittent exercise; (c) resistance exercise; (d) sport-specific exercise and (e) balance tasks. Performances that incorporated a coordinated motor-movement resembling some skill involved in a particular sporting event were categorized as ‘sport-specific’ exercises, whereas non-specific sporting activities (e.g. sprint running) were categorized into one of the remaining groups (where possible). Where more than one type of athletic performance was measured within a single experimental trial (e.g. Walsh et al. [33] examined performance on continuous and resistance exercise tasks), the performances were presented in their respective categories and treated as separate trials. The second primary research outcome was (2) objective indicators of cognitive function, including subjective measurements of mood state. The decision to include mood as a primary research outcome was based on previous suggestions that mood and symptom questionnaires may be more sensitive to subtle changes in hydration status than tests of cognitive ability [34]. Subjective ratings of GI discomfort and thirst following fluid ingestion were intended as the secondary research outcomes. However, very few investigations evaluated GI symptomology [25] or thirst [35–37]. Thus, insufficient data were available to complete secondary analyses.
Other Relevant Data
Other information extracted from relevant research studies included
Participant characteristics: description, age, euhydrated body mass (BM) and maximal oxygen consumption (VO2 max)
The dehydration protocol: mode of dehydration, ambient temperature and relative humidity, protocol duration, level of dehydration and time from finishing the dehydration protocol to receiving fluid
The rehydration protocol: fluid type, volume of fluid consumed, drink time and time from finishing fluid to commencing performance task
Performance task: task description and performance outcomes, ambient temperature, relative humidity, rate of airflow, intensity and duration, where applicable
Percentage of BM loss was used to indicate the level of dehydration attained. If the percentage of BM loss was not directly reported, values were calculated from euhydrated BM (kg) and BM mass loss (kg) using the following formula:
The volume of fluid consumed was expressed as a proportion of BM loss, i.e. relative fluid intake (L kg BM lost−1). If the quantity of fluid consumed was not expressed as a proportion of BM loss, values were calculated from fluid intake (L) and percentage of BM loss using the following formula:
If the volume of fluid consumed was unknown, the BM deficit post-rehydration has been reported.
Time from completing the dehydration protocol to commencing the subsequent performance task (recovery time) and time from commencing fluid ingestion to commencing the subsequent performance task (fluid assimilation time) were approximated from the experimental protocol, where adequate information was provided.
If necessary information was not available from the published article and it was published within the previous 10 years, authors were contacted via email with a request to provide missing data.
Statistical Analyses
Sufficient data were available to perform a meta-analysis examining the impact of fluid consumption following a period of dehydration on subsequent continuous exercise performance. Meta-analyses were not performed on other types of athletic performance or cognitive function because: (1) intermittent and sport-specific exercise performance trials were methodologically heterogeneous, particularly in regards to the exercise protocol and outcomes used to determine a treatment effect; (2) few authors responded to an email request for raw data regarding resistance exercise performance, preventing computation of the correlation coefficient and (3) cognitive performance data was rarely quantified (descriptive terms only).
Meta-analysis on Continuous Exercise Performance
All statistical procedures were performed using IBM SPSS Statistical Software, Version 22.0 and Comprehensive Meta-Analysis, Version 3.0. Repeated measures intervention effect sizes were calculated as Hedges’ g [38], where the mean difference between each intervention and control performance score was standardized against the SD of the performance change and corrected for bias due to small sample size. The magnitude of effect was defined in accordance with Cohen [39]: ES ≤0.2 = small; ≥0.5 ES >0.2 = medium and ≥0.8 = large, where a positive Hedges’ g value indicates a beneficial effect of fluid intake on continuous exercise performance. Where the SD of the performance change was not reported, the missing value was imputed using a correlation coefficient [32] calculated with the following formula:
Where SD∆ is the missing standard deviation of change and R is the correlation coefficient. R was approximated as the mean correlation coefficient (R = 0.84) calculated using raw performance data from nine continuous exercise trials (derived from four separate publications). Sensitivity analysis was performed using R = 0.50, 0.74 and 0.94 to test the robustness of the imputed correlation coefficient. The weighted mean treatment effect was calculated using random-effect models, where trials were weighted by the inverse variance for the standardized performance change. Statistical significance was attained if the 95% CI did not include zero. Data are described as mean ± SD, unless otherwise indicated; articles that reported SEM had their values multiplied by the square root of the sample size to convert to SD. All research studies measuring performance on a continuous exercise task used a single objective measurement to demonstrate the presence or absence of a treatment effect (e.g. time to exhaustion or power output). Hence, no additional precautions were taken to limit data dependency.
Heterogeneity and Sensitivity Analyses
Heterogeneity was assessed using Cochran’s Q and the I 2 index. Low, moderate and high heterogeneity was indicated by an I 2 value of 25, 50 and 75%, respectively [40]. A p value <0.10 for Cochran’s Q was used to indicate significant heterogeneity [32]. Sensitivity analyses were performed by removing individual trials and examining the effect of each study on the results of the weighted mean treatment effect.
Meta-regression Analysis
A priori, we identified the volume of fluid ingested (L kg−1 BM lost) and fluid assimilation time as variables that might moderate the effect of fluid intake on athletic performance. However, prior research indicates that environmental temperature, exercise duration and the ecological validity of the exercise protocol employed may influence the effect of dehydration on athletic performance [29, 41–44], as might level of fluid loss (% BM loss) incurred. Therefore, we explored the relationship between these variables and the magnitude of the treatment effect (Hedges’ g) using a restricted maximum likelihood multiple meta-regression (random effects) model that controlled for potential confounders. Restricted maximum likelihood simple meta-regression was also performed to explore the influence of environmental temperature on Hedges’ g values. The ecological validity of each continuous exercise protocol was defined in accordance with Goulet [43], where fixed-power time to exhaustion (TTE) exercise protocols were considered non-ecologically valid and time-trial type exercise protocols (including protocols measuring work completed within a set timeframe) were classified as ecologically valid. Exercise duration was taken as the mean total exercise time (min) for control and intervention trials. One study did not report total exercise time [29], therefore exercise duration was approximated as per Stewart et al. [37], who performed a comparable performance test.
As per Savoie et al. [2], regression analyses were examined for influential cases and outliers (studentized residuals and cook’s distance). Tests for normality of residuals (Shapiro-Wilk test), multicollinearity (variance inflation factor, VIF), autocorrelation (Durbin-Watson statistic), homoscedasticity and linearity of the relationship between dependent and independent variables (plot of residuals versus predicted values) ensured that analyses did not violate assumptions of meta-regression. Statistical significance was accepted as p < 0.05.
Systematic Review
All athletic and cognitive performances are presented in the systematic review investigating the effect of dehydration and fluid intake on subsequent athletic and cognitive performance. Whilst it was our intention to calculate within-subject intervention effect sizes for all athletic performance outcomes, the vast majority of the publications included in this review did not provide the necessary data to complete a paired analysis. Further, the types of performances investigated varied widely amongst studies, such that the missing SD of change could not be estimated from a known correlation coefficient. To enable comparison of effects across studies, ES were approximated as Hedges’ g for independent groups. The mean difference between each intervention and control performance score was standardized against a pooled SD and corrected for bias due to small sample size using the supplementary spreadsheet by Lakens [45]. This approach will likely underestimate the magnitude of the true effect. Cognitive performance outcomes are presented in descriptive terms only, since few publications quantified the effect of fluid intake numerically. Statistical significance was accepted as p < 0.05 in all studies.
Results
Overview of Studies and Study Quality
Sixty-four repeated measures trials (n = 643 healthy participants, 93% male, excluding Del Coso et al. [46] where gender was not specified, NS) derived from 42 original publications were included in the present systematic review. Methodological quality assessment yielded a median Rosendal Score of 58%. Two trials received a Rosendal Score <50% [47, 48]. Whilst these studies are presented in the systematic review, they were not deemed eligible for inclusion in subsequent meta-analysis. The highest Rosendal Score of 83% was calculated for Rodrigues et al. [49]. Complete results of the quality assessment are displayed in Additional file 1: Table S1.
Study Characteristics
Characteristics of included studies are summarized in Tables 1, 2, 3, 4, 5 and 6. (Full details are presented in Additional file 1: Table S2, S3, S4, S5, S6 and S7). Dehydration and rehydration protocols were heterogeneous amongst included trials. In 57 out of the 61 trials reviewed, dehydration was accomplished via passive heat exposure (n = 11) [42, 47, 50–56] or physical activity (n = 47), conducted in a thermoneutral laboratory ≤25 °C (n = 16) [24, 25, 29, 35, 48, 55, 57–61], heated environmental chamber (n = 28) [22, 29, 33, 36, 37, 41, 46, 49, 62–71] or environmental conditions not specified (n = 3) [72, 73]. The remaining trials reduced body water content through warm water immersion (n = 2) [74, 75] or dietary fluid restriction in combination with 2-h moderate intensity exercise 24 h prior to testing performance (n = 2) [76, 77]. In 46 trials (74%) [22, 24, 29, 36, 37, 41, 42, 46, 47, 49–53, 55–59, 61–63, 66, 67, 71, 73–77], dehydration yielded BM losses ≥2%. Of these, 27 trials (43%) dehydrated participants by ≥3.0% of initial BM [22, 24, 29, 37, 41, 42, 46, 50, 52, 53, 56, 61, 62, 66, 67, 74–77]. Mean BM losses ranged from 1.3 [72] to 4.2% [41]. Ingested fluids were predominantly water or saline solution (0.05–0.50% NaCl). Studies administering carbohydrate-containing fluids were often excluded due to unequal provision of macronutrients on control and intervention trials. Two included studies failed to specify the type of fluid consumed (this was presumed to be water) [42, 56], and one study did not report dietary standardization procedures or specify whether subjects consumed food during the prolonged (>12 h, overnight) period of intervention [22]. The volume of fluid administered ranged from 0.40 [72] to 1.55 L kg−1 BM lost [65]. In 20 trials (33%), participants ingested a volume of fluid to replace <100% of sweat losses [24, 25, 33, 35, 47, 57–59, 62, 68, 70–72, 76, 77]. Only 2 trials [22, 65] provided a volume of fluid that complied with current recommendations for restoring fluid loss (1.25–1.50 L kg BM lost−1) [16, 17]. In 14 trials, dehydrated control subjects ingested a small volume of non-nutritive fluid (≤200 mL) [42, 55, 56, 62, 63, 66, 71] or mouth rinse [33].
Table 1.
Characteristics of research studies evaluating athletic performance on continuous exercise tasks (studies are presented by order of effect)
| Citation | Participants | VO2 max | Dh protocol (exercise intensity); ambient temperature; duration | Total REC (min) | Dh trial BM loss (%) | Fluid type | Fluid assimilation time (min) | Fluid intake (L kg BM lost−1) | Duration (min) | Performance indicator | Ambient temperature; RH; airflow | Hedges’ g |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kenefick et al. (2010a) [41] | 8 M | 43.6 ± 4.1 | EX; 50 °C; 3-h W/R | 120 | 4.1 | NaCl | 300 | 1.05 | 15 | TW (cycle ergometer) | 10 °C | 0.05 |
| Chevront et al. (2005a) [42] | 8 (6 M), physically active | 48 ± 9 | HT | 210 | 3.0 | NS | 390 | 1.00 | 30 | TW (cycle ergometer) | 2 °C; 50%; 2.2 m/s | 0.11 |
| Stewart et al. (2014) [37] | 7 M, recreational cyclists | 52.7 ± 7.9 | EX (H); 37 °C; 120 min | 120 | 3.8 | Water | 120 | 1.15 | 7.2 | TT (cycle ergometer) | 18–25 °C; 20–30% | 0.12 |
| McConell et al. (1999a) [25] | 8 M, well-trained cyclists/triathletes | 63.8 ± 1.2 | EX (V); 21 °C; 45 min | 0 | 1.9 | Water | 45 | 1.00 | 15 | TW (cycle ergometer) | 21 °C; 41%; airflow NS | 0.15 |
| McConell et al. (1999b) [25] | 8 M, well-trained cyclists/triathletes | 63.8 ± 1.2 | EX (V); 21 °C; 45 min | 0 | 1.9 | Water | 45 | 0.50 | 15 | TW (cycle ergometer) | 21 °C; 41%; airflow NS | 0.24 |
| McConell et al. (1997a) [24] | 7 M, well-trained cyclists/triathletes | 68. ± 2.5 | EX (V); 21 °C, 120 min | 0 | 3.2 | Deionized water | 120 | 0.50 | 3.5 | TTE (cycle ergometer) | 21 °C, 43%; airflow NS | 0.24 |
| Kenefick et al. (2006) [36] | 8 M, unacclimated | 63.7 ± 10.2 | EX (M); 36 °C; 75 min | 30 | 2.3 | NaCl + NNS | 20 | 1.00 | 55 | TTE (treadmill running) | 37 °C; 42% | 0.28* |
| Hillman et al. (2011a) [29] | 7 M, unacclimated, competitive cyclists | NS | EX (V); 34 °C; 90 min | 15 | 3.0 | Water | 105 | 1.00 | 7.2 | PO (cycle ergometer) | 23 °C | 0.32 |
| Chevront et al. (2005b) [42] | 8 (6 M) physically active | 48 ± 9 | HT | 210 | 2.9 | NS | 390 | 1.00 | 30 | TW (cycle ergometer) | 20 °C; 50%; 1 m/s | 0.35* |
| Paik et al. (2009) [50] | 10 M, moderately active | 53.6 ± 11.4 | HT | 120 | 3.0 | Water | 120 | 1.00 | 30 | TTE (treadmill running) | NS | 0.43 |
| McConell et al. (1997b) [24] | 7 M, well-trained cyclists/triathletes | 68.4 ± 2.5 | EX (V); 21 °C, 120 min | 0 | 3.2 | Deionized water | 120 | 1.00 | 4.2 | TTE (cycle ergometer) | 21 °C, 43%; airflow NS | 0.58* |
| Hillman et al. (2011b) [29] | 7 M, unacclimated, competitive cyclists | NS | EX (V); 34 °C; 90 min | 15 | 3.8 | Water | 105 | 1.00 | 7.2 | PO (cycle ergometer) | 34 °C | 0.60* |
| Kenefick et al. (2010c) [41] | 8 M | 46.3 ± 5.2 | EX; 50 °C; 3-h W/R | 120 | 4.0 | NaCl | 300 | 1.05 | 15 | TW (cycle ergometer) | 30 °C | 0.64* |
| Castellani et al. (1997) [62] | 8 M, unacclimated | 57.9 ± 4.5 | EX (M); 33 °C; 180 min | 135 | 4.1 | NaCl + NNS | 120 | 0.50 | 72 | TTE (treadmill walking) | 36 °C; 47%; 2.3 m/s | 0.68* |
| Walsh et al. (1994a) [33] | 6 M, endurance cyclists/ triathletes | 61.4 ± 4.4 | EX (V); 30 °C; 60 min | 0 | 1.8 | NaCl + NNS | 50 | 0.90 | 8.2 | TTE (cycle ergometer) | 30 °C; 60%; 0.8 m/s | 0.74* |
| Kenefick et al. (2010b) [41] | 8 M | 45.3 ± 4.6 | EX; 50 °C; 3-h W/R | 120 | 4.2 | NaCl | 300 | 1.05 | 15 | TW (cycle ergometer) | 20 °C | 0.79 |
| Kavouras et al. (2006) [77] | 8 M, acclimated, endurance cyclists | 61.4 ± 2.3 | FR + EX (M); 120 min | >12 h | 3.9 | Water + NNS | 80 | 0.75 | 23 | TTE (cycle ergometer) | 37 °C; 48%; 2.54 m/s | 0.81* |
| Kenefick et al. (2010d) [41] | 8 M | 43.7 ± 7.0 | EX; 50 °C; 3-h W/R | 120 | 4.1 | NaCl | 300 | 1.05 | 15 | TW (cycle ergometer) | 40 °C | 0.87* |
| Below et al. (1994) [63] | 8 M, acclimated, endurance trained | 62.9 ± 2.8 | EX (V); 31 °C; 50 min | 0 | 2.0 | NaCl + NNS | 50 | 1.00 | 11 | TT (cycle ergometer) | 31 °C; 54% | 0.89* |
| Casa et al. (2000) [76] | 8 M, unacclimated, endurance cyclists | 61.4 ± 2.3 | FR + EX (M); 120 min | >12 h | 3.9 | NaCl + NNS | 35 | 0.50 | 27 | TTE (cycle ergometer) | 37 °C; 2.3 m/s | 1.25* |
| Melin et al. (1994) [47] | 6 M, unacclimated, endurance trained | 57.5 ± 4.2 | HT | 60 | 2.6 | Water | NS | 0.50 | 97 | TTE (treadmill marching) | 35 °C; 20–30%; 0.8 m/s | 1.23* |
| Hasegawa et al. (2006) [64] | 9 M, untrained | 48.5 ± 4.5 | EX (M); 32 °C; 60 min | 4 | 1.6 | Water | 65 | 1.00 | 4.4 | TTE (cycle ergometer) | 32 °C; 80% | 4.01* |
Exercise intensity is described as high (H), vigorous (V) or moderate (M), in accordance with classifications outlined by Norton et al. [86]. Values are Hedges’ g effect sizes
Dh dehydration, EX exercise, FR fluid restriction, HT heat, NNS non-nutritive sweetener, NS not specified, PO power output, RH relative humidity, Total REC time from completing the dehydration protocol to commencing the subsequent task, TT time trial, TTE time to exhaustion, TW total work, W/R work rest cycle
*Significant difference between performances undertaken with and without fluid replacement (p < 0.05)
Table 2.
Characteristics of research studies evaluating athletic performance on intermittent exercise tasks
| Citation | Participants | VO2 max | Dh protocol (EX intensity); ambient temperature; duration | Total REC (min) | Dh trial BM loss (%) | Fluid type | Fluid assimilation time (min) | Fluid intake (L kg BM lost−1) | Intermittent exercise task | Ambient temperature; RH; airflow | Performance outcomes(s) | Performance outcomes significantly affected (Hedges’ g) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Walsh et al. (1994b) [33] | 6 M, endurance cyclists/triathletes | 61.4 ± 4.4 | EX (V); 30 °C; 60 min | 15 | 1.8 | NaCl + NNS | 65 | 0.90 | aIST | NS | Max. velocity; lower limb force | No effect |
| Maxwell et al. (1999) [65] | 11 M, untrained | NS | EX (M); 32 °C; 48 min | 120 | 1.5 | NaCl + NNS | 208 | 1.55 | bMART | 32 °C; 73% | Sprint duration | ↑ (0.19) |
| Devlin et al. (2001a) [71] | 7 M, sub-elite cricketers | 56 ± 6 | EX; 28 °C; 60 min | 0 | 2.8 | Water | 60 | 0.80 | cMMRT | 16 °C; 60% | 20-m shuttle runs | ↑ (0.30) |
| Cheuvont et al. (2006) [51] | 8 M, physically active | 52 ± 6 | HT | Testing at 0, 30 and 60 | 2.7 | Water | 185–240 | 1.00 | 15 s WAnT | 22 °C; 65% | Abs. mean PO; Rel. mean PO; Abs. PPO; Rel. PPO; rate of fatigue |
No effect |
| Edwards et al. (2007a) [58] | 11 M, moderately active soccer players | 50.9 ± 4.0 | EX; 19-25 °C; 90 min | 0 | 2.4 | Water | 90 | 0.80 | dYo-Yo Test | NS | Distance covered | ↑ (ES unknown) |
| Maxwell et al. (2009a) [22] | 8 M, unacclimated, game players | 59.9 ± 8.0 | EX (H/M); 36 °C; 90 min | >12 h | 3.9 | Water | >12 h | 1.50 | eIST | 36 °C; 49% | TW; Abs. PPO; Rel. PPO (during the IST, RSB 1 and RSB 2) |
TW (0.97) and Abs. PPO (0.79) ↑ on RSB 2 only. |
| Maxwell et al. (2009b) [22] | 8 M, unacclimated, game players | 59.9 ± 8.0 | EX (H/M); 36 °C; 90 min | >12 h | 3.9 | Water | >12 h | 1.00 | eIST | 36 °C; 49% | TW; Abs. PPO; Rel. PPO (during the IST, RSB 1 and RSB 2) |
No effect |
| Kraft et al. (2011) [75] | 10 M | NS | WI | 45 | 3.0 | Water | 158–178 | 1.00 | fIST | NS | Abs. mean PO; Abs. PPO; s >90 rpm; Rate of fatigue |
No effect |
| Owen et al. (2013a) [59] | 13 M, semi-professional soccer players | 54 ± 3 | EX; 19 °C; 105 min | 5 | 2.5 | Water | 110 | 0.89 | dYo-Yo Test | 19 °C; 59% | Distance covered | No effect |
| Owen et al. (2013b) [59] | 13 M, semi-professional soccer players | 54 ± 3 | EX; 19 °C; 105 min | 5 | 2.5 | Water | 110 | 0.51 | dYo-Yo Test | 19 °C; 59% | Distance covered | No effect |
Exercise intensity is described as high (H), vigorous (V) or moderate (M), in accordance with classifications outlined by Norton et al. [86]. Values are Hedges’ g effect sizes
Abs. absolute, Dh dehydration, ES effect size, EX exercise, HT heat, IST intermittent sprint test, MART maximal anaerobic running test, Max. maximum, MMRT maximal multistage running test, NNS non-nutritive sweetener, NS not specified, PO power output, PPO peak power output, RH relative humidity, Rel. relative, Total REC time from completing the dehydration protocol to commencing the subsequent task, TW total work, WAnT Wingate Anaerobic Test, WI warm water immersion
aThe intermittent sprint test (IST) comprised of five 5-s sprints at 3-min intervals on a cycle ergometer
bThe maximal anaerobic running test (MART) involved repeated 20-s runs on a treadmill, at increasing intensities, with 100-s passive recovery between runs until volitional exhaustion
cThe maximal multistage running test (MMRT) involved repeated 20-m runs between two points, at increasing intensity
dThe Yo-Yo intermittent recovery test is a soccer-specific performance test that comprises of 20-m shuttle runs separated by 10 s jog recovery. Running speed during the test is incremental, and maximal performance is indicated by total distance covered
eThe IST comprised of a 36-min repeated sprint exercise divided into 2-min periods of a 4-s sprint and 100 s of active recovery (35% VO2 max) and 16 s passive rest. A repeated sprint bout (RSB) involving 5 × 2 s sprints with 18 s active recovery was also completed after the 8th and 16th sprints (RSB 1 and RSB 2). All testing was completed on a cycle ergometer
fThe IST comprised of a 3-min warm up followed by 6 × 15 s maximal sprints separated by 30 s active recovery on a cycle ergometer
Table 3.
Characteristics of research studies evaluating athletic performance on resistance exercise tasks
| Citation | Participants | Dh protocol; ambient temperature; duration | Total REC (min) | Dh trial BM loss (%) | Fluid type | Fluid assimilation time (min) | Fluid intake (L kg BM lost−1) | Resistance exercise task | Performance outcomes(s) | Performance outcomes significantly affected (Hedges’ g) |
|---|---|---|---|---|---|---|---|---|---|---|
| Montain et al. (1998) [66] | 8 M, physically active | EX (M); 40 °C; 2–3 h | Testing from 3–8 h |
4.0 | Water | 3–8 h | NS | Knee extension | ET >50% MVC; MVC pre-ET test, 30-s post-ET test and >30-s post-ET test | ET >50% MVC ↑ (2.70) MVC ↓ (ES unknown) |
| Greiwe et al. (1998) [52] | 7 M, unacclimated | HT | 120 | 3.8 | Water | 306 | 1.00 | Knee extension and elbow flexion | Peak torque; ET 100% MVC | No effect |
| Bigard et al. (2001) [53] | 11 M, unacclimated, physically active | HT | 180 | 3.0 | NaCl + water | 120 | 1.00 | Knee extension | MVC, ET 25% MVC; ET 75% MVC | ET 25% MVC ↑ (0.22) |
| Schoffstall et al. (2001) [54] | 10 M, competitive power lifters | HT | 120 | 1.7 | Water | 120 | 1.10 | Bench Press | 1 RM | ↑ (0.24) |
| Del Coso et al. (2008) [46] | 7 (NS), acclimated, endurance cyclists | EX (V); 36 °C; 2 h | 0 | 3.7 | Mineral water | 120 | 0.90 | Knee extension | MVC | No effect |
| Kraft et al. (2010) [74] | 10 M, recreationally strength trained | WI | ≥45 | 3.1 | Water | 165 | 1.00 | gFull body resistance exercise protocol | Repetitions completed at 12 RM | ↑ (0.87) |
| Ali et al. (2013) [57] | 10 M, university-level soccer players | EX (V); 22 °C; 90 min | 0 | 2.9 | Water | 90 | 0.50 | MVC (3.14 and 1.05 rad/s) knee flexion and extension | Peak torque; TW; Abs. mean PO | No effect |
| Knee extension and elbow flexion | Peak torque; mean torque | No effect | ||||||||
| Wilson et al. (2014) [60] | 8 M, licenced jockeys | EX; 20 °C; 45 min | 0 | 1.8 | Water | ~35 | 1.00 | Chest-press and knee flexion | Max. strength | ↑ Chest (5.57) and leg (1.05) max. strength |
| Rodrigues et al. (2014) [49] | 10 M, unacclimated, physically active | EX (V); 37 °C; 91 min | 30 | 2.0 | Water | 121 | NS | Knee extension and elbow flexion | Peak torque | Knee extensor peak torque ↑ (0.85) |
Exercise intensity is described as high (H), vigorous (V) or moderate (M), in accordance with classifications outlined by Norton et al. [86]. Values are Hedges’ g effect sizes
Abs. absolute, Dh dehydration, ES effect size, ET endurance time, EX exercise, HT heat, MVC maximal voluntary contraction, NS not specified, PO power output, RH relative humidity, RM repetition maximum, Total REC time from completing the dehydration protocol to commencing the subsequent task, TW total work, WI warm water immersion
gThe full body resistance exercise protocol measured total repetitions in three sets of bench press, lat pull down, overhead press, barbell curl, triceps and leg press exercise at 12 RM
Table 4.
Characteristics of research studies evaluating athletic performance on sport-specific exercise tasks
| Citation | Participants | VO2 max | Dh protocol; ambient temperature; duration | Total REC (min) | Dh trial BM loss (%) | Fluid type | Fluid assimilation time (min) | Fluid intake (L∙kg BM lost−1) | Sport-specific exercise task | Performance outcomes(s) | Performance outcomes significantly affected (Hedges’ g) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Devlin et al. (2001b) [71] | 7 M, sub-elite cricketers | 56 ± 6 | EX; 28 °C; 60 min | 0 | 2.8 | Water | 60 | 0.80 | Cricket bowling | Accuracy (line and length); velocity | ↑ Bowling accuracy for line (0.85) and length (0.85) |
| Ali et al. (2011) [35] | 10 (0 M), soccer players | 47 ± 4 | EX; 17 °C; 90 min | 0 | 1.4 | Water | 90 | 1.07 | hLSPT | Movement time; penalty time; performance time | No effect |
| Fritz et al. (2013) [72] | 13 M, elite squash players | NS | EX | NS | 1.3 | Water | NS | 0.40 | iGhosting Test | TT | ↓ (0.55) |
| Owen et al. (2013c) [59] | 13 M, semi-professional soccer players | 54 ± 3 | EX; 19 °C; 105 min | 5 | 2.5 | Water | 110 | 0.89 | hLSPT | Movement time; penalty time; performance time | No effect |
| jLSST | Time taken; shot speed; points per shot | No effect | |||||||||
| Owen et al. (2013d) [59] | 13 M, semi-professional soccer players | 54 ± 3 | EX; 19 °C; 105 min | 5 | 2.5 | Water | 110 | 0.51 | hLSPT | Movement time; penalty time; performance time | No effect |
| jLSST | Time taken; shot speed; points per shot | No effect | |||||||||
| Wilson et al. (2014) [60] | 8 M, licenced jockeys | NS | EX; 20 °C; 45 min | 0 | 1.8 | Water | ~35 | 1.00 | Simulated race ride | Pushing frequency | ↑ (1.46) |
| Go-No-Go task | SRT | No effect |
Values are Hedges’ g effect sizes
Dh dehydration, EX exercise, LIST Loughborough Intermittent Sprinting Test, LSPT Loughborough Soccer Passing Test, LSST Loughborough Shooting Test, Max. maximum, NS not specified, RH relative humidity, SRT simple reaction time, Total REC time from completing the dehydration protocol to commencing the subsequent task, TT time trial
Table 5.
Characteristics of research studies evaluating athletic performance on balance exercise tasks
| Citation | Participants | Dh protocol; ambient temperature; duration | Total REC (min) | Dh trial BM loss (%) | Fluid type | Fluid assimilation time (min) | Fluid intake (L kg BM lost−1) | Balance exercise task | Performance outcomes(s) | Performance outcomes significantly affected (Hedges’ g) |
|---|---|---|---|---|---|---|---|---|---|---|
| Erkmen et al. (2010) [61] | 17 M, physically active | EX (V); 21–24 °C; 60 min | Testing at 0 and 20 min | 3.3 | Water | 60/80 | 1.00 | One-leg stand static balance test | Eyes close and eyes open kOSI (0 min post-Dh) Eyes close and eyes open kOSI (20 min post-Dh) |
↓ Eyes open OSI 0 min post-Dh (1.12) |
| Ely et al. (2012a) [67] | 32 M, unacclimated | EX; 50 °C; 3 h work/rest | 90 | 4.1 | NaCl + water | 270 | 1.00 | 20-s dynamic balance test |
kOSI; l mean deflection; time spent stable (Testing at 10 °C, 20 °C, 30 °C and 40 °C) |
No effect |
Exercise intensity is described as high (H), vigorous (V) or moderate (M), in accordance with classifications outlined by Norton et al. [86]. Values are Hedges’ g effect sizes
Dh dehydration, EX exercise, OSI overall stability index, Total REC time from completing the dehydration protocol to commencing the subsequent task
hDuring the Loughborough Soccer Passing Test (LSPT), participants completed a random sequence of eight short and long passes of a soccer ball towards a target, as quickly as possible with the fewest time penalties
iThe ‘Ghosting Test’ is a squash-specific movement test. Participants were instructed to collect a half-ball that was placed on three racquets positioned around the court, move to the ‘T’, and then to the next racquet at the opposite corner as quickly as possible
jDuring the In the Loughborough Shooting Test (LSST), participants were required to sprint ~12 m, then pass, control and shoot the ball at targets within the goal area
kThe overall stability index (OSI) is an indicator of a subject’s ability to balance on a platform. A higher OSI indicates poorer balance performance
lMean deflection was defined as the average position of the subject during the balance test. A higher mean deflection indicates more displacement and poorer balance performance
Table 6.
Characteristics of research studies evaluating cognitive performance
| Citation | Participants | Dh protocol; ambient temperature; duration | Total REC (min) | Dh trial BM loss (%) | Fluid type | Fluid assimilation time (min) | Fluid intake (L∙kg BM lost−1) |
Cognitive domains assessed | Ambient temperature; RH; airflow | Cognitive domains significantly affected by fluid intake (Hedges’ g) |
|---|---|---|---|---|---|---|---|---|---|---|
| Cian et al. (2001a) [55] | 7 M, unacclimated, endurance trained | EX (V); 25 °C; 120 min | Testing at 140 and 240 min | 2.7 | CHO + electrolyte | 80/180 | 1.00 | Memory Perceptive discrimination Psychomotor function/processing speed Mood |
NS | ↑ Memory 2-h post-Dh |
| Cian et al. (2001b) [55] | 7 M, unacclimated, endurance trained | HT | Testing at 140 and 240 min | 2.6 | CHO + electrolyte | 80/180 | 1.00 | Memory Perceptive discrimination Psychomotor function/processing speed Mood |
NS | ↑ Memory 2-h post-Dh ↓ Fatigue |
| Grego et al. (2004) [48] | 8 M, endurance trained cyclists | EX (V); 20–21 °C; 180 min | 5 | 4.1 | Water | 185 | 0.73 | Perceptual discrimination Memory/processing speed |
NS | No effect |
| Serwah et al. (2006a) [68] | 8 M | EX (V); 31 °C; 90 min | 3 | 1.7 | Water | 9 | 1.00 | Psychomotor function/processing speed | NS | No effect |
| Serwah et al. (2006b) [68] | 8 M | EX (V); 31 °C; 90 min | 3 | 1.7 | Water | 90 | 0.50 | Psychomotor function/processing speed | NS | No effect |
| Edwards et al. (2007b) [58] | 11 M, moderately active soccer players | EX; 19–25 °C; 90 min | 0 | 2.4 | Water | 90 | 0.80 | Visual scanning/processing speed | NS | No effect |
| Adam et al. (2008a) [56] | 8 (6 M), physically active soldiers | HT | 120 | 3.0 | NS | 300 | NS | Psychomotor function/processing speed Psychomotor function Perceptive discrimination Visual scanning/vigilance |
20 °C; 50%; 1 m/s | No effect |
| Adam et al. (2008b) [56] | 8 (6 M), physically active soldiers | HT | 120 | 3.0 | NS | 300 | NS | Psychomotor function/processing speed Psychomotor function Perceptive discrimination Visual scanning/vigilance |
2 °C; 50%; 2.2 m/s | No effect |
| D’Anci et al. (2009a) [73] | 16 M, university athletes | EX; 60 min | NS | 2.0 | Water | 60 | NS | Memory Psychomotor function/processing speed Arithmetic/processing speed Visual scanning/vigilance Spatial processing Mood |
NS | ↑ Psychomotor function/processing speed ↓ Anger, fatigue, depression, tension and confusion; ↑ vigour |
| D’Anci et al. (2009b) [73] | 13 (0 M), university athletes | EX; 60–75 min | NS | 1.7 | Water | 60-75 | NS | Memory Psychomotor function/processing speed Arithmetic/processing speed Visual scanning/vigilance Spatial processing Mood |
NS | ↑ Psychomotor function/processing speed ↓ Anger, fatigue, depression, tension and confusion; ↑ vigour |
| Ganio et al. (2011) [69] | 24 M, physically fit | EX; 28 °C; 40 min | 20 | 1.6 | Water | 60 | NS | Psychomotor function/processing speed Visual scanning/vigilance Memory/processing speed Learning/memory Logical reasoning/processing speed Mood |
23 °C | ↑ Psychomotor function/processing speed and memory/processing speed ↓ Fatigue and tension |
| Ely et al. (2012b) [67] | 32 M, unacclimated | EX; 50 °C; 3 h W/R | 90 | 4.1 | NaCl + water | 270 | 1.00 | Psychomotor function/processing speed Memory/processing speed Logical reasoning/processing speed Mood |
Testing at 10, 20, 30 and 40 °C | No effect |
| Wilson et al. (2014) [60] | 8 M, licenced jockeys | EX; 20 °C; 45 min | 0 | 1.8 | Water | ~35 | 1.00 | Response inhibition | NS | No effect |
| Wittbrodt et al. (2015a) [70] | 12 M, recreationally active | EX (V); 32 °C; 50 min | NS | 1.5 | Water | >50 | 1.00 | Psychomotor function/processing speed Memory Perceptive discrimination Visual scanning/processing speed |
32 °C; 65% | No effect |
| Wittbrodt et al. (2015b) [70] | 12 M, recreationally active | EX (V); 32 °C; 50 min | NS | 1.5 | Water | >50 | 0.80 | Psychomotor function/processing speed Memory Perceptive discrimination Visual scanning/processing speed |
32 °C; 65% | No effect |
Exercise intensity is described as high (H), vigorous (V) or moderate (M), in accordance with classifications outlined by Norton et al. [86]. Values are Hedges’ g effect sizes
CHO carbohydrate, Dh dehydration, EX exercise, HT heat, NS not specified, Total REC time from completing the dehydration protocol to commencing the subsequent task, W/R work rest cycle
Athletic Performance
Forty-nine trials (n = 461, 95% male, excluding Del Coso et al. [46] where gender was NS) examined the effect of fluid intake on athletic performance tasks. Findings from research studies that evaluated athletic performance are summarized in Tables 1, 2, 3, 4 and 5. (Full details are presented in Additional file 1: Table S2, S3, S4, S5 and S6).
Continuous Exercise Performance
Twenty-two trials (n = 170 subjects, 98% male) measured the effect of fluid intake on continuous exercise performance (Table 1). The majority of testing was completed on well-trained individuals (mean VO2 max 57.5–68.4 mL kg−1 min−1) [24, 25, 29, 33, 36, 47, 50, 62, 63, 76, 77] (n = 13 trials). In n = 11 trials, exercise was performed in a warm or hot environment (30–40 °C), by acclimated (n = 2) [63, 77] and unacclimated (n = 5) [29, 36, 47, 62, 76] subjects, where environmental adaptation was specified. The remaining trials were completed under thermoneutral (18–25 °C) [24, 25, 29, 37, 41, 42] (n = 7) or cold (2–10 °C) [41, 42] (n = 2) conditions, where ambient temperature was specified. Fluid intake significantly improved continuous exercise performance in 13 out of the 22 trials reviewed.
Meta-analyses and Meta-regression Analyses
Eighteen trials (n = 139 subjects, 97% male) were included in the meta-analysis examining the effect of fluid consumption on continuous exercise performance. Four continuous exercise trials included in the systematic review were omitted from the meta-analysis on the basis of: (1) duration of the TTE performance test was capped (n = 2) [36, 62]; (2) Rosendal score <50% (n = 1) [47] and (3) extreme outlier, exceeding the mean effect estimate by >3 SD with a studentized residual of 2.82 (n = 1) [64], with the results possibly confounded by fatigue. In this study [64], untrained participants completed 1-h dehydrating exercise at 32 °C before commencing a TTE test at 80% VO2 max, without any recovery. All other investigations completed on participants with a VO2 max less than 50.0 mL kg−1 min−1, i.e. untrained or physically active, employed passive methods of dehydration or allocated ~2 h recovery post-active dehydration [41, 42]. Excluding this trial did not influence the results of the meta-analysis (Hedges’ g = 0.48, 95% CI 0.33, 0.63), thus it was removed.
The weighted mean treatment effect summary indicates fluid intake following a period of dehydration significantly improved continuous exercise performance (g = 0.46, 95% CI 0.32, 0.61) (Fig. 3). Data were normally distributed (Shapiro-Wilk Test, p > 0.05). High heterogeneity was evident between trials (I 2 = 80.5, p < 0.01). Subsequent analyses (see below) determined that 82% of variation between trials is due to differences in the ambient environmental temperature at which the exercise was performed. Thus, sensitivity analysis was completed with trials sub-grouped by environmental temperature, where cold-thermoneutral = ≤25 °C and warm-hot = >25 °C. The magnitude and statistical significance of the treatment effect was stable during sensitivity analysis where trials were sequentially removed, with Hedges’ g ranging between 0.29–0.35 and 0.70–0.81 for cold-thermoneutral and warm-hot ambient temperature subgroups, respectively (all ps <0.01). Findings are comparable across different levels of correlation (R = 0.50, 0.74, 0.84 and 0.94), therefore the meta-analysis (and subsequent meta-regression analyses) are robust to the imputed R = 0.84 (full details of sensitivity analyses are presented in Additional file 1: Table S8 and S9).
Fig. 3.
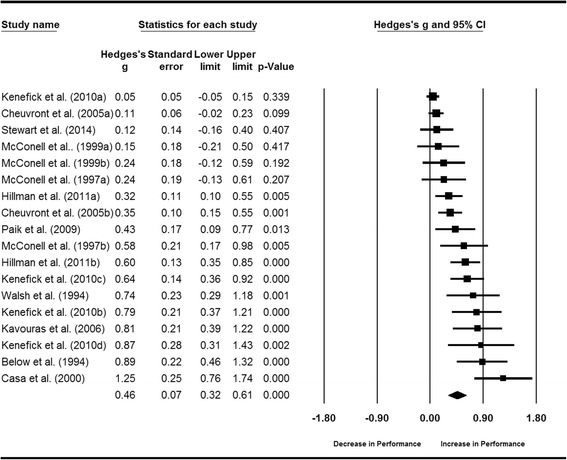
Forest plot displaying the effect of fluid intake on continuous exercise performance. Size of the squares is proportional to the weight of the study
One continuous exercise trial [50] was excluded from the simple meta-regression analysis to determine the relationship between changes in ambient temperature and the magnitude of the weighted mean effect after failing to report environmental temperature at the time of exercise performance. Analyses of the remaining 17 trials (n = 129 subjects, 97% male) detected a strong significant correlation (R 2 = 0.82, p < 0.01) between these parameters (Fig. 4). Therefore, fluid intake may enhance continuous exercise performance to a greater extent at increasing environmental temperatures.
Fig. 4.
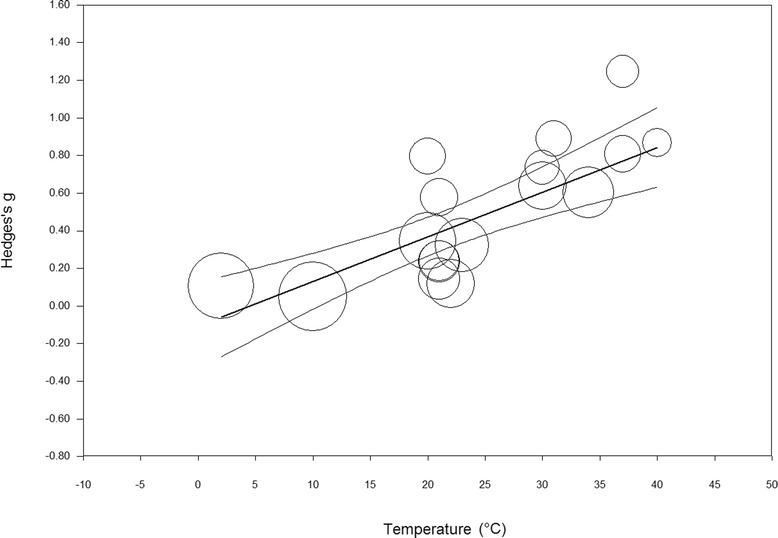
Correlation between change in ambient environmental temperature and change in continuous exercise performance (Hedges’ g). Circle diameter corresponds to the weight of each study. Hedges g = −0.105 + 0.024 × temperature (°C)
The influence of environmental temperature, exercise duration, ecological validity of the exercise protocol and the level of dehydration were controlled in the modelling of the relationship between the volume of fluid consumed (L kg BM lost−1) and the magnitude of the weighted mean effect (Fig. 5). The volume of fluid administered ranged between 0.50–1.15 L kg BM lost−1. No correlation was observed between these parameters (p = 0.625) (Table 7). Mean exercise duration ranged between 4 and 30 min, since no trials involving an exercise task lasting >30 min were eligible for inclusion (as outlined above). There was a trend for fluid intake to improve performance to a greater extent with increasing exercise duration (p = 0.071). The majority of trials (n = 12) measured continuous exercise performance on an ecologically valid exercise protocol, e.g. total work or power output completed within a predefine timeframe (n = 10) [25, 29, 41, 42] or time to complete a set distance (n = 2) [37, 63]. The remaining trials (n = 5) employed a fixed-power TTE exercise protocol with low ecological validity [24, 33, 76, 77]. No significant correlation was observed between the ecological validity of exercise protocol employed and the magnitude of the weighted mean effect (p = 0.188), or the level of dehydration and the magnitude of the weighted mean effect (p = 0.845).
Fig. 5.

Correlation between change in fluid intake (L kg BM lost−1) and change in continuous exercise performance (Hedges’ g) controlling for ambient environmental temperature, exercise duration, level of dehydration and the ecological validity of the exercise protocol. Circle diameter corresponds to the weight of each study. Hedges g = −0.557 + 0.002 × fluid volume (L kg BM lost−1) + 0.025 × temperature (°C) + 0.011 × exercise duration (min) + 0.218, if ecologically valid + 0.0126 × level of dehydration (% BM lost)
Table 7.
Summary of moderator variables for the meta-regression analysis of the effect of fluid volume on the magnitude of the weighted mean treatment effect
| Covariate | Coefficient (95% CI) | p value | R 2 |
|---|---|---|---|
| Fluid volume | 0.002 (−0.006, 0.009) | 0.625 | 0.91 |
| Temperature | 0.025 (0.015, 0.036) | <0.001 | |
| Exercise duration | 0.011 (−0.001, 0.023) | 0.071 | |
| Ecological validity | 0.218 (−0.124, 0.561) | 0.188 | |
| Level of dehydration | 0.013 (−0.126, 0.151) | 0.845 |
One trial [76] failed to report time from commencing fluid ingestion to beginning the subsequent performance task and was excluded from the multiple regression analysis of fluid assimilation time vs. Hedges’ g. Modelling of this relationship corrected for the influence of environmental temperature and type of exercise protocol. Exercise duration was omitted from the model due to collinearity with fluid assimilation time (VIF = 3.18, where all other analyses yielded values ≤1.7). Fluid assimilation time ranged between 45 and 390 min. Analyses of the 16 eligible trials (n = 121 subjects, 94% male) did not detect a significant influence of fluid assimilation time on the weighted mean effect (p = 0.11).
Intermittent Exercise
Ten trials (n = 95 male subjects) evaluated intermittent exercise performance (Table 2). Exercise was undertaken in hot (32–36 °C) [22, 65], thermoneutral (19–22 °C) [51, 59] and cold (16 °C) [71] environmental conditions, where ambient temperature was specified. The majority of testing was done using team sport participants (i.e. individuals who are accustomed to intermittent exercise) [22, 58, 59, 71]. Participants in the remaining trials were untrained [65], physically active [51] or endurance cyclists [33], where the participant population was defined. Fluid intake (0.8–1.55 L kg BM lost−1) significantly improved intermittent exercise performance on 4 out of the 10 trials [22, 58, 65, 71]. The magnitude of improvement ranged from small to large (Hedges g = 0.19–0.97).
Resistance Exercise
Nine trials (n = 83 subjects, 100% male, excluding Del Coso et al. [46] where gender was NS) evaluated resistance exercise performance (Table 3). Across the 8 trials reviewed, 22 separate performance tests were identified. The majority were knee extension or elbow flexion exercise tasks, at variable intensities (n = 18 tasks) [46, 49, 52, 53, 57, 66], although 2 trials measured performance via repetition lifts [54, 74]. Individuals who were accustomed to performing resistance exercise were rarely studied [54, 74]. Fluid intake (1.0–1.10 L kg BM lost−1) significantly improved performance on 7 of 22 resistance exercise tasks completed across 5 trials (Hedges’ g = 0.22–5.57) [49, 53, 54, 60, 66, 75], and significantly decreased performance on 1 task [66].
Sport-Specific Exercise
Six trials (n = 64 subjects, 84% male) evaluated athletic performance on exercise tasks that were specific to either cricket (n = 1) [71], soccer (n = 3) [35, 59], squash (n = 1) [72] or racehorse riding (n = 1) [60] (Table 4). All participants were experienced on the sporting activity for which they were assessed. Fluid intake had no effect on soccer players’ ball-skills (e.g. passing and shooting) [59]. However, squash-specific movements, cricket bowling accuracy and racehorse riding demonstrated moderate to large performance improvements with fluid intake (0.4–1.0 L kg BM lost−1).
Balance Exercise
Two trials (n = 49 male subjects) examined balance performance (Table 5). A significant positive effect of fluid intake was documented for 1 out of 8 balance-related tests completed across both trials.
Cognitive Performance and Mood State Outcomes
Fifteen trials (n = 182 subjects, 90% male) examined the effect of fluid intake on cognitive performance and/or mood. Major findings are summarized in Table 6. (Full details are presented in Additional file 1: Table S7). Across the 15 trials reviewed, 49 neuropsychological tests were identified. Evidence indicating a beneficial effect of fluid intake on cognitive performance was observed on 5 cognitive tests completed across 5 trials [55, 69, 73]. Cognitive domains affected were memory, psychomotor function and processing speed. Four out of the 6 trials evaluating the influence of fluid intake on mood state observed significant positive effects [55, 69, 73], as indicated by decreased subjective ratings of fatigue, anger, depression, tension and confusion and increased vigour.
Discussion
Individuals prone to dehydration (e.g. athletes and manual workers) may have limited opportunity to adequately rehydrate prior to performing physically or cognitively demanding activities. The present systematic review and meta-analysis examines evidence for the effects of fluid intake on subsequent athletic and cognitive performance following dehydrating sweat loss. A beneficial effect for fluid intake was strongest when athletic performance involved continuous exercise tasks. Further, the magnitude of improvement appeared greater when the continuous exercise was performed at elevated environmental temperatures and over longer exercise durations. Whilst the volume of fluid consumed (relative to BM lost) did not appear to influence the size of the treatment effect, fluid intake at levels complying with current recommendations for completely replacing lost fluid (1.25–1.50 L kg BM lost−1) [16, 17] are yet to be thoroughly investigated. Evidence for a beneficial effect of fluid intake on intermittent, resistance and sport-specific exercise performance and cognitive function or mood is less apparent and requires further elucidation.
The weighted mean effect suggests that fluid ingestion following a period of dehydration significantly improves continuous exercise performance, compared to control conditions (no fluid or negligible fluid intake). Individual estimates all indicated a beneficial effect from fluid intake; however, the magnitude of the improvement was heterogeneous (I 2 = 80.5%) which may reflect differences in the methodologies employed between studies. Simple meta-regression determined that 82% of variation between trials can be attributed to differences in the ambient environmental temperature at which subsequent exercise was performed, with fluid intake demonstrating greater efficacy under heat stress conditions. The decline in aerobic performance that occurs with hypohydration has largely been attributed to circulatory strain, whereby reductions in blood volume limit oxygen transport to the exercising muscle [78, 79]. Under elevated environmental temperatures, blood flow is also redirected to the skin facilitating evaporative cooling, augmenting circulatory strain and further impairing exercise performance [41]. These physiological perturbations are typically characterized by increased heart rate and core temperature [41, 78, 79]. Hence, thermoregulatory parameters were monitored in many of the studies reviewed (11/15) [24, 25, 29, 33, 36, 37, 41, 42, 47, 62, 63, 76, 77]. The majority of reviewed studies reported that consumption of fluid was associated with significant reductions in core or rectal temperature (7/11) [36, 41, 42, 47, 62, 63, 76] and heart rate (6/10) [36, 41, 42, 47, 62, 63, 76, 77] at various time points during continuous exercise performance (i.e. for at least one fluid intervention). Thus, fluid intake may offset circulatory strain typically observed when exercise is undertaken in warm environments. The multiple meta-regression analysis also suggests that differences in the duration of the continuous exercise performed may account for a proportion of the heterogeneity observed between experimental trials, with exercise performed over longer durations yielding greater benefit from fluid intake than short duration exercise. However, as the majority of performance tests included in the analysis were relatively short in duration (4–30 min), we cannot be certain that this relationship would hold true over longer exercise durations (i.e. 2–8 h).
Results of the meta-regression failed to indicate a statistically significant relationship between the volume of fluid consumed and continuous exercise performance improvements. However, the majority of trials tested a quantity of fluid that was within a narrow fluid intake range (i.e. 1.0–1.05 L kg BM lost−1, n = 13 out of 18). Hence, the performance effects associated with ingesting a comparably small volume of fluid (e.g. ≤0.75 L kg BM lost−1) or an amount consistent with recommended guidelines (e.g. 1.25–1.50 L kg BM lost−1) remains uncertain. Three experimental investigations have examined the dose-response effect of ingested fluid volume on continuous exercise performance following a period of dehydration with the results demonstrating inconsistent findings [21, 24, 25]. Unfortunately, the investigation with the greatest contrast in fluid volumes (i.e. 0.75 vs. 1.50 L kg BM lost−1 [21]) did not employ a ‘no fluid’ control and was unable to be included in the meta-analysis. Findings from previous studies suggest that fluid intake during exercise exceeding that dictated by thirst may not provide additional performance benefits [80]. However, only three of the publications reviewed measured subjective thirst within the investigation (and these studies did not test different fluid volumes, i.e. only one intervention vs. control). Therefore, it is not clear whether the equivocal effect of fluid intake volume can be attributed to thirst sensation. Based on current evidence, prescribing fluid volumes required to optimize performance on a subsequent continuous exercise task requires clarification.
If relatively small and large fluid intakes elicit comparable treatment effects, individuals who have limited time to rehydrate prior to performing aerobic activities may opt to consume smaller fluid boluses, delaying complete rehydration until circumstances permit (e.g. overnight). This strategy may reduce the probability of the drinker experiencing volume-induced GI discomfort during subsequent activity, which may occur when larger fluid volumes are ingested [25]. Only one of the 42 publications reviewed monitored GI symptomology [25]. In this study, subjective ratings of GI discomfort following different fluid intakes (~0.5 vs. 1.0 L kg BM lost−1) were described as mild to moderate and moderate to high on each trial, respectively. This suggests that larger fluid volumes are likely to induce some degree of participant discomfort which may compromise performance. However, research examining continuous exercise performance following two volumes of fluid intake (i.e. 0.75 vs. 1.50 L kg BM lost−1) demonstrated significantly faster (~3.0%) running performance associated with the larger bolus [21]. Importantly, this study employed a prolonged (i.e. overnight) rehydration period reducing the probability of severe GI disturbance and allowing ingested fluid to equilibrate throughout the body. Further research examining exercise performance (and GI symptoms) when large fluid volumes are ingested over short rehydration periods is warranted.
The effect of fluid intake on intermittent, resistance, sport-specific and balance exercise types remains unclear. It appears that fluid ingestion following a period of dehydration may improve performance on subsequent intermittent, resistance and sport-specific exercise tasks. However, methodological differences make comparison of results across trials challenging.
In regards to intermittent exercise, 4 of 10 trials (n = 95 male subjects) observed a significant positive effect of fluid intake on performance, whilst no trial reported a significant performance decrement. Similarly to the results from continuous exercise, beneficial effects of fluid intake are apparent when intermittent exercise tasks have been completed in warm environments [22, 65]. The impact of task duration may also influence the likelihood of observing performance effects, with longer duration tasks more regularly demonstrating a performance enhancement associated with fluid ingestion [22, 65]. For instance, Maxwell et al. [22] observed that fluid intake only benefited performance on a second repeated sprint bout completed in the latter stages of testing.
Concerning resistance exercise, 6 of 9 trials (n = 83 subjects, 93% male) observed a significant positive effect of fluid intake on performance. One trial reported that fluid intake was detrimental to performance [66]. However, results from this study need to be interpreted with caution as the strength performance task was performed following an endurance task that varied in duration. Evidence indicating a beneficial effect of fluid intake on resistance exercise performance appears stronger when tests of muscular endurance, rather than tests of muscular strength, are employed [53]. Findings from this systematic review demonstrate significantly improved performance on 3 out of the 4 submaximal intensity resistance exercise tasks [53, 66, 74]. In contrast, performance on only 4 out of 15 maximal intensity tests demonstrated improvement with fluid intake [49, 54, 60]. Current evidence is inadequate to determine the influence of other variables (e.g. participant population, mode of dehydration) on the effect of fluid intake. Further research examining the effects of fluid intake on resistance exercise performance using standardized procedures is required.
The 6 trials (n = 64 subjects, 84% male) that evaluated the effect of fluid intake on sport-specific exercise performance exhibited considerable heterogeneity, with tests of cricket [71], soccer [35, 58, 59], squash [72] and racehorse riding [60] performance all being employed. Whilst the majority of sports-specific research has demonstrated no impact of fluid consumption on subsequent performance, the paucity of data and lack of replication studies makes it difficult to determine an overall effect of fluid intake on sport-specific exercise performance.
The present systematic review identified 14 trials (n = 174 subjects, 90% male) examining the effect of fluid intake following a period of dehydration on cognitive function and mood state. Evidence indicating a beneficial effect of fluid intake on cognitive performance was only observed in some studies [55, 73], and there was no clear indication of greater treatment efficacy on a particular cognitive domain. However, some limitations to the current evidence exist. In 4 trials, the cognitive assessment was conducted ≤5 min after concluding the dehydration protocol [48, 58, 68]; further 4 trials did not provide the necessary information to calculate the amount of time between the conclusion of the dehydration protocol and commencement of the cognitive tests [58, 70, 73]. Prior research indicates that acute exercise has a small positive effect on cognitive performance (typically dissipating within ~15 min of exercise cessation) [81], whilst elevated core temperature via heat stress may provide additional cognitive burden [82]. Therefore, the residual effects of physiological stressors used to induce dehydration in these trials may obscure any influence of fluid intake on cognitive performance. Investigations examining the effect of hydration on cognitive performance should also employ neuropsychological tests that have previously demonstrated sensitivity to nutritional interventions [34, 83, 84]. Yet, only two studies included in the present review selected cognitive tests on this basis [56, 73]. The majority did not provide any rationale for their chosen method of assessment [48, 55, 58, 67, 68, 70], increasing the likelihood of false-negative reports. Fluid consumption positively influenced mood state (measured as reduced anger, fatigue, depression, tension and confusion) in 4 out of the 6 trials where it was measured [55, 69, 73]. Whilst this may suggest that self-reported mood state questionnaires are more sensitive to the effects of fluid intake than objective tests of cognitive function, subjective mood ratings were only influenced by fluid intake during trials where significant cognitive effects were also observed, i.e. effects on mood and cognition were not independent of one another. Collectively, it appears that the influence of fluid intake on mood and cognitive performance is still poorly understood and requires further research employing tasks with demonstrated sensitivity.
This review does contain a number of limitations. Firstly, only studies with accessible full text articles written in English were included. Second, three of the studies reviewed [69, 71, 77] examined rehydration in combination with another placebo treatment (studies were excluded if fluids were co-administered with another experimental treatment). Thus, participants’ perceptions regarding the expected treatment may have influenced these results. Third, as oral fluid replacement cannot be blinded, it is conceivable that the placebo effect may account for a small amount of benefit observed with rehydration. However, it was necessary to exclude research studies that blinded participants to hydration status using intravenous methods because the infusion does not accurately mimic the physiological effects of oral rehydration. Fourth, the present review elected to compare against a “no fluid” or “negligible fluid” control condition, because a euhydrated control may be confounded by the effects of the dehydration protocol itself (i.e. hyperthermia or fatigue). However, using this comparison, we cannot determine whether fluid intake fully or partially restored performance to euhydrated levels. Similarly, fluid ingestion may have minimal or no effect on athletic or cognitive performance if the outcome measured is not sensitive to the effects of modest fluid losses. Fifth, where fluid was administered at the time of dehydration (i.e. concurrent fluid intake), rather than following dehydration (i.e. subsequent fluid intake), different physiologic responses to the dehydration protocol may occur on control and intervention trials, e.g. decreased core temperature leading to reduced central fatigue. This could have implications for subsequent athletic performance, and consequently, the magnitude of the overall treatment effect. Sixth, fluid intake ≤200 mL was considered ‘negligible’ and included within the definition of control conditions. However, one study has reported that ingesting 100 mL of fluid (25 mL boluses at 5-min intervals during exercise) increased TTE following exercise-induced dehydration [85]. Thus, trials administering ≤200 mL fluid to dehydrated control subjects may underestimate the true magnitude of the treatment effect.
Conclusions
Collectively, the results of the present review suggest that individuals who have limited opportunity to adequately rehydrate prior to performing continuous exercise in a heated environment should consume fluid, even if the body water deficit is modest (1.3% reduction in BM) and fluid intake is inadequate for complete rehydration (0.5 L kg BM lost−1). The influence of fluid intake for those individuals performing intermittent, resistance and sport-specific exercise or undertaking cognitively demanding tasks is not as well understood, and this review serves to highlight areas for future research.
Acknowledgments
Funding
No funding was received for the preparation of this manuscript.
Authors’ Contributions
All authors (DM, BD and CI) were involved in the conception and design of this review. DM and CI were responsible for collating the manuscripts and retrieving the data. DM conducted the analysis of the data. All authors (DM, BD and CI) contributed to drafting and revising the article and the final approval of the published version of the manuscript.
Competing Interests
Danielle McCartney, Ben Desbrow and Christopher Irwin declare that they have no conflict of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional File
Supplementary Table S1 - S9. (DOC 725 kb)
References
- 1.Gigou P, Lamontagne-Lacasse M, Goulet EDB, editors. Meta-analysis of the effects of pre-exercise hypohydration on endurance performance, lactate threshold and vo(2max). 57th Annual Meeting of the American-College-Sports-Medicine/Inaugural World Congress on Exercise is Medicine; 2010. Baltimore (USA): Med Sci Sport Exer. 2010;42(5):361-62.
- 2.Savoie F-A, Kenefick RW, Ely BR, Cheuvront SN, Goulet EDB. Effect of hypohydration on muscle endurance, strength, anaerobic power and capacity and vertical jumping ability: a meta-analysis. Sports Med (Auckland, NZ) 2015;45(8):1207–27. doi: 10.1007/s40279-015-0349-0. [DOI] [PubMed] [Google Scholar]
- 3.Gamage JP, De Silva AP, Nalliah AK, Galloway SD. Effects of dehydration on cricket specific skill performance in hot and humid conditions. Int J Sport Nutr Exerc Metab. 2016. doi:10.1123/ijsnem.2016-0015 [DOI] [PubMed]
- 4.Baker LB, Dougherty KA, Chow M, Kenney WL. Progressive dehydration causes a progressive decline in basketball skill performance. Med Sci Sport Exer. 2007;39(7):1114–23. doi: 10.1249/mss.0b013e3180574b02. [DOI] [PubMed] [Google Scholar]
- 5.Dougherty KA, Baker LB, Chow M, Kenney WL. Two percent dehydration impairs and six percent carbohydrate drink improves boys basketball skills. Med Sci Sport Exer. 2006;38(9):1650–8. doi: 10.1249/01.mss.0000227640.60736.8e. [DOI] [PubMed] [Google Scholar]
- 6.Smith MF, Newell AJ, Baker MR. Effect of acute mild dehydration on cognitive-motor performance in golf. J Strength Con Res. 2012;26(11):3075–80. doi: 10.1519/JSC.0b013e318245bea7. [DOI] [PubMed] [Google Scholar]
- 7.MacLeod H, Sunderland C. Previous-day hypohydration impairs skill performance in elite female field hockey players. Scand J Med Sci Spor. 2012;22(3):430–8. doi: 10.1111/j.1600-0838.2010.01230.x. [DOI] [PubMed] [Google Scholar]
- 8.Carrasco A. Effects of exercise-induced dehydration on cognitive ability, muscular endurance and surfing performance. Auckland: Massey University; 2008. [Google Scholar]
- 9.Benton D. Dehydration influences mood and cognition: a plausible hypothesis? Nutrients. 2011;3(5):555–73. doi: 10.3390/nu3050555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masento NA, Golightly M, Field DT, Butler LT, van Reekum CM. Effects of hydration status on cognitive performance and mood. Brit J Nutr. 2014;111(10):1841–52. doi: 10.1017/S0007114513004455. [DOI] [PubMed] [Google Scholar]
- 11.Castro-Sepúlveda M, Ramírez-Campillo R, Astudillo S, Burgos C, Henríquez-Olguín C. Prevalence of dehydration and fluid intake practices in elite rally dakar drivers. Sci Sports. 2014;29(6):327–30. doi: 10.1016/j.scispo.2014.04.005. [DOI] [Google Scholar]
- 12.Ferreira AM, de Oliveira AB, Marostica MR, Rosendo da Silva MDD, Carvalho da Rocha OA, Kherlakian R, et al. Swat rate and hydration status on swimmers. Brazilian J Sports Nutr. 2015;9(51):247–54. [Google Scholar]
- 13.Gatterer H, Schenk K, Ferrari P, Faulhaber M, Schopp E, Burtscher M. Changes in hydration status of soccer players competing in the 2008 European Championship. J Sport Med Phys Fit. 2011;51(1):89–94. [PubMed] [Google Scholar]
- 14.Minton DM, Torres-McGehee TM, Emerson CC, LaSalle TL. Chronic hypohydration in minor professional ice hockey players. Med Sci Sport Exer. 2010;42(5):642. doi: 10.1249/01.MSS.0000385790.78778.c3. [DOI] [Google Scholar]
- 15.Kenefick RW, Sawka MN. Hydration at the work site. J Am Coll Nutr. 2007;26(5 Suppl):597s–603s. doi: 10.1080/07315724.2007.10719665. [DOI] [PubMed] [Google Scholar]
- 16.Sawka MN, Burke LM, Eichner ER, Maughan RJ, Montain SJ. American College of Sports Medicine position stand. Exercise and fluid replacement. Med Sci Sport Exer. 2007;39(2):377–90. doi: 10.1249/mss.0b013e31802ca597. [DOI] [PubMed] [Google Scholar]
- 17.Thomas DT, Erdman KA, Burke LM. Position of the academy of nutrition and dietetics, dietitians of Canada, and the American college of sports medicine: nutrition and athletic performance. J Acad Nutr Diet. 2016;116(3):501–28. doi: 10.1016/j.jand.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Desbrow B, Jansen S, Barrett A, Leveritt MD, Irwin C. Comparing the rehydration potential of different milk-based drinks to a carbohydrate-electrolyte beverage. Appl Physiol Nutr Me. 2014;39(12):1366–72. doi: 10.1139/apnm-2014-0174. [DOI] [PubMed] [Google Scholar]
- 19.Kwiatek MA, Menne D, Steingoetter A, Goetze O, Forras-Kaufman Z, Kaufman E, et al. Effect of meal volume and calorie load on postprandial gastric function and emptying: studies under physiological conditions by combined fiber-optic pressure measurement and MRI. Am J Physiol-Gastr L. 2009;297(5):G894–G901. doi: 10.1152/ajpgi.00117.2009. [DOI] [PubMed] [Google Scholar]
- 20.Deibert P, Koenig D, Dickhuth HH, Berg A. The gastrointestinal system: the relationship between an athlete’s health and sport performance. Int Sportmed J. 2005;6(3):130–40. [Google Scholar]
- 21.Davis BA, Thigpen LK, Hornsby JH, Green JM, Coates TE, O'Neal EK. Hydration kinetics and 10-km outdoor running performance following 75% versus 150% between bout fluid replacement. Eur J Sport Sci. 2014;14(7):703–10. doi: 10.1080/17461391.2014.894578. [DOI] [PubMed] [Google Scholar]
- 22.Maxwell NS, McKenzie RW, Bishop D. Influence of hypohydration on intermittent sprint performance in the heat. Int J Sports Physiol Perform. 2009;4(1):54–67. doi: 10.1123/ijspp.4.1.54. [DOI] [PubMed] [Google Scholar]
- 23.Davis JK, Laurent CM, Allen KE, Green JM, Stolworthy NI, Welch TR, et al. Influence of dehydration on intermittent sprint performance. J Strength Cond Res. 2015;29(9):2586–93. doi: 10.1519/JSC.0000000000000907. [DOI] [PubMed] [Google Scholar]
- 24.McConell GK, Burge CM, Skinner SL, Hargreaves M. Influence of ingested fluid volume on physiological responses during prolonged exercise. Acta Physiol Scand. 1997;160(2):149–56. doi: 10.1046/j.1365-201X.1997.00139.x. [DOI] [PubMed] [Google Scholar]
- 25.McConell GK, Stephens TJ, Canny BJ. Fluid ingestion does not influence intense 1-h exercise performance in a mild environment. Med Sci Sport Exer. 1999;31(3):386–92. doi: 10.1097/00005768-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (prisma-p) 2015 statement. Syst Rev. 2015;4(1):1–9. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bardis CN, Kavouras SA, Arnaoutis G, Panagiotakos DB, Sidossis LS. Mild dehydration and cycling performance during 5-kilometer hill climbing. J Athl Training. 2013;48(6):741–7. doi: 10.4085/1062-6050-48.5.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baker LB, Conroy DE, Kenney WL. Dehydration impairs vigilance-related attention in male basketball players. Med Sci Sport Exer. 2007;39(6):976–83. doi: 10.1097/mss.0b013e3180471ff2. [DOI] [PubMed] [Google Scholar]
- 29.Hillman A, Vince R, Taylor L, McNaughton L, Mitchell N, Siegler J. Exercise-induced dehydration with and without environmental heat stress results in increased oxidative stress. Appl Physiol Nutr Me. 2011;36(5):698–706. doi: 10.1139/h11-080. [DOI] [PubMed] [Google Scholar]
- 30.van Rosendal SP, Osborne MA, Fassett RG, Coombes JS. Guidelines for glycerol use in hyperhydration and rehydration associated with exercise. Sports Med (Auckland, NZ) 2010;40(2):113–29. doi: 10.2165/11530760-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 31.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 32.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. Wiley online library. 2008
- 33.Walsh RM, Noakes TD, Hawley JA, Dennis SC. Impaired high-intensity cycling performance time at low levels of dehydration. Int J Sports Med. 1994;15(7):392–8. doi: 10.1055/s-2007-1021076. [DOI] [PubMed] [Google Scholar]
- 34.Lieberman HR. Methods for assessing the effects of dehydration on cognitive function. Nutr Rev. 2012;70(Suppl 2):S146. doi: 10.1111/j.1753-4887.2012.00524.x. [DOI] [PubMed] [Google Scholar]
- 35.Ali A, Gardiner R, Foskett A, Gant N. Fluid balance, thermoregulation and sprint and passing skill performance in female soccer players. Scan J Med Sci Spor. 2011;21(3):437–45. doi: 10.1111/j.1600-0838.2009.01055.x. [DOI] [PubMed] [Google Scholar]
- 36.Kenefick RW, O'Moore KM, Mahood NV, Castellani JW. Rapid iv versus oral rehydration: responses to subsequent exercise heat stress. Med Sci Sport Exer. 2006;38(12):2125–31. doi: 10.1249/01.mss.0000235358.39555.80. [DOI] [PubMed] [Google Scholar]
- 37.Stewart CJ, Whyte DG, Cannon J, Wickham J, Marino FE. Exercise-induced dehydration does not alter time trial or neuromuscular performance. Int J Sports Med. 2014;35(9):725–30. doi: 10.1055/s-0033-1364022. [DOI] [PubMed] [Google Scholar]
- 38.Hedges LV. Distribution theory for glass’s estimator of effect size and related estimators. J Educ Stat. 1981;6:107–28. doi: 10.2307/1164588. [DOI] [Google Scholar]
- 39.Cohen J. Statistical power analysis for behavioral sciences. New York, NY: Routledge; 1988. [Google Scholar]
- 40.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Brit Med J. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kenefick RW, Cheuvront SN, Palombo LJ, Ely BR, Sawka MN. Skin temperature modifies the impact of hypohydration on aerobic performance. J Appl Physiol. 2010;109(1):79–86. doi: 10.1152/japplphysiol.00135.2010. [DOI] [PubMed] [Google Scholar]
- 42.Cheuvront SN, Carter R, III, Castellani JW, Sawka MN. Hypohydration impairs endurance exercise performance in temperate but not cold air. J Appl Physiol. 2005;99(5):1972–6. doi: 10.1152/japplphysiol.00329.2005. [DOI] [PubMed] [Google Scholar]
- 43.Goulet EDB. Effect of exercise-induced dehydration on endurance performance: evaluating the impact of exercise protocols on outcomes using a meta-analytic procedure. Brit J Sports Med. 2013;47(11):679–86. doi: 10.1136/bjsports-2012-090958. [DOI] [PubMed] [Google Scholar]
- 44.Goulet ED. Effect of exercise-induced dehydration on time-trial exercise performance: a meta-analysis. Br J Sports Med. 2011;45(14):1149–56. doi: 10.1136/bjsm.2010.077966. [DOI] [PubMed] [Google Scholar]
- 45.Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013;4:863. doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Del Coso J, Estevez E, Baquero RA, Mora-Rodriguez R. Anaerobic performance when rehydrating with water or commercially available sports drinks during prolonged exercise in the heat. Appl Physiol Nutr Me. 2008;33(2):290–8. doi: 10.1139/H07-188. [DOI] [PubMed] [Google Scholar]
- 47.Melin B, Curé M, Jimenez C, Koulmann N, Savourey G, Bittel J. Effect of ingestion pattern on rehydration and exercise performance subsequent to passive dehydration. Eur J Appl Physiol O. 1994;68(4):281–4. doi: 10.1007/BF00571443. [DOI] [PubMed] [Google Scholar]
- 48.Grego F, Vallier JM, Collardeau M, Rousseu C, Cremieux J, Brisswalter J. Influence of exercise duration and hydration status on cognitive function during prolonged cycling exercise. Int J Sports Med. 2005;26(1):27–33. doi: 10.1055/s-2004-817915. [DOI] [PubMed] [Google Scholar]
- 49.Rodrigues R, Baroni BM, Pompermayer MG, De Oliveira LR, Geremia JM, Meyer F, et al. Effects of acute dehydration on neuromuscular responses of exercised and nonexercised muscles after exercise in the heat. J Strength Cond Res. 2014;28(12):3531–6. doi: 10.1519/JSC.0000000000000578. [DOI] [PubMed] [Google Scholar]
- 50.Paik IY, Jeong MH, Jin HE, Kim YI, Suh AR, Cho SY, et al. Fluid replacement following dehydration reduces oxidative stress during recovery. Biochem Bioph Res Co. 2009;383(1):103–7. doi: 10.1016/j.bbrc.2009.03.135. [DOI] [PubMed] [Google Scholar]
- 51.Cheuvront SN, Carter R, III, Haymes EM, Sawka MN. No effect of moderate hypohydration or hyperthermia on anaerobic exercise performance. Med Sci Sport Exer. 2006;38(6):1093–7. doi: 10.1249/01.mss.0000222838.74015.15. [DOI] [PubMed] [Google Scholar]
- 52.Greiwe JS, Staffey KS, Melrose DR, Narve MD, Knowlton RG. Effects of dehydration on isometric muscular strength and endurance. Med Sci Sport Exer. 1998;30(2):284–8. doi: 10.1097/00005768-199802000-00017. [DOI] [PubMed] [Google Scholar]
- 53.Bigard AX, Sanchez H, Claveyrolas G, Martin S, Thimonier B, Arnaud MJ. Effects of dehydration and rehydration on EMG changes during fatiguing contractions. Med Sci Sport Exer. 2001;33(10):1694–700. doi: 10.1097/00005768-200110000-00013. [DOI] [PubMed] [Google Scholar]
- 54.Schoffstall JE, Branch JD, Leutholtz BC, Swain DE. Effects of dehydration and rehydration on the one-repetition maximum bench press of weight-trained males. J Strength Cond Res. 2001;15(1):102–8. [PubMed] [Google Scholar]
- 55.Cian C, Barraud PA, Melin B, Raphel C. Effects of fluid ingestion on cognitive function after heat stress or exercise-induced dehydration. Int J Psychophysiol. 2001;42(3):243–51. doi: 10.1016/S0167-8760(01)00142-8. [DOI] [PubMed] [Google Scholar]
- 56.Adam GE, Carter R, III, Cheuvront SN, Merullo DJ, Castellani JW, Lieberman HR, et al. Hydration effects on cognitive performance during military tasks in temperate and cold environments. Physiol Behav. 2008;93(4-5):748–56. doi: 10.1016/j.physbeh.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 57.Ali A, Williams C. Isokinetic and isometric muscle function of the knee extensors and flexors during simulated soccer activity: effect of exercise and dehydration. J Sport Sci. 2013;31(8):907–16. doi: 10.1080/02640414.2012.753635. [DOI] [PubMed] [Google Scholar]
- 58.Edwards AM, Mann ME, Marfell-Jones MJ, Rankin DM, Noakes TD, Shillington DP. Influence of moderate dehydration on soccer performance: physiological responses to 45 min of outdoor match-play and the immediate subsequent performance of sport-specific and mental concentration tests. Brit J Sport Med. 2007;41(6):385–91. doi: 10.1136/bjsm.2006.033860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Owen JA, Kehoe SJ, Oliver SJ. Influence of fluid intake on soccer performance in a temperate environment. J Sport Sci. 2013;31(1):1–10. doi: 10.1080/02640414.2012.720701. [DOI] [PubMed] [Google Scholar]
- 60.Wilson G, Hawken MB, Poole I, Sparks A, Bennett S, Drust B, et al. Rapid weight-loss impairs simulated riding performance and strength in jockeys: implications for making-weight. J Sport Sci. 2014;32(4):383–91. doi: 10.1080/02640414.2013.825732. [DOI] [PubMed] [Google Scholar]
- 61.Erkmen N, Taskin H, Kaplan T, Sanioglu A. Balance performance and recovery after exercise with water intake, sport drink intake and no fluid. J Exerc Sci Fit. 2010;8(2):105–12. doi: 10.1016/S1728-869X(10)60016-0. [DOI] [Google Scholar]
- 62.Castellani JW, Maresh CM, Armstrong LE, Kenefick RW, Riebe D, Echegaray M, et al. Intravenous vs oral rehydration: effects on subsequent exercise heat stress. J Appl Physiol. 1997;82(3):799–806. doi: 10.1152/jappl.1997.82.3.799. [DOI] [PubMed] [Google Scholar]
- 63.Below PR, Morarodriguez R, Gonzalezalonso J, Coyle EF. Fluid and carbohydrate ingestion independently improve performance during 1-h of intense exercise. Med Sci Sport Exer. 1995;27(2):200–10. doi: 10.1249/00005768-199502000-00009. [DOI] [PubMed] [Google Scholar]
- 64.Hasegawa H, Takatori T, Komura T, Yamasaki M. Combined effects of pre-cooling and water ingestion on thermoregulation and physical capacity during exercise in a hot environment. J Sport Sci. 2006;24(1):3–9. doi: 10.1080/02640410400022185. [DOI] [PubMed] [Google Scholar]
- 65.Maxwell NS, Gardner F, Nimmo MA. Intermittent running: muscle metabolism in the heat and effect of hypohydration. Med Sci Sport Exer. 1999;31(5):675–83. doi: 10.1097/00005768-199905000-00009. [DOI] [PubMed] [Google Scholar]
- 66.Montain SJ, Smith SA, Mattot RP, Zientara GP, Jolesz FA, Sawka MN. Hypohydration effects on skeletal muscle performance and metabolism: a p-31-mrs study. J Appl Physiol. 1998;84(6):1889–94. doi: 10.1152/jappl.1998.84.6.1889. [DOI] [PubMed] [Google Scholar]
- 67.Ely BR, Sollanek KJ, Cheuvront SN, Lieberman HR, Kenefick RW. Hypohydration and acute thermal stress affect mood state but not cognition or dynamic postural balance. Eur J Appl Physiol. 2013;113(4):1027–34. doi: 10.1007/s00421-012-2506-6. [DOI] [PubMed] [Google Scholar]
- 68.Serwah N, Marino FE. The combined effects of hydration and exercise heat stress on choice reaction time. J Sci Med Sport. 2006;9(1-2):157–64. doi: 10.1016/j.jsams.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 69.Ganio MS, Armstrong LE, Casa DJ, McDermott BP, Lee EC, Yamamoto LM, et al. Mild dehydration impairs cognitive performance and mood of men. Brit J Nutr. 2011;106(10):1535–43. doi: 10.1017/S0007114511002005. [DOI] [PubMed] [Google Scholar]
- 70.Wittbrodt MT, Millard-Stafford M, Sherman RA, Cheatham CC. Fluid replacement attenuates physiological strain resulting from mild hypohydration without impacting cognitive performance. Int J Sport Nutr Exe. 2015;25(5):439–47. doi: 10.1123/ijsnem.2014-0173. [DOI] [PubMed] [Google Scholar]
- 71.Devlin LH, Fraser SF, Barras NS, Hawley JA. Moderate levels of hypohydration impairs bowling accuracy but not bowling velocity in skilled cricket players. J Sci Med Sport. 2001;4(2):179–87. doi: 10.1016/S1440-2440(01)80028-1. [DOI] [PubMed] [Google Scholar]
- 72.Fritz S, Toriola AL, Neveling N. Effects of hydration strategies on competitive squash performance. Med Sport. 2013;66(3):363–74. [Google Scholar]
- 73.D'Anci KE, Vibhakar A, Kanter JH, Mahoney CR, Taylor HA. Voluntary dehydration and cognitive performance in trained college athletes. Percept Motor Skill. 2009;109(1):251–69. doi: 10.2466/pms.109.1.251-269. [DOI] [PubMed] [Google Scholar]
- 74.Kraft JA, Green JM, Bishop PA, Richardson MT, Neggers YH, Leeper JD. Impact of dehydration on a full body resistance exercise protocol. Eur J Appl Physiol. 2010;109(2):259–67. doi: 10.1007/s00421-009-1348-3. [DOI] [PubMed] [Google Scholar]
- 75.Kraft JA, Green JM, Bishop PA, Richardson MT, Neggers YH, Leeper JD. Effects of heat exposure and 3% dehydration achieved via hot water immersion on repeated cycle sprint performance. J Strength Cond Res. 2011;25(3):778–86. doi: 10.1519/JSC.0b013e3181c1f79d. [DOI] [PubMed] [Google Scholar]
- 76.Casa DJ, Maresh CM, Armstrong LE, Kavouras SA, Herrera JA, Hacker FT, et al. Intravenous versus oral rehydration during a brief period: responses to subsequent exercise in the heat. Med Sci Sport Exer. 2000;32(1):124–33. doi: 10.1097/00005768-200001000-00019. [DOI] [PubMed] [Google Scholar]
- 77.Kavouras SA, Armstrong LE, Maresh CM, Casa DJ, Herrera-Soto JA, Scheett TP, et al. Rehydration with glycerol: endocrine, cardiovascular, and thermoregulatory responses during exercise in the heat. J Appl Physiol. 2006;100(2):442–50. doi: 10.1152/japplphysiol.00187.2005. [DOI] [PubMed] [Google Scholar]
- 78.Nixon PGF. Human circulation regulation during physical stress. Stress Medicine. 1988;4(2):124–5. doi: 10.1002/smi.2460040213. [DOI] [Google Scholar]
- 79.Cheuvront SN, Kenefick RW, Montain SJ, Sawka MN. Mechanisms of aerobic performance impairment with heat stress and dehydration. J Appl Physiol. 2010;109(6):1989–95. doi: 10.1152/japplphysiol.00367.2010. [DOI] [PubMed] [Google Scholar]
- 80.Goulet ED. Dehydration and endurance performance in competitive athletes. Nutr Rev. 2012;70(Suppl 2):S132–6. doi: 10.1111/j.1753-4887.2012.00530.x. [DOI] [PubMed] [Google Scholar]
- 81.Chang YK, Labban JD, Gapin JI, Etnier JL. The effects of acute exercise on cognitive performance: a meta-analysis. Brain Res. 2012;1453:87–101. doi: 10.1016/j.brainres.2012.02.068. [DOI] [PubMed] [Google Scholar]
- 82.Bandelow S, Maughan R, Shirreffs S, Ozgunen K, Kurdak S, Ersoz G, et al. The effects of exercise, heat, cooling and rehydration strategies on cognitive function in football players. Scand J Med Sci Spor. 2010;20:148–60. doi: 10.1111/j.1600-0838.2010.01220.x. [DOI] [PubMed] [Google Scholar]
- 83.Lieberman HR. Hydration and cognition: a critical review and recommendations for future research. J Am Coll Nutr. 2007;26(5):555S–61S. doi: 10.1080/07315724.2007.10719658. [DOI] [PubMed] [Google Scholar]
- 84.Edmonds CJ, Crombie R, Gardner MR. Subjective thirst moderates changes in speed of responding associated with water consumption. Front Hum Neurosci. 2013;7:363. doi: 10.3389/fnhum.2013.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arnaoutis G, Kavouras SA, Christaki I, Sidossis LS. Water ingestion improves performance compared with mouth rinse in dehydrated subjects. Med Sci Sport Exer. 2012;44(1):175–9. doi: 10.1249/MSS.0b013e3182285776. [DOI] [PubMed] [Google Scholar]
- 86.Norton K, Norton L, Sadgrove D. Position statement on physical activity and exercise intensity terminology. J Sci Med Sport. 2010;13(5):496–502. doi: 10.1016/j.jsams.2009.09.008. [DOI] [PubMed] [Google Scholar]


