Abstract
Sirtuins (SIRT1–SIRT7) are unique histone deacetylases (HDACs) whose activity depends on NAD+ levels and thus on the cellular metabolic status. SIRTs regulate energy metabolism and mitochondrial function. They orchestrate the stress response and damage repair. Through these functions sirtuins modulate the course of aging and affect neurodegenerative diseases. SIRTSs interact with multiple signaling proteins, transcription factors (TFs) and poly(ADP-ribose) polymerases (PARPs) another class of NAD+-dependent post-translational protein modifiers. The cross-talk between SIRTs TFs and PARPs is a highly promising research target in a number of brain pathologies. This review describes updated results on sirtuins in brain aging/neurodegeneration. It focuses on SIRT1 but also on the roles of mitochondrial SIRTs (SIRT3, 4, 5) and on SIRT6 and SIRT2 localized in the nucleus and in cytosol, respectively. The involvement of SIRTs in regulation of insulin-like growth factor signaling in the brain during aging and in Alzheimer’s disease was also focused. Moreover, we analyze the mechanism(s) and potential significance of interactions between SIRTs and several TFs in the regulation of cell survival and death. A critical view is given on the application of SIRT activators/modulators in therapy of neurodegenerative diseases.
Keywords: Sirtuins, Brain aging, Alzheimer’s disease, Parkinson’s disease, Neuroprotection, Transcription factors
Introduction
Sirtuins (SIRT1–SIRT7) belong to the family of histone deacetylases. These enzymes modulate the properties and functions of proteins (e.g. histones, kinases, and transcription factors-TFs) [1, 2] by removing acetyl groups post-translationally attached to their lysine residues by acetyltransferases. Sirtuins are class III HDACs and differ from other classes in that they require NAD+ for their activity. This feature couples sirtuin activity to the cellular metabolic status [3], in turn allowing these enzymes to modulate the crucial proteins of the electron transport chain (ETC), stress response, and life/death signaling. Some sirtuins also possess additional enzymatic activities such as mono(ADP-ribosyl)ation (SIRT3, SIRT4, SIRT6), the ability to remove a wide array of other lysine modifications (e.g. desuccinylation and demalonylation—SIRT5; decrotonylation—SIRT1–3), and/or lack detectable deacetylation capability (SIRT4) [1, 2]. Sirtuins are engaged in cross-talk with a wide spectrum of transcription factors, including forkhead box subgroup O (FOXOs), p53, and NF-κB, and with proteins engaged in DNA repair such as DNA-dependent protein kinase (DNA-PK) [1, 4]. The versatile and ubiquitous family of poly(ADP-ribose) polymerases (PARPs) shares the feature of NAD+-dependence with sirtuins; the two classes of enzymes compete for the substrate and interact in numerous ways, influencing a very broad range of cellular functions [1, 4]. Sirtuins display complex cellular localization in the cytoplasm, nucleus, and mitochondria [2]. All sirtuins are present in the brain in a highly regulated, spatiotemporal pattern and may influence the course of aging and pathological changes [4, 5].
Sirtuins and Their Roles in Mitochondria: Biogenesis, Energy Production, and Survival/Death Signaling
The presence of sirtuins (SIRT3, 4, 5) in mitochondria appears to undergo precise regulation. The exact localization of SIRT3 seems to be species-specific; human SIRT3 is a mitochondrial matrix protein, but its mouse ortholog resides in the inner membrane [6, 7]. SIRT4 and SIRT-5 are also present in the mitochondrial matrix. Human SIRT5 has an additional membrane insertion sequence; its mitochondrial presence depends on the isoform [8]. Mitochondrial localization of sirtuins is mutually interdependent. It is proposed that SIRT3 is present in mitochondria only when the expression of SIRT5 is low [9]. This scattered evidence suggests the possibility of a complex network of regulation for the level and localization of various sirtuins. The results published thus far point to the involvement of sirtuins in the regulation of mitochondrial turnover, fusion and fission, and of mitochondrial cell death signaling. Sirtuins also influence mitochondrial respiratory machinery and ROS production in multiple tissues (Fig. 1). Importantly, the significance of mitochondrial regulation for CNS homeostasis extends well beyond brain neurons, as they are extremely sensitive to the effects of metabolic deregulation in the periphery (with the arginine/urea metabolism being an example of a sirtuin-dependent pathway strongly linked to neurodegenerative conditions). SIRT3 can also enhance via FOXO3 the expression of antioxidant enzymes including the mitochondrial manganese superoxide dismutase (Mn-SOD), peroxiredoxins, or thioredoxin 2 [10, 11].
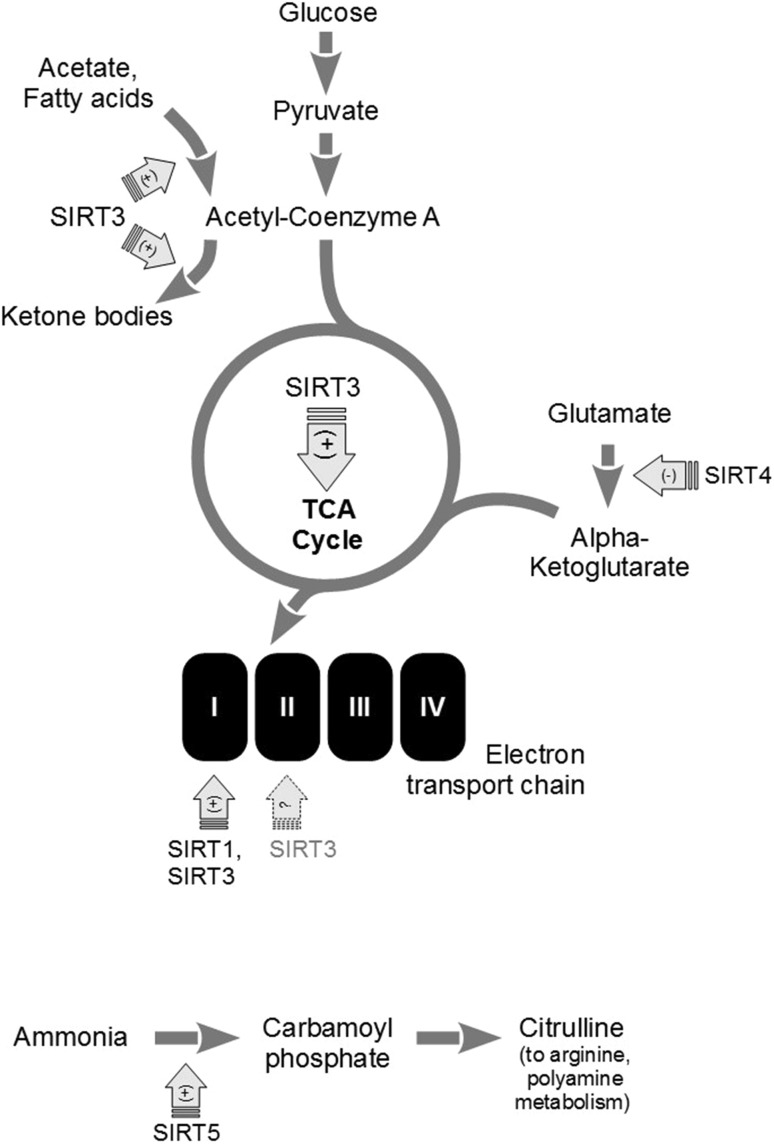
Fig. 1.
Mitochondrial targets of sirtuin signalling. Depending on the organ and cell type, sirtuins may affect multiple stages of glucose-based energy metabolism, the production of ketone bodies, glutamate usage, or arginine, citrulline, and polyamine biosynthesis. While numerous metabolites have direct roles in the CNS, others not produced locally, may dramatically impact brain health (as in the case of e.g. urea cycle, which is typically incomplete in the central neurons, but its deregulation in peripheral tissues leads to neurodegeneration in the CNS). According to [40], modified
SIRT1, mainly a nuclear enzyme, can be also present in mitochondria [12]. Moreover, it has been shown to be engaged in mitochondrial biogenesis [13–15]; reviewed in [12, 16]. Exercise training increases SIRT1 mRNA level and the amount of mtDNA indicating intensified mitogenesis in most brain regions, with potential cognitive significance [15]. SIRT1 seems to exert this beneficial influence via peroxisome proliferator-activated receptor γ co-activator-1α (PGC-1α) [17]. PGC-1α is a crucial regulator of mitochondrial biogenesis and energy metabolism [18, 19]. PGC-1α is also engaged in antioxidant defense, for example via regulation of Mn-SOD and glutathione peroxidase [20]. Impaired PGC-1α function may contribute to the pathogenesis of neurodegenerative diseases such as Alzheimer’s and Parkinson’s (AD and PD, respectively), Huntington’s disease, or ischemic damage [21–24]. SIRT3 is also involved in the regulation of mitochondrial biogenesis in a manner mediated by its target FOXO3 and Parkin. SIRT3 activates FOXO3a and its target PTEN-induced kinase-1 (PINK-1), a protein known to modulate the cellular redox status and mitochondrial function. PINK-1 in turn enhances Parkin activity, potentiating the fusion of mitochondria and mitophagy [25]. SIRT3 overexpression has led to a significant increase in cellular mtDNA content, while shRNA against SIRT3 has reduced the PGC-1α-mediated rise of mtDNA [26].
The influence of sirtuins on the energy metabolism may also come from their direct interactions with the respiratory machinery (Fig. 1). SIRT3 regulates pyruvate dehydrogenase that is acetylated by the acetyl-CoA acetyltransferase 1 (ACAT1); acetylation/deacetylation status of the dehydrogenase is important for the regulation of glycolysis in cancer cells [27]. Moreover, SIRT3 deacetylates and stimulates isocitrate dehydrogenase 2, an enzyme of the tricarboxylic acid cycle [28]. Complex I constituent, NADH dehydrogenase 1α subcomplex subunit 9 is deacetylated and activated by SIRT3 [29]. SIRT1 has been shown to enhance the function of complex I in insulin-resistant cells, possibly via SIRT3. Overexpression of SIRT1 attenuated high-fat diet-induced insulin resistance in the skeletal muscle, and restored the levels of SIRT3, mitochondrial antioxidant enzymes and DNA [30]. The part of complex II, succinate dehydrogenase subunit A is also suggested as a SIRT3 substrate [31]. Thus, sirtuins appear to influence several stages of energy metabolism. SIRT4 generally falls in the same scenario. Loss of its expression in several cell types (hepatocytes, muscle) leads to lower ATP production. SIRT4 has been implicated in the regulation of mitochondrial uncoupling. It is also involved in signaling to the nucleus via AMPK, PGC1α and acetyl-CoA carboxylase, which adjusts mitochondrial ATP production to the energetic demands of the cell [32]. SIRT5, too, has been found to be linked to AMPK, PGC1α and mitochondrial ATP generation [33].
Mitochondrial sirtuins are involved in the usage of alternative energy sources. The change of energetic substrates is accomplished in hepatocytes by SIRT3 through deacetylation of acyl-CoA dehydrogenases, glutamate dehydrogenase, and the mitochondrial acetyl-CoA synthetase [34–36]. These activities allow sustained energy production in the conditions of disturbed supply of the basal substrates. SIRT4 has been found to shift the balance in lipid usage from fatty acid oxidation towards lipid anabolism, by inhibiting malonyl-CoA decarboxylase [37]. Mitochondrial lipid metabolism can be also affected by SIRT5 via its desuccinylase activity directed towards liver mitochondrial proteins engaged in β-oxidation and ketogenesis [38]. SIRT5 might also influence other aspects of mitochondrial energy production such as the tricarboxylic acid cycle [39].
Besides the ATP generation, SIRT5 regulates the detoxication of ammonia. Through deacetylation, SIRT5 activates the carbamoyl phosphate synthase 1, intensifying the conversion of ammonia into carbamyl phosphate and then citrulline, which is metabolized in the urea cycle (Fig. 1; [40]).
Sirtuins exert their influence on the antioxidative defenses in mitochondria. While PGC-1α is induced by SIRT1 in rat hippocampus [41], Kong et al. [26] have shown that SIRT3 is an important mediator of the PGC-1α-dependent induction of SOD2 and glutathione peroxidase-1 (in skeletal muscle cells). A number of articles confirmed the role of SIRT3 in the positive regulation of the level and activity of MnSOD in various tissues [42–44]. SIRT3 also plays a role in the mitochondrial unfolded protein response, which is activated to cope with oxidatively damaged proteins [45].
Sirtuins have been shown to be involved in the regulation of mitochondrial membrane permeability. In cardiac muscle, SIRT3 deacetylates mitochondrial protein cyclophilin D, which is a regulatory component of the permeability transition pore (mPTP) [46]. SIRT5 deacetylates cytochrome c in vitro [47]. However, the outcome of these phenomena is unclear.
Sirtuins in Aging
The emerging involvement of sirtuins and their targets in the longevity effects of caloric restriction (CR) may be an excellent recapitulation of their roles in the organism’s struggle to control and counter stress and macromolecular damage [48]. Sirtuins are bi-directionally linked to the signaling pathways of insulin and insulin-like growth factor-I (IGF-I), collectively termed IIS (insulin/IGF signaling). IGF-I increases SIRT1 expression via JNK1 (c-Jun N-terminal kinase 1 [49]). In turn, SIRT1 and SIRT2 restore the activity of the IGF/insulin receptor target Akt, and SIRT1 supports the IIS signal by deacetylation of insulin receptor substrate 2 (IRS-2). However, SIRT1 and SIRT6 could also suppress the expression of IGF, its receptor, and IIS-dependent genes in some circumstances [49]. IIS plays highly regulated, important roles in the CNS. IGF-I synthesis declines in old organisms, weakening IGF’s trophic action and most probably causing a significant proportion of observed age-related disturbances in brain function [50–52].
Despite the generally trophic role of IGF-I the IIS pathway turns out to be a crucial element of longevity-inhibiting signaling [53]. In invertebrate models of aging, IIS-dependent suppression of FOXO ortholog (DAF-16) is relieved in conditions of stress such as oxidative damage, starvation, or CR. This de-repression leads to the activation of DAF-16/FOXO-responsive genes, enhancing the resistance to broad range of stress conditions [54–57].
Data obtained in vertebrates also suggest the involvement of IIS in the modulation of stress resistance and, possibly, longevity [53, 58–60]. The effect was dependent on neuronal action of IIS [61, 62]. However, the matter is still not fully settled suggesting that the much higher complexity and redundancy of IIS in mammals requires far more in-depth analysis [63, 64].Significant side-effects of reduced IIS also complicate the matters [65–67].
Sirtuins appear to be involved in the longevity-modulating role of IIS; the impact of SIRT1 on long-term survival occurs again through signaling events in specific regions of the CNS [68]. SIRT1 also appears to be involved in the role of IIS in the CR, but sirtuins might also affect the calorie intake itself—again, through the influence on FOXO [54, 69]. A drop in hippocampal SIRT1 level or activity (Fig. 2) has been noted in the aged rat brain, although the results are inconsistent with some works showing reduced activity despite elevated protein [70, 71].
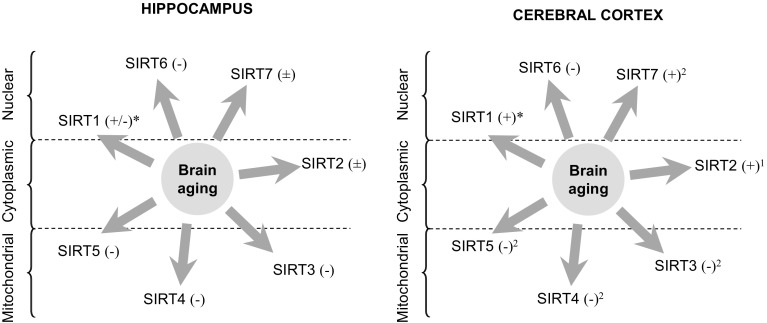
Fig. 2.
Changes in the protein levels of various sirtuins in the aged rat brain. The influence of physiological brain aging on the protein levels of various sirtuins in the rodent model. 24 months old rats are compared to adults (3 months old). Predominant cellular localizations of sirtuin proteins are marked in hippocampus and cerebral cortex. ±No change. *Increased protein but lower activity [71]. 1Change observed only in the occipital but not frontal or temporal lobes of the cerebral cortex. 2Only in frontal but not occipital or temporal lobe
The expression of SIRT3-SIRT7 undergoes changes in the aging brain in a region-specific manner (Fig. 2; [71, 72]). Single-nucleotide polymorphisms in SIRT3, SIRT5 and SIRT6 genes have been noted to correlate with human lifespan [73].
The potential role for SIRT2 in aging is suggested by the association found between human longevity and a polymorphism in the probable regulatory elements of its gene [74]. Isoform-/region-specific increase of brain SIRT2 content has been observed during aging in mice and rats [71, 75]. Deacetylation by SIRT2 of the life-span modulating cell cycle checkpoint kinase BubR1 has been shown to preserve its cellular levels while loss of BubR1 is observed in aging muscle due to NAD+ decline [76, 77]. This makes SIRT2 a good candidate for another longevity modulator although it does not seem to be the sole BubR1 regulating factor [78].
SIRT3 single nucleotide polymorphism also seems to associate with human longevity [79, 80], although the data still needs further elaboration [81]. SIRT3 reacts to nutritional status and has been shown to mediate some of the beneficial effects of CR, including many of the CR-induced transcriptional changes in numerous tissues [28, 82, 83]. SIRT3 is increasingly viewed as a modulator of metabolic adaptation to caloric restriction, making it a promising target [84]. Its protein expression changes in a number of mouse peripheral organs during aging, including mouse hematopoietic stem cells where its decrease limits their regenerative potential [85, 86]. Intense oxidative stress reduces SIRT3 in human mesenchymal stromal/stem cells, which renders them more vulnerable as SIRT3 supports the activity of the catalase-SOD ensemble [87, 88]. Disturbances in the SIRT3 role as an important free radical defense supporter also appear to contribute to aging of the central auditory system [89]. Moreover, the repertoire of SIRT3 interacting partners suggest further aspects of its role in longevity. Deacetylation by SIRT3 supports the stability and activity of 8-oxoguanine-DNA glycosylase-1 (OGG1), a base excision DNA repair enzyme. This protects mtDNA against accumulation of the mutagenic damage product 8-oxoguanine [90]. SIRT3 also deacetylates DNA repair regulator Ku70 [91]. In addition, SIRT3 binds the heat shock protein HSP70 and causes its increased nuclear presence [92]. These interactions are potentially linked to the mechanisms of age-related neurodegeneration.
Corresponding with SIRT6 role in glucose metabolism and IGF-I homeostasis, results have been obtained suggesting its involvement in CR [93, 94]. Animal models provide somewhat conflicting results on SIRT6 levels during aging [95–97]; some of the age dependency may be explained by the regulatory loop that links SIRT6 with the age-modulated microRNA miR-766 [98]. The potential engagement of SIRT6 disturbances in the aging process is otherwise among the best documented. Suppression of SIRT6 protein levels mediates premature senescence-like phenotype in cells under H2O2-induced oxidative stress [99]. Premature cell senescence in Hutchinson–Gilford progeria syndrome (HGPS) and chronic obstructive pulmonary disease is linked with lower SIRT6 expression; its restoration remedies a number of senescence-linked traits, in the latter case through modulation of IIS–mTor signaling [100, 101]. The restoration of falling SIRT6 levels also rescues the diminished efficiency of DNA base excision repair in human foreskin fibroblasts from aged donors [102]. Likewise, in the aged brain diminished SIRT6 binding could lead to genomic instability [103]. In turn, some peripheral tissues display an age-related rise of SIRT6; its inhibition by physical exercise improved oxidative damage resistance in muscle [96]. SIRT6−/− mice develop (possibly IGF-I-linked) progeroid-like phenotype, while SIRT6 overexpression supports male longevity in mice which is accompanied by a reduction in serum IGF-I, dramatic increase in the expression of IGF-binding protein-1 mRNA, and changed phosphorylation levels of Akt and FOXO1 [104, 105]. Moreover, SIRT6 binds c-Jun and inhibits its IGF-dependent transcriptional activity [106]. Analysis of SIRT6 interactions (PARP-1, DNA-PK catalytic subunit, other DNA repair proteins, histones) also supports its role in aging, probably through the regulation of chromatin assembly state to facilitate DNA repair in a way somewhat reminiscent of the role of its partner PARP-1 [107]. SIRT6 localizes early to double-strand DNA breaks and is needed for their efficient removal via both pathways: homologous recombination (HR) and non-homologous end-joining (NHEJ) [108, 109]. The mentioned drop in SIRT6 expression during cellular senescence is accompanied by HR deficiency and SIRT6 overexpression largely rescued this phenotype [110]. Cells deficient in SIRT6 enzymatic activity display defects in base excision DNA repair, increased sensitivity to ionizing radiation (but not UV) and multiple chromosomal aberrations though the results clearly need further elucidation [104]. The links between SIRT6, DNA repair, and aging also extend to telomere maintenance. SIRT6 localizes to telomeric chromatin and facilitates the binding of Werner syndrome (WS) protein (WRN) there. WRN is a DNA helicase crucial for genome stability, mutated in the WS. SIRT6 deficiency leads to replicative senescence and telomere dysfunction resembling the pathology seen in WS [111].
The engagement of SIRT6 in the mitigation of aging and oxidative stress also occurs through its interactions with several crucial pathways of transcriptional regulation. SIRT6 has been found to support the transactivation of anti-oxidant genes by nuclear factor erythroid 2-related factor 2 (NRF-2). SIRT-6 deficiency has led to oxidative stress and accelerated decay of human mesenchymal stem cells [112]. NF-κB, another SIRT6 partner, potentially belongs to the crucial modulators of age-related phenotypes [113]. The interaction of SIRT6 with NF-κB subunit RelA recruits SIRT6 to NF-κB target sequences and allows it to repress promoter activities; many of these belong to a group of genes that show increased expression with age [113, 114]. Experimental SIRT6 deficiency led to hyperacetylation of histones bound to NF-κB target promoters. This increased the activity of these promoters, augmenting NF-κB-dependent cellular senescence. This role of NF-κB has been confirmed in vivo [114]. Hypoxia-inducible factor (HIF) transcription factors are another family of SIRT6 (and SIRT1) interaction partners. The vast significance of HIFs for the regulation of oxygen + glucose/lactate metabolism suggests their engagement of in the course of aging. In invertebrates HIF-related modulation of the lifespan has been shown, though conflicting views exist whether the pathway is separate from CR- and IGF-dependent longevity modulation [115, 116]. The above mentioned data and the shortened lifespan of SIRT6-deficient rodents (accompanied by disturbed glucose metabolism) [117] suggest that SIRT-HIF cross-talk might potentially be also engaged in vertebrate longevity. It is known that SIRT1 can inhibit HIF1 and activate HIF2, and that SIRT6 may be a co-repressor for HIF-1α [117–119]. HIF transactivation targets include genes with known neuroprotective influence, although their role in neurodegeneration is still ambiguous [120, 121].
Sirt7 has been recently noted to support the regenerative potential hematopoietic stem cells via regulation of mitochondrial stress signaling [122]. Its numerous interactions with enzymes of nucleic acid metabolism strengthen the possible association with life-long maintenance, necessitating further research in the topic [107].
The signaling targets of sirtuin-regulated FOXOs with potential anti-aging significance are still rather unclear; candidates include thioredoxin-interacting protein (Txnip), which is repressed by FOXO1a [123]. Txnip1 suppresses the stress response, correlates negatively with longevity and is viewed as a SIRT1 antagonist [124, 125]. FOXOs also target microRNAs that might modulate stress resistance and long-lived dormant invertebrate developmental states [126]. Several other TFs have been suggested as mediators of the pro-longevity SIRT1 action, but their significance needs further elucidation [127].
Sirtuins in Neurodegeneration and Neuroprotection
Sirtuins in AD
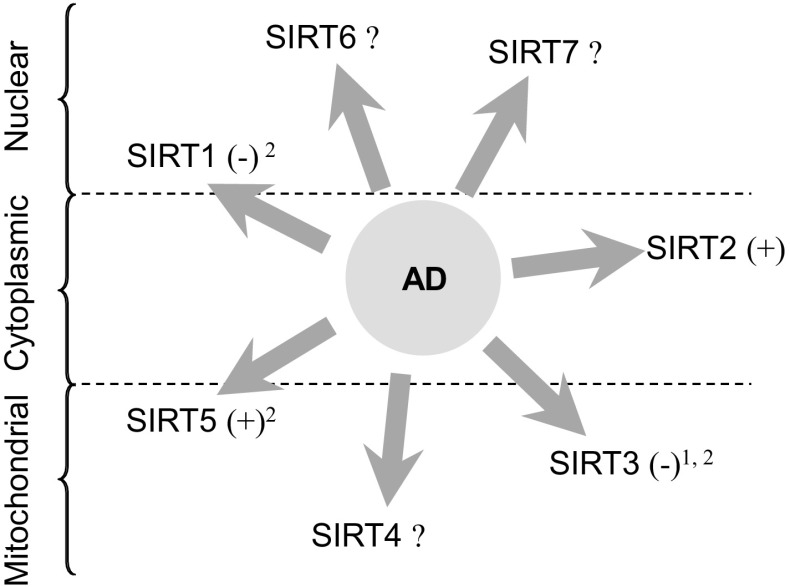
A number of works have shown the potential role of sirtuins in AD (Fig. 3) and other neurodegenerative disorders. The reduction of SIRT1 and SIRT3 mRNA/protein levels observed in AD brain correlates with the stage/duration of the disease [128, 129], and can be mimcked in vitro by the influence of Aβ25-35 on SIRT1 [130]. In turn, up-regulation of SIRT3 mRNA that followed the spatial and temporal profiles of Aβ accumulation has been shown in mice, and higher SIRT3 mRNA was observed in the temporal cortex of AD cases (Braak tangle stage III–VI, average age 82.5 ± 2.3) [131]. SIRT5 is induced in activated microglia of AD brains [129]. In vitro Aβ1-42 treatment also led to increased SIRT-3, -4, and -5 [132]. However, overexpression of APP and presenilin 1 has led to reduction in SIRT3 mRNA and protein in a mouse model, suggesting more complex relations [133].
Fig. 3.
Changes in the levels of various sirtuins in the course of AD. The influence of AD pathology on the expression levels of various sirtuins in the human brain. Predominant cellular localizations of sirtuin proteins are marked. ±No change. 1Increased mRNA expression observed in the temporal cortex [131]. 2Negative (SIRT1, SIRT3) and positive correlation (SIRT5) of immunoreactivity in the hippocampus with Braak neuropathology staging [129]
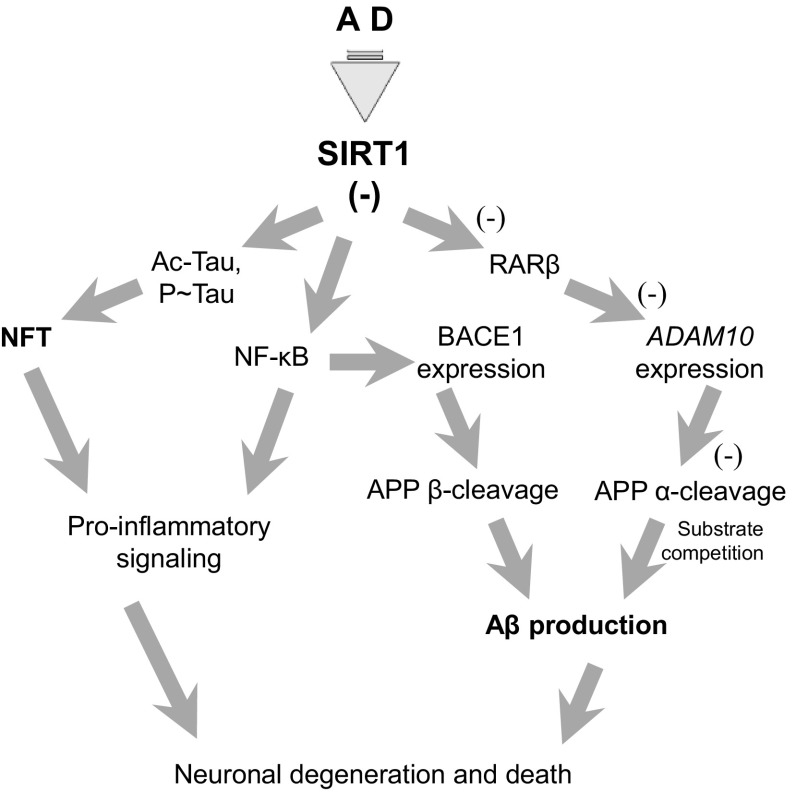
It has been reported that SIRT1 shifts the balance between amyloidogenic and non-amyloidogenic processing of APP in vitro and in transgenic mouse models [134]. SIRT1 up-regulates the α-secretase ADAM10, and through inhibition of NF-κB down-regulates the expression of the β-secretase β-site AβPP-cleaving enzyme 1 (BACE1) (Fig. 4; [135–141]). Moreover, Aβ degradation via autophagy may also be dependent on SIRT1 [142]. Thus, SIRT1 appears to reduce the levels of Aβ, oxidative stress and the resulting neuronal loss [139]. Activation or overexpression of SIRT1 is also reported to interfere with Aβ toxicity mediated by microglia through its ability to inhibit NF-κB signaling [143, 144, 148]. SIRT1 might also protect against synapse loss, a more subtle and earlier effect of Aβ pathology [139]. In turn, small-molecule SIRT2 inhibitors 3-(1-azepanylsulfonyl)-N-(3-bromphenyl) benzamide (AK-7) and 2-cyano-3-[5-(2,5-dichlorophenyl)-2-furanyl]-N-5-quinolinyl-2-propenamide (AGK2) have shifted the balance between α- and β-secretase reducing the Aβ load and led to cognitive improvement in two transgenic mouse models [145]. AGK-2 also reduced glial activation by Aβ1-42 [144]. Thus, SIRT1 and SIRT2 seem to influence the APP cleavage in approximately opposing ways.
Fig. 4.
The significance of SIRT1 in Alzheimer’s disease. SIRT1 modulates multiple pathways that adjust the metabolism of Aβ to keep its levels within physiological limits. The sequence of events occurring in AD reduces SIRT1-dependent effects: tau deacetylation, inhibition of the NF-κB pathway, and the α-cleavage of APP, leading to elevaed Aβ and to intensified pro-inflammatory signaling
Less data is available for other sirtuins. It has been found that short-term treatment with extracellular Aβ1-42 oligomers enhanced the expression of SIRT4 gene but prolonged treatment affected all three mitochondrial isoforms (SIRT3 to SIRT5), suggesting that links between APP/Aβ and SIRTs might be more complex, possibly reciprocal [132].
Intracellular accumulation of pathologically modified microtubule associated protein tau may be another highly promising target in AD research and therapy [146]. Sirtuins mediate the leptin-dependent inhibition of tau phosphorylation [147]. SIRT1also removes acetyl groups from tau, thus relieving the p300-mediated inhibition of phospho-tau degradation [148]. Manipulations of sirtuin activity could therefore influence tau, potentially changing the number of neurofibrillary tangles (NFT) [149, 150]. Moreover, SIRT1 and tau share common upstream regulation mechanism, as both are targets of microRNA-132 [151] and of ademosine monophosphate-activated kinase (AMPK, which leads to the inhibition of the crucial tau kinase GSK-3β, and modulates SIRT1 signaling in a complex manner) [152–154]. These might contribute to the observed inverse correlation between abnormal tau deposition and SIRT1 mRNA and protein levels in AD [128].
Besides Aβ and tau, the two crucial elements of molecular AD pathology, sirtuin signaling is able to influence pathways engaged in neuroprotection and brain tissue renewal. The SIRT1/retinoic acid receptor β target ADAM10 not only cleaves APP but also induces Notch receptor cleavage [155]. The release of Notch intracellular domain activates the transcription of neurogenesis-related genes, and Notch pathway has been shown to be a necessary element of neurogenesis and differentiation of the newly created cells in response to pathological insults [156, 157]. Moreover, Notch targets include genes crucial for synaptic plasticity, learning and memory, and generation of neurites and synapses [155]. Thus, the protection offered by SIRT1 appears to be multi-tiered and stem both from Notch activation and influences on APP and tau metabolism.
A neuroprotective role of SIRT1 has been also observed in prion diseases [158]. Somewhat surprisingly, numerous results point to detrimental roles played by SIRT2 in neurodegenerative disorders, and in other pathological conditions. SIRT2 is increased in AD; its knock-out or inhibition reduces the cytoskeletal pathology and improves autophagy [159]. A meta-analysis has found an association between a polymorphism in an intron of SIRT2 gene and AD susceptibility [160].
Sirtuin partners FOXOs and the IIS have vast potential significance for AD and other diseases linked to disturbed somatic maintenance. The significance of brain IGF-I signaling and its targets for neuronal survival and death is still poorly known and appears to be fundamentally different from their peripheral roles [161].
IIS has recently become a focus in the research deciphering metabolic disturbances that co-occur with (and possibly precede) AD, raising some hopes for the search of early, measurable symptoms of developing pathology [162]. IIS can suppress Aβ production [163] and resulting tissue damage [164] although its full role in AD is still unclear [165, 166]. Deeper understanding is necessary as it may become an attractive target in the future treatment of AD and PD [167]. However, despite the discrepancies IGF-I replacement therapies have been proposed and tested [161, 168].
FOXOs themselves are capable of extensively modulating protein turnover and oxidative stress, both crucial for Aβ/ASN accumulation and toxicity [169]. FOXOs might also mediate the inhibition of neuroprotective PI3K/Akt signaling by Aβ [170]. These TFs have been thus suggested as potential integrating factors in AD metabolic deregulation [171]. The expression of FOXO1 is altered with increased AD severity [172]. FOXO3a might also mediate the toxic effect of Aβ-dependent inhibition of neuroprotective PI3K/Akt signaling [170], and the impact of age on FOXO3 has been suggested as a crucial step changing relatively benign protein aggregates into neurotoxic Aβ deposits [169]. FOXO3a also modulates toxic aggregation of ASN [173] and is found in Lewy bodies/Lewy neurites [174].
Sirtuins in PD
The course of PD, another neurodegenerative disorder that impacts the dopaminergic system also is affected by SIRT signaling. SIRT1 displays neuroprotective properties in experimental PD models [175, 176]. It was reported that oxyresveratrol protected dopaminergic SH-SY5Y cells against the toxicity of the Parkinsonian mimetic 6-hydroxydopamine through countering the-down regulation of SIRT1. Resveratrol whose functions include activation of SIRT1 also offered protection in this model, as well as in MPTP-induced mouse Parkinsonism [177, 178]. Moreover, genetic variants that result in reduced SIRT1 expression co-occurred with sporadic PD [179].
SIRT1 might exert its protective effects in PD through several pathways linked to general stress resistance and more specifically to α-synuclein (ASN) metabolism. The activation of PGC-1α, a protein considered a central element of oxidative stress resistance, by SIRT1 in response to resveratrol may render MPTP-treated mice less prone to neurodegeneration [180]. The protective effect of resveratrol in a rotenone-induced human neuroblastoma cell model of PD has been largely attributed to its ability to induce autophagic degradation of ASN via SIRT1 [181]. Molecular chaperones may also be valuable targets in protein misfolding-related diseases; Hsp70 has been found to protect against ASN aggregation and toxicity [182, 183]. SIRT1 deacetylated the heat shock factor 1 (HSF1) facilitating prolonged binding to its target sequence in the gene coding for Hsp70. This led to elevated expression of Hsp70 in stress conditions [184] raising the possibility that HSF1 and Hsp70 might indeed mediate the protective effect of SIRT1 as it does for example in an amyotrophic lateral sclerosis model [185].
On the contrary, inhibition of SIRT2 with AK-7 reduces MPTP-induced loss of dopaminergic neurons in a mouse model [186]. SIRT2 inhibition improves neurological and behavioral deficits in a PD model induced by MPTP in old mice [187]. siRNA against SIRT2 or its inhibitor AGK2 block the toxic effect of α-synuclein in a Parkinsonian primary midbrain culture model (mutant ASN transfection) and modifies the pattern of α-synuclein inclusions in cells transfected with ASN and its interaction partner synphilin 1 [188]. SIRT2 inhibition improves neurological and behavioral deficits in a PD model induced by MPTP in old mice [187].
SIRT2 inhibition also blocked the apoptosis of an oligodendroglial cell line in a model of another ASN-linked disorder, multiple system atrophy [189]. Results in cerebral ischemia are less clear [186, 190]. However, SIRT2 has also been shown to contribute to the pathology of the vascular system and to the effects of oxidative stress in the endothelium, which have immediate impact on brain oxygen supply [191, 192].
Sirtuins in HD
Huntington’s disease (HD) is an autosomal trinucleotide repeat disorder characterized by striatal and cortical neurodegeneration leading to motor and cognitive dysfunction. The CAG (polyglutamine) expansion affects the open reading frame of the HTT gene coding for huntingtin. This leads to pathological deposition of huntingtin protein, and disruption of gene regulation, metabolic, and signaling pathways [193]. Weakened trophic support of neurons and the resulting nuclear accumulation of FOXO3a transcription factor might be an important aspect [194]. The role of sirtuins in neuronal survival and the known interactions with huntingtin [195] and FOXOs [1] made them a plausible research target. However, sirtuins’ role in HD is somewhat controversial, likely stemming from their wide, pleiotropic spectrum of signaling interactions [193]. Till recently, SIRT1 appeared to protect most species from glutamine repeat toxicity, with the notable exception of the Drosophila model [193]. Mutant huntingtin reduces SIRT1 activity, weakening its positive role in neuronal survival. It is possible that the structural similarity between mutant huntingtin and sirtuin-interacting transcription factors might play a role [193]. SIRT1 binds and activates the promoter of brain-derived neurotrophic factor (BDNF); it can also augment the expression of crucial genes such as superoxide dismutase 2, or mitochondrial biogenesis modulators, and can impact Bax signaling via modulation of its binding to Ku70 [196]. However, evidence for neuroprotective influence of selective SIRT1 inhibition in several HD models including mice has been published in recent years; it has been suggested that this approach might augment the clearance of mutant huntingtin [196, 197]. The neuroprotection achieved by SIRT2 inhibition is much more consistent with the current views on its role [193]. The question of possible therapeutic application of sirtuin modulators appears to be tough and highly selective approaches seem necessary.
Pharmacological Manipulation of Sirtuin Activities for Research and Therapeutic Purposes
A number of pharmacological agents are used to influence the activity of sirtuins for research purposes [198]. HDAC inhibitors display significant level of class specificity: sirtuin inhibitors usually do not affect class I, II or IV enzymes, although the selectivity between sirtuins is a frequent issue [199, 200]. Novel indole compounds seems to offer good specificity and potency while also offering good bioavailability and cell permeability [196]. A new inhibitor 6-chloro-2,3,4,9-tetrahydro-1H-carbazole-1-carboxamide (EX-527) has been shown to be potent and selective towards SIRT1 [201]. The inhibitor has been used to investigate the role of this isoform in cell physiology and pathology, for example in the regulation of inflammatory responses [202, 203]. In a work on oxidative mitochondrial damage evoked by hyperglycemia the SIRT1 inhibitor has been compared to the effects of siRNA-mediated SIRT1 knock-down [204]. EX-527 has been entered into clinical trials [196]. AGK2, an inhibitor selective towards SIRT2 has been used in a study to assess the role of this sirtuin in the toxicity of α-synuclein, mutant huntingtin, and of SIRT2 in cellular energy metabolism [188, 205, 206]. SIRT2 inhibitor AK-7 was also able to offer neuroprotection in a mouse HD model [193]. 1,2-dihydro-3H-naphtho[2,1-b]pyran-3-one (splitomicin) [200]; reviewed in [207] has been used as a basis for an array of derivatives with preferential action against SIRT2 versus SIRT1 [208]. The specificity of the widely employed polyphenolic inhibitor 2-[(2-hydroxynaphthalen-1-ylmethylene)amino]-N-(1-phenethyl)benzamide (sirtinol) [207] has been recently questioned [209]. 3,4′,5-trihydroxy-trans-stilbene, 5-[(1E)-2-(4-hydroxyphenyl)ethenyl]-1,3-benzenediol (resveratrol), a polyphenol with still unclear mechanism of action has been used to activate sirtuins, with beneficial effects on metabolic regulation, energy metabolism, and organism survival [17, 210]. However, its lack of specificity makes it highly problematic as a research tool [211]. It influences the expression and activity of nitric oxide synthases, catalase, superoxide dismutase, glutathione metabolism, and apoptotic signaling to name a few; only some of these effects are mediated by sirtuins [212]. Despite its shortcomings resveratrol has entered into clinical trials aimed at sirtuins’ role in healthy aging and gender-specific longevity mechanisms, in AD-related cognitive decline, in muscle function in old age, and in the status of a cytoprotective enzyme heme oxygenase-1 [213–216]. Polyphenolic activators of sirtuins also include the powerful and pleiotropic curcumin. The clear need for more specific and selective compounds has led to the identification of a number of new activators such as N-(2-(3-(piperazin-1-ylmethyl)imidazo[2,1-b]thiazol-6-yl)phenyl)quinoxaline-2-carboxamide (SRT1720), 4-methyl-N-[2-[3-(morpholinomethyl)imidazo[2,1-b]thiazol-6-yl]phenyl]-2-(pyridin-3-yl)thiazole-5-carboxamide (SRT2104), which has already been shown to protect against neurodegeneration and motor impairment in a mouse HD model [217]. However, despite their therapeutic potential revealed in animal studies and despite some clinical trials on the improvement of the peripheral metabolic health, clinical CNS data are currently lacking [10, 218, 219].
Conclusion
During the past decade, there has been significant progress in understanding the role of sirtuins in brain aging and in neurodegenerative disorders such as AD [1, 16]. Till now relatively little is known about the role of SIRTs in PD or Huntighton’s disease [5, 196]. The role of SIRT1 in the regulation of APP metabolism and tau deacetylation/phosphorylation should be stressed [147, 148]. SIRT1 expression and activity may significantly affect the course of AD pathology and may be a promising therapeutic target. Recently, studies focused on mitochondrial SIRTs and their roles in antioxidative defense [2]. In oxidative stress and in brain aging/neurodegeration down-regulation of the nuclear SIRT6 may influence DNA repair machinery and probably also telomere maintenance. SIRT6 participates in homologus recombinationation, in non-homologus end-joining, and in base excision DNA repair pathways. It interacts with the transcription factor NF-κB, with PARP and with other proteins engaged in DNA repair; this suggests SIRT6 as another promising target in the regulation of longevity [73, 105]. Till now controversial findings are published on the role of SIRT2 which might be important for longevity but also seems to take part in Aβ production, α-synuclein toxicity, and neuronal cell death [74, 145, 188]. Insufficient data are available on SIRT4 and SIRT5 in mitochondria; the knowledge on sirtuin interactions in the regulation of cell survival and death in physiology and pathology is also leaving something to be desired. Hopefully, further studies will expand our knowledge about application of sirtuin modulators in the therapy of neurodegenerative diseases.
Acknowledgements
Authors’ work is supported by National Science Centre Grant 2013/09/B/NZ3/01350.
References
- 1.Jęśko H, Strosznajder RP. Sirtuins and their interactions with transcription factors and poly(ADP-ribose) polymerases. Folia Neuropathol. 2016;54:212–233. doi: 10.5114/fn.2016.62531. [DOI] [PubMed] [Google Scholar]
- 2.Kupis W, Pałyga J, Tomal E, Niewiadomska E. The role of sirtuins in cellular homeostasis. J Physiol Biochem. 2016;72:371–380. doi: 10.1007/s13105-016-0492-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Covington JD, Bajpeyi S. The sirtuins: markers of metabolic health. Mol Nutr Food Res. 2016;60:79–91. doi: 10.1002/mnfr.201500340. [DOI] [PubMed] [Google Scholar]
- 4.Wątroba M, Szukiewicz D. The role of sirtuins in aging and age-related diseases. Adv Med Sci. 2016;61:52–62. doi: 10.1016/j.advms.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Tang BL. Sirtuins as modifiers of Parkinson’s disease pathology. J Neurosci Res. 2016 doi: 10.1002/jnr.23806. [DOI] [PubMed] [Google Scholar]
- 6.Schwer B, North BJ, Frye RA, et al. The human silent information regulator (Sir)2 homologue hSIRT3 is a mitochondrial nicotinamide adenine dinucleotide-dependent deacetylase. J Cell Biol. 2002;158:647–657. doi: 10.1083/jcb.200205057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi T, Wang F, Stieren E, Tong Q. SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J Biol Chem. 2005;280:13560–13567. doi: 10.1074/jbc.M414670200. [DOI] [PubMed] [Google Scholar]
- 8.Matsushita N, Yonashiro R, Ogata Y, et al. Distinct regulation of mitochondrial localization and stability of two human Sirt5 isoforms. Genes Cells. 2011;16:190–202. doi: 10.1111/j.1365-2443.2010.01475.x. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura Y, Ogura M, Tanaka D, Inagaki N. Localization of mouse mitochondrial SIRT proteins: shift of SIRT3 to nucleus by co-expression with SIRT5. Biochem Biophys Res Commun. 2008;366:174–179. doi: 10.1016/j.bbrc.2007.11.122. [DOI] [PubMed] [Google Scholar]
- 10.Kida Y, Goligorsky MS. Sirtuins, Cell Senescence, and Vascular Aging. Can J Cardiol. 2016;32:634–641. doi: 10.1016/j.cjca.2015.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tseng AH-H, Wu L-H, Shieh S-S, Wang DL. SIRT3 interactions with FOXO3 acetylation, phosphorylation and ubiquitinylation mediate endothelial cell responses to hypoxia. Biochem J. 2014;464:157–168. doi: 10.1042/BJ20140213. [DOI] [PubMed] [Google Scholar]
- 12.Tang BL. Sirt1 and the Mitochondria. Mol Cells. 2016;39:87–95. doi: 10.14348/molcells.2016.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomes AP, Duarte FV, Nunes P, et al. Berberine protects against high fat diet-induced dysfunction in muscle mitochondria by inducing SIRT1-dependent mitochondrial biogenesis. Biochim Biophys Acta. 2012;1822:185–195. doi: 10.1016/j.bbadis.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seo D-B, Jeong HW, Lee S-J, Lee S-J. Coumestrol induces mitochondrial biogenesis by activating Sirt1 in cultured skeletal muscle cells. J Agric Food Chem. 2014;62:4298–4305. doi: 10.1021/jf404882w. [DOI] [PubMed] [Google Scholar]
- 15.Steiner JL, Murphy EA, McClellan JL, et al. Exercise training increases mitochondrial biogenesis in the brain. J Appl Physiol. 2011;111:1066–1071. doi: 10.1152/japplphysiol.00343.2011. [DOI] [PubMed] [Google Scholar]
- 16.Yuan Y, Cruzat VF, Newsholme P, et al. Regulation of SIRT1 in aging: roles in mitochondrial function and biogenesis. Mech Ageing Dev. 2016;155:10–21. doi: 10.1016/j.mad.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Lagouge M, Argmann C, Gerhart-Hines Z, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Villena JA. New insights into PGC-1 coactivators: redefining their role in the regulation of mitochondrial function and beyond. FEBS J. 2015;282:647–672. doi: 10.1111/febs.13175. [DOI] [PubMed] [Google Scholar]
- 19.Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006;27:728–735. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- 20.St-Pierre J, Drori S, Uldry M, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 21.Sweeney G, Song J. The association between PGC-1α and Alzheimer’s disease. Anat Cell Biol. 2016;49:1–6. doi: 10.5115/acb.2016.49.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corona JC, Duchen MR. PPARγ and PGC-1α as therapeutic targets in Parkinson’s. Neurochem Res. 2015;40:308–316. doi: 10.1007/s11064-014-1377-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johri A, Chandra A, Beal MF. PGC-1α, mitochondrial dysfunction, and Huntington’s disease. Free Radic Biol Med. 2013;62:37–46. doi: 10.1016/j.freeradbiomed.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen S-D, Yang D-I, Lin T-K, et al. Roles of oxidative stress, apoptosis, PGC-1α and mitochondrial biogenesis in cerebral ischemia. Int J Mol Sci. 2011;12:7199–7215. doi: 10.3390/ijms12107199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Das S, Mitrovsky G, Vasanthi HR, Das DK. Antiaging properties of a grape-derived antioxidant are regulated by mitochondrial balance of fusion and fission leading to mitophagy triggered by a signaling network of Sirt1-Sirt3-Foxo3-PINK1-PARKIN. Oxid Med Cell Longev. 2014;2014:345105. doi: 10.1155/2014/345105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Kong X, Wang R, Xue Y, et al. Sirtuin 3, a new target of PGC-1alpha, plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS ONE. 2010;5:e11707. doi: 10.1371/journal.pone.0011707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan J, Shan C, Kang H-B, et al. Tyr phosphorylation of PDP1 toggles recruitment between ACAT1 and SIRT3 to regulate the pyruvate dehydrogenase complex. Mol Cell. 2014;53:534–548. doi: 10.1016/j.molcel.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Someya S, Yu W, Hallows WC, et al. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010;143:802–812. doi: 10.1016/j.cell.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahn B-H, Kim H-S, Song S, et al. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci USA. 2008;105:14447–14452. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang H-H, Qin G-J, Li X-L, et al. SIRT1 overexpression in skeletal muscle in vivo induces increased insulin sensitivity and enhanced complex I but not complex II–V functions in individual subsarcolemmal and intermyofibrillar mitochondria. J Physiol Biochem. 2015;71:177–190. doi: 10.1007/s13105-015-0396-x. [DOI] [PubMed] [Google Scholar]
- 31.Finley LWS, Haas W, Desquiret-Dumas V, et al. Succinate dehydrogenase is a direct target of sirtuin 3 deacetylase activity. PLoS ONE. 2011;6:e23295. doi: 10.1371/journal.pone.0023295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ho L, Titus AS, Banerjee KK, et al. SIRT4 regulates ATP homeostasis and mediates a retrograde signaling via AMPK. Aging. 2013;5:835–849. doi: 10.18632/aging.100616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buler M, Aatsinki S-M, Izzi V, et al. SIRT5 is under the control of PGC-1α and AMPK and is involved in regulation of mitochondrial energy metabolism. FASEB J. 2014;28:3225–3237. doi: 10.1096/fj.13-245241. [DOI] [PubMed] [Google Scholar]
- 34.Hirschey MD, Shimazu T, Goetzman E, et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464:121–125. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lombard DB, Alt FW, Cheng H-L, et al. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol. 2007;27:8807–8814. doi: 10.1128/MCB.01636-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwer B, Bunkenborg J, Verdin RO, et al. Reversible lysine acetylation controls the activity of the mitochondrial enzyme acetyl-CoA synthetase 2. Proc Natl Acad Sci USA. 2006;103:10224–10229. doi: 10.1073/pnas.0603968103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laurent G, German NJ, Saha AK, et al. SIRT4 coordinates the balance between lipid synthesis and catabolism by repressing malonyl CoA decarboxylase. Mol Cell. 2013;50:686–698. doi: 10.1016/j.molcel.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rardin MJ, He W, Nishida Y, et al. SIRT5 regulates the mitochondrial lysine succinylome and metabolic networks. Cell Metab. 2013;18:920–933. doi: 10.1016/j.cmet.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park J, Chen Y, Tishkoff DX, et al. SIRT5-mediated lysine desuccinylation impacts diverse metabolic pathways. Mol Cell. 2013;50:919–930. doi: 10.1016/j.molcel.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang J-Y, Hirschey MD, Shimazu T, et al. Mitochondrial sirtuins. Biochim Biophys Acta. 2010;1804:1645–1651. doi: 10.1016/j.bbapap.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 41.Wang S-J, Zhao X-H, Chen W, et al. Sirtuin 1 activation enhances the PGC-1α/mitochondrial antioxidant system pathway in status epilepticus. Mol Med Rep. 2015;11:521–526. doi: 10.3892/mmr.2014.2724. [DOI] [PubMed] [Google Scholar]
- 42.Sundaresan NR, Gupta M, Kim G, et al. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest. 2009;119:2758–2771. doi: 10.1172/JCI39162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tao R, Coleman MC, Pennington JD, et al. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell. 2010;40:893–904. doi: 10.1016/j.molcel.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qiu X, Brown K, Hirschey MD, et al. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12:662–667. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 45.Papa L, Germain D. SirT3 regulates the mitochondrial unfolded protein response. Mol Cell Biol. 2014;34:699–710. doi: 10.1128/MCB.01337-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hafner AV, Dai J, Gomes AP, et al. Regulation of the mPTP by SIRT3-mediated deacetylation of CypD at lysine 166 suppresses age-related cardiac hypertrophy. Aging. 2010;2:914–923. doi: 10.18632/aging.100252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schlicker C, Gertz M, Papatheodorou P, et al. Substrates and regulation mechanisms for the human mitochondrial sirtuins Sirt3 and Sirt5. J Mol Biol. 2008;382:790–801. doi: 10.1016/j.jmb.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 48.Speakman JR, Mitchell SE. Caloric restriction. Mol Aspects Med. 2011;32:159–221. doi: 10.1016/j.mam.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 49.Ng F, Tang BL. When is Sirt1 activity bad for dying neurons? Front Cell Neurosci. 2013;7:186. doi: 10.3389/fncel.2013.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Belfiore A, Frasca F, Pandini G, et al. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev. 2009;30:586–623. doi: 10.1210/er.2008-0047. [DOI] [PubMed] [Google Scholar]
- 51.Pardo J, Uriarte M, Cónsole GM, et al. Insulin-like growth factor-i gene therapy increases hippocampal neurogenesis, astrocyte branching and improves spatial memory in female aging rats. Eur J Neurosci. 2016 doi: 10.1111/ejn.13278. [DOI] [PubMed] [Google Scholar]
- 52.Ashpole NM, Sanders JE, Hodges EL, et al. Growth hormone, insulin-like growth factor-1 and the aging brain. Exp Gerontol. 2015;68:76–81. doi: 10.1016/j.exger.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holzenberger M, Dupont J, Ducos B, et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 54.Lee S-H, Min K-J. Caloric restriction and its mimetics. BMB Rep. 2013;46:181–187. doi: 10.5483/BMBRep.2013.46.4.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hyun M, Lee J, Lee K, et al. Longevity and resistance to stress correlate with DNA repair capacity in Caenorhabditis elegans. Nucleic Acids Res. 2008;36:1380–1389. doi: 10.1093/nar/gkm1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lithgow GJ, Walker GA. Stress resistance as a determinate of C. elegans lifespan. Mech Ageing Dev. 2002;123:765–771. doi: 10.1016/S0047-6374(01)00422-5. [DOI] [PubMed] [Google Scholar]
- 57.Vermeulen CJ, Loeschcke V. Longevity and the stress response in Drosophila. Exp Gerontol. 2007;42:153–159. doi: 10.1016/j.exger.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 58.Bonafè M, Barbieri M, Marchegiani F, et al. Polymorphic variants of insulin-like growth factor I (IGF-I) receptor and phosphoinositide 3-kinase genes affect IGF-I plasma levels and human longevity: cues for an evolutionarily conserved mechanism of life span control. J Clin Endocrinol Metab. 2003;88:3299–3304. doi: 10.1210/jc.2002-021810. [DOI] [PubMed] [Google Scholar]
- 59.Deelen J, Uh H-W, Monajemi R, et al. Gene set analysis of GWAS data for human longevity highlights the relevance of the insulin/IGF-1 signaling and telomere maintenance pathways. Age (Dordr) 2013;35:235–249. doi: 10.1007/s11357-011-9340-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suh Y, Atzmon G, Cho M-O, et al. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc Natl Acad Sci USA. 2008;105:3438–3442. doi: 10.1073/pnas.0705467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taguchi A, Wartschow LM, White MF. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science. 2007;317:369–372. doi: 10.1126/science.1142179. [DOI] [PubMed] [Google Scholar]
- 62.Kappeler L, De Magalhaes Filho C, Dupont J, et al. Brain IGF-1 receptors control mammalian growth and lifespan through a neuroendocrine mechanism. PLoS Biol. 2008;6:e254. doi: 10.1371/journal.pbio.0060254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Selman C, Lingard S, Gems D, et al. Comment on “Brain IRS2 signaling coordinates life span and nutrient homeostasis.”. Science. 2008 doi: 10.1126/science.1152366. [DOI] [PubMed] [Google Scholar]
- 64.Bokov AF, Garg N, Ikeno Y, et al. Does reduced IGF-1R signaling in Igf1r+/− mice alter aging? PLoS ONE. 2011;6:e26891. doi: 10.1371/journal.pone.0026891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Akanji AO, Suresh CG, Al-Radwan R, Fatania HR. Insulin-like growth factor (IGF)-I, IGF-II and IGF-binding protein (IGFBP)-3 levels in Arab subjects with coronary heart disease. Scand J Clin. Lab Invest. 2007;67:553–559. doi: 10.1080/00365510601173153. [DOI] [PubMed] [Google Scholar]
- 66.Juul A, Scheike T, Davidsen M, et al. Low serum insulin-like growth factor I is associated with increased risk of ischemic heart disease: a population-based case-control study. Circulation. 2002;106:939–944. doi: 10.1161/01.CIR.0000027563.44593.CC. [DOI] [PubMed] [Google Scholar]
- 67.Yamaguchi H, Komamura K, Choraku M, et al. Impact of serum insulin-like growth factor-1 on early prognosis in acute myocardial infarction. Intern Med. 2008;47:819–825. doi: 10.2169/internalmedicine.47.0736. [DOI] [PubMed] [Google Scholar]
- 68.Satoh A, Brace CS, Rensing N, et al. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 2013;18:416–430. doi: 10.1016/j.cmet.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sasaki T, Kim H-J, Kobayashi M, et al. Induction of hypothalamic Sirt1 leads to cessation of feeding via agouti-related peptide. Endocrinology. 2010;151:2556–2566. doi: 10.1210/en.2009-1319. [DOI] [PubMed] [Google Scholar]
- 70.Quintas A, de Solís AJ, Díez-Guerra FJ, et al. Age-associated decrease of SIRT1 expression in rat hippocampus: prevention by late onset caloric restriction. Exp Gerontol. 2012;47:198–201. doi: 10.1016/j.exger.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 71.Braidy N, Poljak A, Grant R, et al. Differential expression of sirtuins in the aging rat brain. Front Cell Neurosci. 2015;9:167. doi: 10.3389/fncel.2015.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wong DW, Soga T, Parhar IS. Aging and chronic administration of serotonin-selective reuptake inhibitor citalopram upregulate Sirt4 gene expression in the preoptic area of male mice. Front Genet. 2015;6:281. doi: 10.3389/fgene.2015.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.TenNapel MJ, Lynch CF, Burns TL, et al. SIRT6 minor allele genotype is associated with >5-year decrease in lifespan in an aged cohort. PLoS ONE. 2014;9:e115616. doi: 10.1371/journal.pone.0115616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Crocco P, Montesanto A, Passarino G, Rose G. Polymorphisms falling within putative miRNA target sites in the 3′UTR region of SIRT2 and DRD2 genes are correlated with human longevity. J Gerontol A Biol Sci Med Sci. 2015 doi: 10.1093/gerona/glv058. [DOI] [PubMed] [Google Scholar]
- 75.Maxwell MM, Tomkinson EM, Nobles J, et al. The Sirtuin 2 microtubule deacetylase is an abundant neuronal protein that accumulates in the aging CNS. Hum Mol Genet. 2011;20:3986–3996. doi: 10.1093/hmg/ddr326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baker DJ, Dawlaty MM, Wijshake T, et al. Increased expression of BubR1 protects against aneuploidy and cancer and extends healthy lifespan. Nat Cell Biol. 2013;15:96–102. doi: 10.1038/ncb2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.North BJ, Rosenberg MA, Jeganathan KB, et al. SIRT2 induces the checkpoint kinase BubR1 to increase lifespan. EMBO J. 2014;33:1438–1453. doi: 10.15252/embj.201386907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cosentino C, Mostoslavsky R. Sirtuin to the rescue: SIRT2 extends life span of BubR1 mice. EMBO J. 2014;33:1417–1419. doi: 10.15252/embj.201488983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bellizzi D, Rose G, Cavalcante P, et al. A novel VNTR enhancer within the SIRT3 gene, a human homologue of SIR2, is associated with survival at oldest ages. Genomics. 2005;85:258–263. doi: 10.1016/j.ygeno.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 80.Albani D, Ateri E, Mazzuco S, et al. Modulation of human longevity by SIRT3 single nucleotide polymorphisms in the prospective study “Treviso Longeva (TRELONG).”. Age (Dordr) 2014;36:469–478. doi: 10.1007/s11357-013-9559-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Giblin W, Skinner ME, Lombard DB. Sirtuins: guardians of mammalian healthspan. Trends Genet. 2014;30:271–286. doi: 10.1016/j.tig.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Palacios OM, Carmona JJ, Michan S, et al. Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1alpha in skeletal muscle. Aging. 2009;1:771–783. doi: 10.18632/aging.100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Barger JL, Anderson RM, Newton MA, et al. A conserved transcriptional signature of delayed aging and reduced disease vulnerability is partially mediated by SIRT3. PLoS One. 2015 doi: 10.1371/journal.pone.0120738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kincaid B, Bossy-Wetzel E. Forever young: SIRT3 a shield against mitochondrial meltdown, aging, and neurodegeneration. Front Aging Neurosci. 2013;5:48. doi: 10.3389/fnagi.2013.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kwon Y, Kim J, Lee C-Y, Kim H. Expression of SIRT1 and SIRT3 varies according to age in mice. Anat Cell Biol. 2015;48:54–61. doi: 10.5115/acb.2015.48.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brown K, Xie S, Qiu X, et al. SIRT3 reverses aging-associated degeneration. Cell Rep. 2013;3:319–327. doi: 10.1016/j.celrep.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Iwahara T, Bonasio R, Narendra V, Reinberg D. SIRT3 functions in the nucleus in the control of stress-related gene expression. Mol Cell Biol. 2012;32:5022–5034. doi: 10.1128/MCB.00822-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang X-Q, Shao Y, Ma C-Y, et al. Decreased SIRT3 in aged human mesenchymal stromal/stem cells increases cellular susceptibility to oxidative stress. J Cell Mol Med. 2014;18:2298–2310. doi: 10.1111/jcmm.12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zeng L, Yang Y, Hu Y, et al. Age-related decrease in the mitochondrial sirtuin deacetylase Sirt3 expression associated with ROS accumulation in the auditory cortex of the mimetic aging rat model. PLoS ONE. 2014;9:e88019. doi: 10.1371/journal.pone.0088019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cheng Y, Ren X, Gowda ASP, et al. Interaction of Sirt3 with OGG1 contributes to repair of mitochondrial DNA and protects from apoptotic cell death under oxidative stress. Cell Death Dis. 2013;4:e731. doi: 10.1038/cddis.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sundaresan NR, Samant SA, Pillai VB, et al. SIRT3 is a stress-responsive deacetylase in cardiomyocytes that protects cells from stress-mediated cell death by deacetylation of Ku70. Mol Cell Biol. 2008;28:6384–6401. doi: 10.1128/MCB.00426-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Law IKM, Liu L, Xu A, et al. Identification and characterization of proteins interacting with SIRT1 and SIRT3: implications in the anti-aging and metabolic effects of sirtuins. Proteomics. 2009;9:2444–2456. doi: 10.1002/pmic.200800738. [DOI] [PubMed] [Google Scholar]
- 93.Zhang P, Tu B, Wang H, et al. Tumor suppressor p53 cooperates with SIRT6 to regulate gluconeogenesis by promoting FoxO1 nuclear exclusion. Proc Natl Acad Sci USA. 2014;111:10684–10689. doi: 10.1073/pnas.1411026111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kanfi Y, Shalman R, Peshti V, et al. Regulation of SIRT6 protein levels by nutrient availability. FEBS Lett. 2008;582:543–548. doi: 10.1016/j.febslet.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ghiraldini FG, Crispim ACV, Mello MLS. Effects of hyperglycemia and aging on nuclear sirtuins and DNA damage of mouse hepatocytes. Mol Biol Cell. 2013;24:2467–2476. doi: 10.1091/mbc.E13-04-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Koltai E, Szabo Z, Atalay M, et al. Exercise alters SIRT1, SIRT6, NAD and NAMPT levels in skeletal muscle of aged rats. Mech Ageing Dev. 2010;131:21–28. doi: 10.1016/j.mad.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ren Y, Shan TZ, Zhu LN, et al. Effect of breed on the expression of Sirtuins (Sirt1-7) and antioxidant capacity in porcine brain. Animal. 2013;7:1994–1998. doi: 10.1017/S175173111300164X. [DOI] [PubMed] [Google Scholar]
- 98.Sharma A, Diecke S, Zhang WY, et al. The role of SIRT6 protein in aging and reprogramming of human induced pluripotent stem cells. J Biol Chem. 2013;288:18439–18447. doi: 10.1074/jbc.M112.405928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu R, Liu H, Ha Y, et al. Oxidative stress induces endothelial cell senescence via downregulation of Sirt6. Biomed Res Int. 2014 doi: 10.1155/2014/902842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Endisha H, Merrill-Schools J, Zhao M, et al. Restoring SIRT6 expression in Hutchinson-Gilford progeria syndrome cells impedes premature senescence and formation of dysmorphic nuclei. Pathobiology. 2015;82:9–20. doi: 10.1159/000368856. [DOI] [PubMed] [Google Scholar]
- 101.Takasaka N, Araya J, Hara H, et al. Autophagy induction by SIRT6 through attenuation of insulin-like growth factor signaling is involved in the regulation of human bronchial epithelial cell senescence. J Immunol. 2014;192:958–968. doi: 10.4049/jimmunol.1302341. [DOI] [PubMed] [Google Scholar]
- 102.Xu Z, Zhang L, Zhang W, et al. SIRT6 rescues the age related decline in base excision repair in a PARP1-dependent manner. Cell Cycle. 2015;14:269–276. doi: 10.4161/15384101.2014.980641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Van Meter M, Kashyap M, Rezazadeh S, et al. SIRT6 represses LINE1 retrotransposons by ribosylating KAP1 but this repression fails with stress and age. Nat Commun. 2014;5:5011. doi: 10.1038/ncomms6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mostoslavsky R, Chua KF, Lombard DB, et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124:315–329. doi: 10.1016/j.cell.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 105.Kanfi Y, Naiman S, Amir G, et al. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012;483:218–221. doi: 10.1038/nature10815. [DOI] [PubMed] [Google Scholar]
- 106.Sundaresan NR, Vasudevan P, Zhong L, et al. The sirtuin SIRT6 blocks IGF-Akt signaling and development of cardiac hypertrophy by targeting c-Jun. Nat Med. 2012;18:1643–1650. doi: 10.1038/nm.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee N, Kim D-K, Kim E-S, et al. Comparative interactomes of SIRT6 and SIRT7: implication of functional links to aging. Proteomics. 2014;14:1610–1622. doi: 10.1002/pmic.201400001. [DOI] [PubMed] [Google Scholar]
- 108.Toiber D, Erdel F, Bouazoune K, et al. SIRT6 recruits SNF2H to DNA break sites, preventing genomic instability through chromatin remodeling. Mol Cell. 2013;51:454–468. doi: 10.1016/j.molcel.2013.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mao Z, Hine C, Tian X, et al. SIRT6 promotes DNA repair under stress by activating PARP1. Science. 2011;332:1443–1446. doi: 10.1126/science.1202723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mao Z, Tian X, Van Meter M, et al. Sirtuin 6 (SIRT6) rescues the decline of homologous recombination repair during replicative senescence. Proc Natl Acad Sci USA. 2012;109:11800–11805. doi: 10.1073/pnas.1200583109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Michishita E, McCord RA, Berber E, et al. SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature. 2008;452:492–496. doi: 10.1038/nature06736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pan H, Guan D, Liu X, et al. SIRT6 safeguards human mesenchymal stem cells from oxidative stress by coactivating NRF2. Cell Res. 2016 doi: 10.1038/cr.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Adler AS, Sinha S, Kawahara TLA, et al. Motif module map reveals enforcement of aging by continual NF-kappaB activity. Genes Dev. 2007;21:3244–3257. doi: 10.1101/gad.1588507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kawahara TLA, Michishita E, Adler AS, et al. SIRT6 links histone H3 lysine 9 deacetylation to control of NF-κB dependent gene expression and organismal lifespan. Cell. 2009;136:62–74. doi: 10.1016/j.cell.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen D, Thomas EL, Kapahi P. HIF-1 modulates dietary restriction-mediated lifespan extension via IRE-1 in Caenorhabditis elegans. PLoS Genet. 2009;5:e1000486. doi: 10.1371/journal.pgen.1000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mehta R, Steinkraus KA, Sutphin GL, et al. Proteasomal regulation of the hypoxic response modulates aging in C. elegans. Science. 2009;324:1196–1198. doi: 10.1126/science.1173507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhong L, D’Urso A, Toiber D, et al. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell. 2010;140:280–293. doi: 10.1016/j.cell.2009.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lim J-H, Lee Y-M, Chun Y-S, et al. Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1alpha. Mol Cell. 2010;38:864–878. doi: 10.1016/j.molcel.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 119.Dioum EM, Chen R, Alexander MS, et al. Regulation of hypoxia-inducible factor 2alpha signaling by the stress-responsive deacetylase sirtuin 1. Science. 2009;324:1289–1293. doi: 10.1126/science.1169956. [DOI] [PubMed] [Google Scholar]
- 120.Trollmann R, Gassmann M. The role of hypoxia-inducible transcription factors in the hypoxic neonatal brain. Brain Dev. 2009;31:503–509. doi: 10.1016/j.braindev.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 121.Fan X, Heijnen CJ, van der Kooij MA, et al. The role and regulation of hypoxia-inducible factor-1α expression in brain development and neonatal hypoxic–ischemic brain injury. Brain Res Rev. 2009;62:99–108. doi: 10.1016/j.brainresrev.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 122.Mohrin M, Shin J, Liu Y, et al. Stem cell aging. A mitochondrial UPR-mediated metabolic checkpoint regulates hematopoietic stem cell aging. Science. 2015;347:1374–1377. doi: 10.1126/science.aaa2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.de Candia P, Blekhman R, Chabot AE, et al. A combination of genomic approaches reveals the role of FOXO1a in regulating an oxidative stress response pathway. PLoS ONE. 2008;3:e1670. doi: 10.1371/journal.pone.0001670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dunn LL, Buckle AM, Cooke JP, Ng MKC. The emerging role of the thioredoxin system in angiogenesis. Arterioscler Thromb Vasc Biol. 2010;30:2089–2098. doi: 10.1161/ATVBAHA.110.209643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chondrogianni N, de C MSimoesD, Franceschi C, Gonos ES. Cloning of differentially expressed genes in skin fibroblasts from centenarians. Biogerontology. 2004;5:401–409. doi: 10.1007/s10522-004-3188-1. [DOI] [PubMed] [Google Scholar]
- 126.Baugh LR, Sternberg PW. DAF-16/FOXO regulates transcription of cki-1/Cip/Kip and repression of lin-4 during C. elegans L1 arrest. Curr Biol. 2006;16:780–785. doi: 10.1016/j.cub.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 127.Ng F, Wijaya L, Tang BL. SIRT1 in the brain-connections with aging-associated disorders and lifespan. Front Cell Neurosci. 2015;9:64. doi: 10.3389/fncel.2015.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Julien C, Tremblay C, Emond V, et al. Sirtuin 1 reduction parallels the accumulation of tau in Alzheimer disease. J Neuropathol Exp Neurol. 2009;68:48–58. doi: 10.1097/NEN.0b013e3181922348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lutz MI, Milenkovic I, Regelsberger G, Kovacs GG. Distinct patterns of sirtuin expression during progression of Alzheimer’s disease. Neuromolecular Med. 2014;16:405–414. doi: 10.1007/s12017-014-8288-8. [DOI] [PubMed] [Google Scholar]
- 130.Lattanzio F, Carboni L, Carretta D, et al. Treatment with the neurotoxic Aβ (25–35) peptide modulates the expression of neuroprotective factors Pin1, Sirtuin 1, and brain-derived neurotrophic factor in SH-SY5Y human neuroblastoma cells. Exp Toxicol Pathol. 2016;68:271–276. doi: 10.1016/j.etp.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 131.Weir HJM, Murray TK, Kehoe PG, et al. CNS SIRT3 expression is altered by reactive oxygen species and in Alzheimer’s disease. PLoS ONE. 2012;7:e48225. doi: 10.1371/journal.pone.0048225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cieślik M, Czapski GA, Strosznajder JB. The molecular mechanism of amyloid β42 peptide toxicity: the role of sphingosine kinase-1 and mitochondrial sirtuins. PLoS ONE. 2015;10:e0137193. doi: 10.1371/journal.pone.0137193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yang W, Zou Y, Zhang M, et al. Mitochondrial Sirt3 expression is decreased in APP/PS1 double transgenic mouse model of Alzheimer’s disease. Neurochem Res. 2015;40:1576–1582. doi: 10.1007/s11064-015-1630-1. [DOI] [PubMed] [Google Scholar]
- 134.Qin W, Yang T, Ho L, et al. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J Biol Chem. 2006;281:21745–21754. doi: 10.1074/jbc.M602909200. [DOI] [PubMed] [Google Scholar]
- 135.Tippmann F, Hundt J, Schneider A, et al. Up-regulation of the alpha-secretase ADAM10 by retinoic acid receptors and acitretin. FASEB J. 2009;23:1643–1654. doi: 10.1096/fj.08-121392. [DOI] [PubMed] [Google Scholar]
- 136.Wang J, Fivecoat H, Ho L, et al. The role of Sirt1: at the crossroad between promotion of longevity and protection against Alzheimer’s disease neuropathology. Biochim Biophys Acta. 2010;1804:1690–1694. doi: 10.1016/j.bbapap.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 137.Lee HR, Shin HK, Park SY, et al. Cilostazol suppresses β-amyloid production by activating a disintegrin and metalloproteinase 10 via the upregulation of SIRT1-coupled retinoic acid receptor-β. J Neurosci Res. 2014;92:1581–1590. doi: 10.1002/jnr.23421. [DOI] [PubMed] [Google Scholar]
- 138.Marwarha G, Raza S, Meiers C, Ghribi O. Leptin attenuates BACE1 expression and amyloid-β genesis via the activation of SIRT1 signaling pathway. Biochim Biophys Acta. 2014;1842:1587–1595. doi: 10.1016/j.bbadis.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 139.Godoy JA, Zolezzi JM, Braidy N, Inestrosa NC. Role of Sirt1 during the ageing process: relevance to protection of synapses in the brain. Mol Neurobiol. 2014;50:744–756. doi: 10.1007/s12035-014-8645-5. [DOI] [PubMed] [Google Scholar]
- 140.Guo P, Wang D, Wang X, et al. Effect and mechanism of fuzhisan and donepezil on the sirtuin 1 pathway and amyloid precursor protein metabolism in PC12 cells. Mol Med Rep. 2016;13:3539–3546. doi: 10.3892/mmr.2016.4957. [DOI] [PubMed] [Google Scholar]
- 141.Gao R, Wang Y, Pan Q, et al. Fuzhisan, a chinese herbal medicine, suppresses beta-secretase gene transcription via upregulation of SIRT1 expression in N2a-APP695 cells. Int J Clin Exp Med. 2015;8:7231–7240. [PMC free article] [PubMed] [Google Scholar]
- 142.Park SY, Lee HR, Lee WS, et al. Cilostazol modulates autophagic degradation of β-amyloid peptide via SIRT1-coupled LKB1/AMPKα signaling in neuronal cells. PLoS ONE. 2016;11:e0160620. doi: 10.1371/journal.pone.0160620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chen J, Zhou Y, Mueller-Steiner S, et al. SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J Biol Chem. 2005;280:40364–40374. doi: 10.1074/jbc.M509329200. [DOI] [PubMed] [Google Scholar]
- 144.Scuderi C, Stecca C, Bronzuoli MR, et al. Sirtuin modulators control reactive gliosis in an in vitro model of Alzheimer’s disease. Front Pharmacol. 2014;5:89. doi: 10.3389/fphar.2014.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Biella G, Fusco F, Nardo E, et al. Sirtuin 2 inhibition improves cognitive performance and acts on amyloid-β protein precursor processing in two Alzheimer’s disease mouse models. J Alzheimers Dis. 2016;53:1193–1207. doi: 10.3233/JAD-151135. [DOI] [PubMed] [Google Scholar]
- 146.Chesser AS, Pritchard SM, Johnson GVW. Tau clearance mechanisms and their possible role in the pathogenesis of Alzheimer disease. Front Neurol. 2013;4:122. doi: 10.3389/fneur.2013.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Greco SJ, Hamzelou A, Johnston JM, et al. Leptin boosts cellular metabolism by activating AMPK and the sirtuins to reduce tau phosphorylation and β-amyloid in neurons. Biochem Biophys Res Commun. 2011;414:170–174. doi: 10.1016/j.bbrc.2011.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Min S-W, Cho S-H, Zhou Y, et al. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron. 2010;67:953–966. doi: 10.1016/j.neuron.2010.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Du L-L, Xie J-Z, Cheng X-S, et al. Activation of sirtuin 1 attenuates cerebral ventricular streptozotocin-induced tau hyperphosphorylation and cognitive injuries in rat hippocampi. Age (Dordr) 2014;36:613–623. doi: 10.1007/s11357-013-9592-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Corpas R, Revilla S, Ursulet S, et al. SIRT1 overexpression in mouse hippocampus induces cognitive enhancement through proteostatic and neurotrophic mechanisms. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-0087-9. [DOI] [PubMed] [Google Scholar]
- 151.Hernandez-Rapp J, Rainone S, Goupil C, et al. microRNA-132/212 deficiency enhances Aβ production and senile plaque deposition in Alzheimer’s disease triple transgenic mice. Sci Rep. 2016;6:30953. doi: 10.1038/srep30953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Salminen A, Kaarniranta K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res Rev. 2012;11:230–241. doi: 10.1016/j.arr.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 153.Kim H-S, Moon S, Paik J-H, et al. Activation of the 5′-AMP-activated protein kinase in the cerebral cortex of young senescence-accelerated P8 mice and association with GSK3β- and PP2A-dependent inhibition of p-tau396 expression. J Alzheimers Dis. 2015;46:249–259. doi: 10.3233/JAD-150035. [DOI] [PubMed] [Google Scholar]
- 154.Park H, Kam T-I, Kim Y, et al. Neuropathogenic role of adenylate kinase-1 in Aβ-mediated tau phosphorylation via AMPK and GSK3β. Hum Mol Genet. 2012;21:2725–2737. doi: 10.1093/hmg/dds100. [DOI] [PubMed] [Google Scholar]
- 155.Costa RM, Drew C, Silva AJ. Notch to remember. Trends Neurosci. 2005;28:429–435. doi: 10.1016/j.tins.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 156.Bonda DJ, Lee H-G, Camins A, et al. The sirtuin pathway in ageing and Alzheimer disease: mechanistic and therapeutic considerations. Lancet Neurol. 2011;10:275–279. doi: 10.1016/S1474-4422(11)70013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Xiao M-J, Han Z, Shao B, Jin K. Notch signaling and neurogenesis in normal and stroke brain. Int J Physiol Pathophysiol Pharmacol. 2009;1:192–202. [PMC free article] [PubMed] [Google Scholar]
- 158.Seo J-S, Moon M-H, Jeong J-K, et al. SIRT1, a histone deacetylase, regulates prion protein-induced neuronal cell death. Neurobiol Aging. 2012;33:1110–1120. doi: 10.1016/j.neurobiolaging.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 159.Silva DF, Esteves AR, Oliveira CR, Cardoso SM. Mitochondrial metabolism power SIRT2-dependent deficient traffic causing Alzheimer’s-disease related pathology. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-9951-x. [DOI] [PubMed] [Google Scholar]
- 160.Wei W, Xu X, Li H, et al. The SIRT2 polymorphism rs10410544 and risk of Alzheimer’s disease: a meta-analysis. Neuromolecular Med. 2014;16:448–456. doi: 10.1007/s12017-014-8291-0. [DOI] [PubMed] [Google Scholar]
- 161.Piriz J, Muller A, Trejo JL, Torres-Aleman I. IGF-I and the aging mammalian brain. Exp Gerontol. 2011;46:96–99. doi: 10.1016/j.exger.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 162.Trueba-Sáiz A, Cavada C, Fernandez AM, et al. Loss of serum IGF-I input to the brain as an early biomarker of disease onset in Alzheimer mice. Transl. Psychiatry. 2013;3:e330. doi: 10.1038/tp.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hu Y-K, Wang X, Li L, et al. MicroRNA-98 induces an Alzheimer’s disease-like disturbance by targeting insulin-like growth factor 1. Neurosci Bull. 2013;29:745–751. doi: 10.1007/s12264-013-1348-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Poirier R, Fernandez AM, Torres-Aleman I, Metzger F. Early brain amyloidosis in APP/PS1 mice with serum insulin-like growth factor-I deficiency. Neurosci Lett. 2012;509:101–104. doi: 10.1016/j.neulet.2011.12.048. [DOI] [PubMed] [Google Scholar]
- 165.Gontier G, George C, Chaker Z, et al. Blocking IGF signaling in adult neurons alleviates Alzheimer’s disease pathology through amyloid-β clearance. J Neurosci. 2015;35:11500–11513. doi: 10.1523/JNEUROSCI.0343-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.de Bruijn RFAG, Janssen JAMJL, Brugts MP, et al. Insulin-like growth factor-I receptor stimulating activity is associated with dementia. J Alzheimers Dis. 2014;42:137–142. doi: 10.3233/JAD-140186. [DOI] [PubMed] [Google Scholar]
- 167.Bassil F, Fernagut P-O, Bezard E, Meissner WG. Insulin, IGF-1 and GLP-1 signaling in neurodegenerative disorders: targets for disease modification? Prog Neurobiol. 2014;118:1–18. doi: 10.1016/j.pneurobio.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 168.Carro E, Trejo JL, Gomez-Isla T, et al. Serum insulin-like growth factor I regulates brain amyloid-beta levels. Nat Med. 2002;8:1390–1397. doi: 10.1038/nm1202-793. [DOI] [PubMed] [Google Scholar]
- 169.Perez FP, Bose D, Maloney B, et al. Late-onset Alzheimer’s disease, heating up and foxed by several proteins: pathomolecular effects of the aging process. J Alzheimers Dis. 2014;40:1–17. doi: 10.3233/JAD-131544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Akhter R, Sanphui P, Biswas SC. The essential role of p53-up-regulated modulator of apoptosis (Puma) and its regulation by FoxO3a transcription factor in β-amyloid-induced neuron death. J Biol Chem. 2014;289:10812–10822. doi: 10.1074/jbc.M113.519355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Manolopoulos KN, Klotz L-O, Korsten P, et al. Linking Alzheimer’s disease to insulin resistance: the FoxO response to oxidative stress. Mol Psychiatry. 2010;15:1046–1052. doi: 10.1038/mp.2010.17. [DOI] [PubMed] [Google Scholar]
- 172.Gómez Ravetti M, Rosso OA, Berretta R, Moscato P. Uncovering molecular biomarkers that correlate cognitive decline with the changes of hippocampus’ gene expression profiles in Alzheimer’s disease. PLoS One. 2010 doi: 10.1371/journal.pone.0010153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pino E, Amamoto R, Zheng L, et al. FOXO3 determines the accumulation of α-synuclein and controls the fate of dopaminergic neurons in the substantia nigra. Hum Mol Genet. 2014;23:1435–1452. doi: 10.1093/hmg/ddt530. [DOI] [PubMed] [Google Scholar]
- 174.Su B, Liu H, Wang X, et al. Ectopic localization of FOXO3a protein in Lewy bodies in Lewy body dementia and Parkinson’s disease. Mol Neurodegener. 2009;4:32. doi: 10.1186/1750-1326-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Albani D, Polito L, Batelli S, et al. The SIRT1 activator resveratrol protects SK-N-BE cells from oxidative stress and against toxicity caused by alpha-synuclein or amyloid-beta (1–42) peptide. J Neurochem. 2009;110:1445–1456. doi: 10.1111/j.1471-4159.2009.06228.x. [DOI] [PubMed] [Google Scholar]
- 176.Okawara M, Katsuki H, Kurimoto E, et al. Resveratrol protects dopaminergic neurons in midbrain slice culture from multiple insults. Biochem Pharmacol. 2007;73:550–560. doi: 10.1016/j.bcp.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 177.Chao J, Yu M-S, Ho Y-S, et al. Dietary oxyresveratrol prevents parkinsonian mimetic 6-hydroxydopamine neurotoxicity. Free Radic Biol Med. 2008;45:1019–1026. doi: 10.1016/j.freeradbiomed.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 178.Blanchet J, Longpré F, Bureau G, et al. Resveratrol, a red wine polyphenol, protects dopaminergic neurons in MPTP-treated mice. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1243–1250. doi: 10.1016/j.pnpbp.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 179.Zhang A, Wang H, Qin X, et al. Genetic analysis of SIRT1 gene promoter in sporadic Parkinson’s disease. Biochem Biophys Res Commun. 2012;422:693–696. doi: 10.1016/j.bbrc.2012.05.059. [DOI] [PubMed] [Google Scholar]
- 180.Mudò G, Mäkelä J, Di Liberto V, et al. Transgenic expression and activation of PGC-1α protect dopaminergic neurons in the MPTP mouse model of Parkinson’s disease. Cell Mol Life Sci. 2012;69:1153–1165. doi: 10.1007/s00018-011-0850-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wu Y, Li X, Zhu JX, et al. Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson’s disease. Neurosignals. 2011;19:163–174. doi: 10.1159/000328516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.McLean PJ, Klucken J, Shin Y, Hyman BT. Geldanamycin induces Hsp70 and prevents alpha-synuclein aggregation and toxicity in vitro. Biochem Biophys Res Commun. 2004;321:665–669. doi: 10.1016/j.bbrc.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 183.Klucken J, Shin Y, Masliah E, et al. Hsp70 reduces alpha-synuclein aggregation and toxicity. J Biol Chem. 2004;279:25497–25502. doi: 10.1074/jbc.M400255200. [DOI] [PubMed] [Google Scholar]
- 184.Westerheide SD, Anckar J, Stevens SM, et al. Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science. 2009;323:1063–1066. doi: 10.1126/science.1165946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Watanabe S, Ageta-Ishihara N, Nagatsu S, et al. SIRT1 overexpression ameliorates a mouse model of SOD1-linked amyotrophic lateral sclerosis via HSF1/HSP70i chaperone system. Mol Brain. 2014;7:62. doi: 10.1186/s13041-014-0062-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chen X, Wales P, Quinti L, et al. The sirtuin-2 inhibitor AK7 is neuroprotective in models of Parkinson’s disease but not amyotrophic lateral sclerosis and cerebral ischemia. PLoS ONE. 2015;10:e0116919. doi: 10.1371/journal.pone.0116919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Guan Q, Wang M, Chen H, et al. Aging-related 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurochemial and behavioral deficits and redox dysfunction: improvement by AK-7. Exp Gerontol. 2016;82:19–29. doi: 10.1016/j.exger.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 188.Outeiro TF, Kontopoulos E, Altmann SM, et al. Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson’s disease. Science. 2007;317:516–519. doi: 10.1126/science.1143780. [DOI] [PubMed] [Google Scholar]
- 189.Hasegawa T, Baba T, Kobayashi M, et al. Role of TPPP/p25 on α-synuclein-mediated oligodendroglial degeneration and the protective effect of SIRT2 inhibition in a cellular model of multiple system atrophy. Neurochem Int. 2010;57:857–866. doi: 10.1016/j.neuint.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 190.Krey L, Lühder F, Kusch K, et al. Knockout of silent information regulator 2 (SIRT2) preserves neurological function after experimental stroke in mice. J Cereb Blood Flow Metab. 2015;35:2080–2088. doi: 10.1038/jcbfm.2015.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Narayan N, Lee IH, Borenstein R, et al. The NAD-dependent deacetylase SIRT2 is required for programmed necrosis. Nature. 2012;492:199–204. doi: 10.1038/nature11700. [DOI] [PubMed] [Google Scholar]
- 192.Liu J, Wu X, Wang X, et al. Global gene expression profiling reveals functional importance of Sirt2 in endothelial cells under oxidative stress. Int J Mol Sci. 2013;14:5633–5649. doi: 10.3390/ijms14035633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Naia L, Rego AC. Sirtuins: double players in Huntington’s disease. Biochim Biophys Acta. 2015;1852:2183–2194. doi: 10.1016/j.bbadis.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 194.Kannike K, Sepp M, Zuccato C, et al. Forkhead transcription factor FOXO3a levels are increased in Huntington disease because of overactivated positive autofeedback loop. J Biol Chem. 2014;289:32845–32857. doi: 10.1074/jbc.M114.612424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Jiang M, Wang J, Fu J, et al. Neuroprotective role of Sirt1 in mammalian models of Huntington’s disease through activation of multiple Sirt1 targets. Nat Med. 2012;18:153–158. doi: 10.1038/nm.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Duan W. Targeting sirtuin-1 in Huntington’s disease: rationale and current status. CNS Drugs. 2013;27:345–352. doi: 10.1007/s40263-013-0055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Smith MR, Syed A, Lukacsovich T, et al. A potent and selective Sirtuin 1 inhibitor alleviates pathology in multiple animal and cell models of Huntington’s disease. Hum Mol Genet. 2014;23:2995–3007. doi: 10.1093/hmg/ddu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Carafa V, Rotili D, Forgione M, et al. Sirtuin functions and modulation: from chemistry to the clinic. Clin Epigenetics. 2016 doi: 10.1186/s13148-016-0224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Pontiki E, Hadjipavlou-Litina D. Histone deacetylase inhibitors (HDACIs). Structure–activity relationships: history and new QSAR perspectives. Med Res Rev. 2012;32:1–165. doi: 10.1002/med.20200. [DOI] [PubMed] [Google Scholar]
- 200.Bedalov A, Gatbonton T, Irvine WP, et al. Identification of a small molecule inhibitor of Sir2p. Proc Natl Acad Sci USA. 2001;98:15113–15118. doi: 10.1073/pnas.261574398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Napper AD, Hixon J, McDonagh T, et al. Discovery of indoles as potent and selective inhibitors of the deacetylase SIRT1. J Med Chem. 2005;48:8045–8054. doi: 10.1021/jm050522v. [DOI] [PubMed] [Google Scholar]
- 202.Orecchia A, Scarponi C, Di Felice F, et al. Sirtinol treatment reduces inflammation in human dermal microvascular endothelial cells. PLoS ONE. 2011;6:e24307. doi: 10.1371/journal.pone.0024307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Jung YJ, Lee JE, Lee AS, et al. SIRT1 overexpression decreases cisplatin-induced acetylation of NF-κB p65 subunit and cytotoxicity in renal proximal tubule cells. Biochem Biophys Res Commun. 2012;419:206–210. doi: 10.1016/j.bbrc.2012.01.148. [DOI] [PubMed] [Google Scholar]
- 204.Xu Y, Nie L, Yin Y-G, et al. Resveratrol protects against hyperglycemia-induced oxidative damage to mitochondria by activating SIRT1 in rat mesangial cells. Toxicol Appl Pharmacol. 2012;259:395–401. doi: 10.1016/j.taap.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 205.Luthi-Carter R, Taylor DM, Pallos J, et al. SIRT2 inhibition achieves neuroprotection by decreasing sterol biosynthesis. Proc Natl Acad Sci USA. 2010;107:7927–7932. doi: 10.1073/pnas.1002924107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Nie H, Chen H, Han J, et al. Silencing of SIRT2 induces cell death and a decrease in the intracellular ATP level of PC12 cells. Int J Physiol Pathophysiol Pharmacol. 2011;3:65–70. [PMC free article] [PubMed] [Google Scholar]
- 207.Villalba JM, Alcaín FJ. Sirtuin activators and inhibitors. Biofactors. 2012;38:349–359. doi: 10.1002/biof.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Neugebauer RC, Uchiechowska U, Meier R, et al. Structure–activity studies on splitomicin derivatives as sirtuin inhibitors and computational prediction of binding mode. J Med Chem. 2008;51:1203–1213. doi: 10.1021/jm700972e. [DOI] [PubMed] [Google Scholar]
- 209.Wang TTY, Schoene NW, Kim E-K, Kim YS. Pleiotropic effects of the sirtuin inhibitor sirtinol involves concentration-dependent modulation of multiple nuclear receptor-mediated pathways in androgen-responsive prostate cancer cell LNCaP. Mol Carcinog. 2013;52:676–685. doi: 10.1002/mc.21906. [DOI] [PubMed] [Google Scholar]
- 210.Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Chung S, Yao H, Caito S, et al. Regulation of SIRT1 in cellular functions: role of polyphenols. Arch Biochem Biophys. 2010;501:79–90. doi: 10.1016/j.abb.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Aquilano K, Vigilanza P, Baldelli S, et al. Peroxisome Proliferator-activated Receptor γ Co-activator 1α (PGC-1α) and Sirtuin 1 (SIRT1) Reside in Mitochondria. J Biol Chem. 2010;285:21590–21599. doi: 10.1074/jbc.M109.070169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Resveratrol for Alzheimer’s Disease—Tabular View—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/record/NCT01504854?term=sirtuin&rank=14. Accessed 3 Feb 2016
- 214.Influence of Caloric Restriction and Resveratrol in the Sirtuin System in Women and Men Aged 55–65 Years—Full Text View—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT01668836?term=sirtuin&rank=1&submit_fld_opt=. Accessed 3 Feb 2016
- 215.Resveratrol to Enhance Vitality and Vigor in Elders—Full Text View—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02123121?term=sirtuin&rank=18&submit_fld_opt=. Accessed 3 Feb 2016
- 216.Reresveratrol Administered to Healthy Male Subjects—Full Text View—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/study/NCT01768507?term=sirtuin&rank=5. Accessed 3 Feb 2016
- 217.Jiang M, Zheng J, Peng Q, et al. Sirtuin 1 activator SRT2104 protects Huntington’s disease mice. Ann Clin Transl Neurol. 2014;1:1047–1052. doi: 10.1002/acn3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Venkatasubramanian S, Noh RM, Daga S, et al. Cardiovascular effects of a novel SIRT1 activator, SRT2104, in otherwise healthy cigarette smokers. J Am Heart Assoc. 2013;2:e000042. doi: 10.1161/JAHA.113.000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.D’Onofrio N, Vitiello M, Casale R, et al. Sirtuins in vascular diseases: emerging roles and therapeutic potential. Biochim Biophys Acta. 2015;1852:1311–1322. doi: 10.1016/j.bbadis.2015.03.001. [DOI] [PubMed] [Google Scholar]