Abstract
Parkinson's disease (PD) is the second most prevalent neurodegenerative disease worldwide, affecting more than four million people. Typically, it affects individuals above 45, when they are still productive, compromising both aging and quality of life. Therefore, the cost of the disease must be identified, so that the use of resources can be rational and efficient. Additionally, in Brazil, there is a lack of research on the costs of neurodegenerative diseases, such as PD, a gap addressed in this study. This systematic review critically addresses the various methodologies used in original research around the world in the last decade on the subject, showing that costs are hardly comparable. Nonetheless, the economic and social impacts are implicit, and important information for public health agents is provided.
1. Introduction
Health and the economy are related intrinsically. The purpose of the studies on the costs of diseases is describing them, estimating costs, comparing established programs, and projecting these costs based on clinical, demographic, epidemiological, and technological factors. In fact, over the past decade, there has been a growing number of studies, which are presumed to be valuable decision tools, because the limited amount of resources must be used rationally and efficiently as not to miss opportunities to improve overall population health [1].
In neurodegenerative diseases, such as Parkinson's disease (PD), whose prevention is still impossible, the burden borne by society, whether it is financial, social, or even psychological, is often heavy. Being the second most prevalent neurodegenerative disease worldwide, it generally affects individuals between 40 and 50 (late-onset PD) [2, 3], compromising their productive life and aging. As such, research needs to be directed to reducing their costs.
Studies on disease costs may have several approaches, such as economic assessment, epidemiological design, or even type of cost involved, as well as the viewpoint of defining resource use strategies. The diversity in methods is a significant factor why cost estimates differ between studies, opening the discussion about which public policies are most appropriate for PD.
This systematic review provides introductory concepts on the types of studies on costs and analyzes results in selected articles critically, highlighting the benefits and limitations of their methods. Moreover, this study identifies the most common studies regarding DP costs worldwide over the past 10 years, showing possibilities for studies being carried out in Brazil, where there is a lack of this type of analysis because most studies only involve the clinical aspects of the disease.
2. Methodology
In March 2016, two online bibliographic information services were accessed—SCOPUS and PubMed—with the aim of selecting original articles about the cost of PD over the past decade.
The following terms were used for access: (parkinson disease) AND TITLE-ABS-KEY (economics) OR TITLE-ABS-KEY (costs of illness) OR TITLE-ABS-KEY (health expenditures) OR TITLE-ABS-KEY (cost effectiveness analysis) OR TITLE-ABS-KEY (cost benefit analysis) OR TITLE-ABS - CUT (cost utility analysis) OR TITLE-ABS-KEY (cost minimization analysis) OR TITLE-ABS-KEY (direct costs) OR TITLE-ABS-KEY (CB costs) OR TITLE-ABS-KEY (out of pockets) AND DOCTYPE (air OR re) AND PUBYEAR > 2004 AND (LIMIT-TO (LANGUAGE, “English”)) AND (LIMIT-TO (DOCTYPE, “air”)).
This method identified 522 papers. The inclusion criterion was that articles refer to costs related to this disease in general and/or regarding the use of medication. Papers that compared procedures and/or medicines, dealt with specific therapies, as well as PD surgeries, or were related to patient caregivers or already selected papers but were neither applicable to the research nor available to access were excluded. Revisions were also not selected (380 papers were excluded because of the title, for not being compatible, having been duplicated, and/or being reviews). Once the first levels of inclusion were satisfied, 142 papers were selected for reading the abstract. Although 35 of these had a suggestive title, that is, they did not tackle general costs of disease exclusively, 107 were separated for complete reading, and 30 met the criteria for research (Figure 1).
Figure 1.
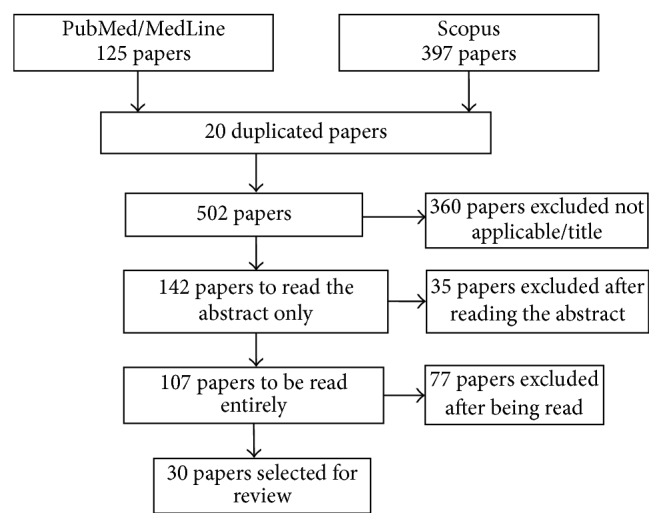
Search, selection, and inclusion of papers for critical analysis of studies on economic PD evaluation in online platforms.
2.1. Basic Concepts of Health Studies
The determination of the costs of a disease facilitates learning what its burden to society is, assessing its degree of efficiency, and understanding how the market tends to organize itself regarding certain values [4].
2.1.1. Economic Assessment
The basic function of any economic assessment is to identify, measure, value, and compare the costs and consequences of alternative proposals [4–8].
In this case, four techniques are possible (Table 1):
Table 1.
Types of economic evaluation and their main characteristics.
| Type of economic analysis | Costs | Advantages | Disadvantages |
|---|---|---|---|
| Cost minimization (CMA) | Monetary | This technique only measures costs | It does not describe results, and it has little applicability to health |
| Cost-effectiveness (CEA) | Monetary | It allows comparisons between health programs | Difficulty in comparison of results |
| Cost-benefit (CBA) | Monetary | This analysis allows comparisons between strategies because it works with the same monetary unit | Difficulty of valuing human life |
| Cost-utility (CUA) | Monetary | This analysis considers the level of well-being and preferences of the individual | The scales of measurement of quality are arbitrary |
(1) Cost-Minimization. Cost-minimization is the least used technique, because it only compares costs of interventions that produce the same outcomes with different costs. For chronic diseases, such as PD, there are no studies using this type of analysis.
(2) Cost-Effectiveness. Cost-effectiveness is the technique most used in literature, which assesses the impact of different alternatives that bring better results with lower costs; these are always comparative and explicit and designed to select the best option to achieve what is perceived in clinical practice.
(3) Cost-Benefit. This analysis determines whether a new health technology or intervention generates net benefits to society. However, due to its difficulty, complexity, and controversies in valuing human life and certain health conditions in monetary terms, this analysis is rarely found in the literature.
(4) Cost-Utility. This analysis assesses the impacts on survival and quality of life, which are determining criteria to judge the effects of strategies in health care, that is, the level of well-being and preferences of the individual.
2.1.2. Study Designs
The epidemiological study designs define how the research will be performed in relation to adopted method [9–13]. The most discussed study designs in PD research are as follows (Table 2).
Table 2.
Main study designs on costs.
| Approaches | Description | Advantages | Disadvantages |
|---|---|---|---|
| Prevalence | Frequency measure It evaluates all existing cases in a given period |
Ample results Specific policy planning Fast study and recommended for chronic diseases |
Considered weak at estimating the risk of developing disease |
| Incidence | Frequency measure Assesses the number of new cases in a given period |
Implementation of measures to reduce new cases It is used more for acute diseases, since it estimates the risk of developing the disease |
Not recommended for chronic diseases |
| Top-down | It measures the proportion of a disease attributed to several risk factors. It involves a study directed from total to lower levels | When the scope of study is well understood | More comprehensive, it hampers the study on the details of the disease |
| Bottom-up | Related to the unit costs of inputs used. It involves the study directed from individual levels to the total. | More detailed | Risk of double counting |
| Prospective | Temporal study, performed during disease. Probes the effect through the cause | Used in chronic diseases | Time-consuming and expensive |
| Retrospective | Temporal study performed with preexisting data. Probes the cause through the effect |
Quick and cheaper | Risk of memory bias |
| Econometric | Comparison of groups | Minor amount of data required Cost difference between the two populations |
Long study, requiring that the control group be paired to the study group |
| Markov models | Stochastic process Used in prospective studies. Patients stratified in stages of disease |
Dynamic model aiming at studying the transition from one stage to another, evaluating the costs of each step | Transition of stages is independent, without considering the previous one |
(1) Prevalence and Incidence. The prevalence estimates the number of deaths, hospitalizations, prevention, and research attributable to a disease in a given period (usually a year), to subsequently estimate the costs incurred by these consequences. Incidence refers to the number of new cases in a predefined period, and it foresees the associated costs from the onset of the disease until its disappearance (usually cure or death), through a rough projection of the flow of these values.
Studies on prevalence display greater results than incidence ones, for diseases are usually long-term sequelae, as is the case of PD, and they are of great importance in planning specific policies on certain diseases when their economic burden was underestimated. Therefore, they can identify the main components of current expenses and uncharged resources.
(2) Top-Down and Bottom-Up. Top-down approaches are normally used in prevalence studies, when the expenses of a disease are widely known from national or regional statistics. In bottom-up studies, cost estimates are more detailed. The data depend on the scope of the study, and they are intrinsically related to the unit costs of inputs used, through interviews, questionnaires or chart review, and assessment of individual cost. The average cost per person is then obtained by means of the number of times the service was used and the number of people with the disease. Although bottom-up studies are more complete when it comes to resources and more precise regarding patient selection, they run a high risk of double counting costs (e.g., if a patient has more than one disease and costs of comorbidities are confused and/or grouped). The majority of studies with PD adopt this approach.
(3) Prospective and Retrospective Approaches. There is a temporal relationship, where in prospective studies the relevant events have not happened yet, that is, studying the patient over time, formalizing a system of data collection focused on the purpose of the research, such as questionnaires designed specifically for patients and/or their caregivers, where everything is recorded in “real time.” In retrospective studies, all events had already occurred when the study was initiated. They are usually employed in long-term chronic diseases, as is the case of PD. In this case, research efficiency can only be possible with enough observational datasets. It would be best if the data were stored electronically to minimize memory bias due to omission of facts or values.
(4) Econometric Approaches. Econometric approaches estimate differences between groups. One of the groups has the disease and the other does not; however, both have the same characteristics, which are assessed by several regression analyses involving demographic factors such as sex, age, marital status, ethnicity, relationship between patient and caregiver, housing, and duration of the disease.
(5) Markov Models. Markov models are used in several studies of chronic diseases, when patients are studied over time, and they are stratified according to disease scale. In the case of PD, the scale of Hoehn and Yahr (it assesses the degree of disability due to the disease in scores) is key to building this model. These are typically prospective studies, proposing cost increases with disease severity.
Several approaches may be featured in the same study; that is, we may have a retrospective, prevalent, and bottom-up study, for instance, because its purpose, most of the time, is to maximize the content of information, contributing to enriching knowledge.
2.1.3. Classification of Costs
The costs of a disease are typically stratified as follows [4, 7, 10, 14] (Table 3).
Table 3.
Classification of costs.
| Types of costs | Description |
|---|---|
| Direct medical | Directly related to the disease. Hospitalization, medication, medical appointments, treatments, laboratory tests, and diagnosis |
| Direct nonmedical | Directly related to the disease. Transport, domestic modifications, food |
| Indirect | Loss of productivity: partial, temporary, or permanent They may affect the patient and/or caregiver Early retirement |
| Intangible | Psychological and psychosocial and costs, difficult measurement |
| Personal | Costs incurred by the patient and/or their family, when there is no support from private and/or public health care. Private consultations, medication, treatments, and domestic modifications. Linked with direct costs |
(1) Direct Costs. Direct costs are related to the disease and its equation; their charges may concern public administration, insurance companies, the patient, the patient's family, or even a combination of all or some of these determinants. The estimates of direct costs associated with chronic diseases are higher than those associated with acute and communicable diseases, on the condition that better treatments and methods of prevention are adopted. This group can be divided into direct medical costs and nonmedical ones, although not all studies adopt this division.
(2) Indirect Costs. Indirect costs refer to the loss of income and/or productivity; they are caused by disease. Additionally, they can incur costs to both the patient and the employer. Depending on the disease, this loss may be partial, temporary, or permanent, and it may be restricted to the patient and/or caregiver (as in the case of advanced stage PD), frequently leading to early retirement. If there is a possibility of returning to regular activities, this disease may not occur on the same productivity level as before, or lead to frequent absences (absenteeism), incurring additional costs, such as loss of promotions.
(3) Intangible Costs. Intangible costs are virtually impossible to measure, since they incur psychological and psychosocial costs imposed to the patients, their family, and acquaintances due to the disease, as well as pain, behavioral changes, and everyday activities. They depend on the perception that the patient's health problems lead to social consequences, such as isolation.
(4) Personal Costs. Personal costs are the costs borne by the patient and/or their family and friends due to consultations with health professionals, medication, laboratory tests, domestic adjustments, locomotion resources, and the need for home care. Depending on the country, these costs, also called copayments, are borne by the government, health insurance, or religious or private health institutions. Sometimes, these payments are also designated as direct costs because they are associated with the disease. They may be redeemable or not, implying an additional expense to the patient, who has to spend a certain amount of money in advance.
2.1.4. Perspectives
Who bears the costs related to the program defines which costs are included for analysis [6, 7, 10] (Table 4).
Table 4.
Description of the main perspectives used in cost studies.
| Perspective | Description |
|---|---|
| Industry | Related to human capital. Considers the individual as an investment target |
| Society | More common in the literature. It is comprehensive and based on health-related decisions. It represents the public interest |
| Patient/family | Less common, only addresses the patient's and their family's costs |
| Public/private health care | To identify and quantify all inputs used in the production of the service/procedure. Important to form the cost of illness |
(1) Industry (Human Capital). The industry bears the costs due to absenteeism or loss of productivity, and early retirement due to the disease.
(2) Society. A more comprehensive perspective considers all costs related to the program, regardless of who will pay the expenses (patient, government, or insurance companies). This approach is thought to be the most appropriate to support health-related decisions. Most research on PD addresses this perspective, although some studies include more than one viewpoint.
(3) Patients and Their Family. Costs are borne by the patient concerning appointments, transport used for his treatment, purchase of medication, expenses with caregivers, domestic changes, and so on.
(4) Programs, Public Health, and/or Insurance Companies. When there is a need to identify all the inputs related to the disease, for which monetary value explains the base period and the form of assessment used should be assigned, this perspective is highly likely to underestimate the cost of disease, especially when greater profit or lower production costs are targeted.
2.2. Studies on the Socioeconomic Impact of PD
Although PD affects more than four million people worldwide [15], little is known about their progression rates, the costs of medical care, and the management of resources specific to this disease [16]. In Brazil, although its notification is not compulsory, unofficial data estimate 220,000 PD sufferers. Considering local records of patients with PD, in a study conducted in the city of Bambuí, Minas Gerais, it was found that 3% of the population above 64 had the disease, a result similar to the prevalence rates found in elderly studies in European and American countries and slightly above the rates in Eastern countries [17].
PD was considered among the most prevalent and costly diseases of the brain, being the fourth most expensive second study in 28 European countries [18]. However, the level of socioeconomic development, budget availability of health systems, and culture of each country or region determine research methodologies, which are directed to a subset of expenses, using only a few components and all expenditure resulting from a disease, which would be practically impossible. Therefore, there is no one method more or less appropriate for this type of study, as the costs of PD in all countries involved cannot be compared and the information cannot be simply transferred from one country to another without having any evidence to support the use of the data.
Over the past decade, there has been a significant increase in the number of papers related to costs of diseases. Figure 2 shows the evolution for PD, between 2005 and February 2016, with a higher concentration between 2011 and 2013, triggered by the need to investigate the values involved in the cost of this disease. The demographic transition is a reality, and health managers need data that can enable their strategies towards public policies. The graph shows the 522 identified papers (125 Pubmed, 397 Scopus) and the 30 selected among them for this review.
Figure 2.
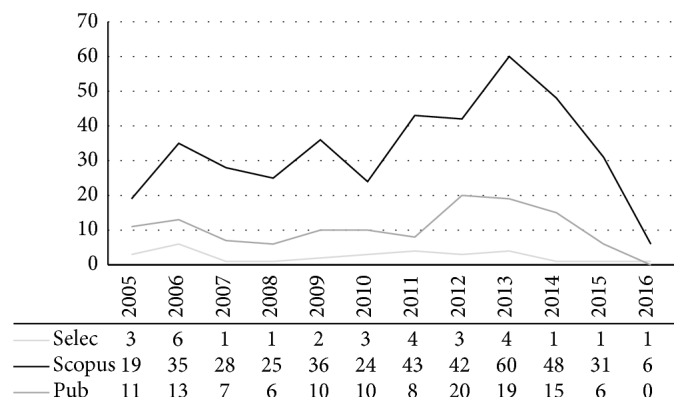
Number of publications on PD costs over the past 10 years. Scopus = 397 papers; Pubmed = 125 papers; Selected = 30.
3. Discussion
The papers selected for this review are summarized in Table 5. The PD cost may be very different from one country to another. Nonetheless, the monetary value of the year in which the study happened should be considered. All values were converted to US dollars ($) and daily, quarterly, or half-yearly results were converted to annual. Only one article had values by period of life [19], and another took into consideration those 40 to 79 years of age [20].
Table 5.
Comparison of findings on costs of PD in selected studies.
| Author | Country/Region | Year | n | Design | Costs studied | Perspective | Value/year US$ |
Comments |
|---|---|---|---|---|---|---|---|---|
| Yoritaka et al. [40] | Japan | 2016 | 715 | SPO | D | S | 5,828 | Direct cost |
| Martínez-Martin et al., [44] | Spain | 2015 | 174 | PO/BU | D/I | S | 13,720.24/year 4 | Magnitude of disease and quality of life |
| Tamás et al. [39] | Hungary | 2014 | 110 | PE/BU | D/I/OOP | S/CH | 6,831 | Costs of illness and quality of life |
| Kowal et al. [22] | USA | 2013 | 630,000 | PE | D/I | S | 22,800 | Economic load current and projected (by 2050) in the USA |
| Zhao et al. [19] | Singapore | 2013 | 195 | PE/MK/BU | D/I | S | 68,519 (over the lifetime period) | Cost of illness |
| Johnson et al. [41] | USA | 2013 | 1,151 | RE | D/I | CS | 43,506 PDINST (cohort) | Cost of illness x several cohorts |
| Bhattacharjee and Sambamoorthi [29] | USA | 2013 | 350 | RE | D/OOP | S | 15,404 | Cost of illness/over expenditure associated with PD |
| Kaltenboeck et al. [23] | USA | 2012 | 25,577 | RE | D | G | 78,042 (ambulatory pac. PD) | Survival rates and costs of patients of health programs |
| Bach et al. [43] | Germany | 2012 | 1,449 | PE | D/I | G | 6.00 (2190) to 12.69 (4631.85) | Cost of illness/drugs/comorbidities |
| Lökk et al. [25] | Sweden | 2012 | 4,163 | PE/RE | D | S | 9,333 | Cost of illness/drugs |
| Johnson et al. [20] | USA | 2011 | 278 | PO | I | S/CH/CS | 569,393 (45 years), 188,590 (55), 35,496 (65), 2,451 (75) (from 40 to 79 years) | Indirect costs |
| Jennum et al. [26] | Denmark | 2011 | 13,400 | RE/PO | D/I | S | 7,763 | Cost of illness |
| Zhao et al. [38] | Singapore | 2011 | 195 | PE/BU | D/I/OOP | S | 10,129 | Cost of illness |
| von Campenhausen et al. [45] | Europe (6 countries) | 2011 | 486 | PE/RE/BU | D/I/OOP | S | 2,968 to 11,124 | Cost of illness |
| Winter et al. [30] | Italy | 2010 | 70 | PO/BU | D/I/OOP | S | 19,574 | Cost of illness/drugs |
| Winter et al. [46] | Germany | 2010 | 145 | PO/PE/BU | D/I/OOP | G | 22,763 | Cost of illness |
| Winter et al. [47] | Germany | 2010 | 145/133 | PE/RE | D/I | S | 21,138 to 35,864 | Cost of illness |
| Winter et al. [32] | Czech Rep. | 2009 | 100 | PE/RE/BU | D/I | S/CH/P | 12,483 | Cost of illness |
| Winter et al. [37] | Russia | 2009 | 100 | PE/PO/BU | D/I | S/CH | 5,935 | Cost of illness |
| Vargas et al. [42] | Brazil | 2008 | 144 | PE/PO/BU | IN | NA | NA | Resource use X incapacity |
| McCrone et al. [33] | UK | 2007 | 175 | PE/RE | D/OOP | CS/P | 19,861 | Cost of illness |
| Leibson et al. [35] | USA | 2006 | 92 | PE/RE | D | NA | Unclear | Cost of illness per groups |
| Ragothaman et al. [36] | India | 2006 | 175 | PE/PO | D | S | 707 | Cost of illness/direct costs |
| Wang et al. [48] | China | 2006 | 190 | PE/RE/BU | D/I | S | 925 | Cost of illness |
| Vossius et al. [31] | Germany/Norway | 2006 | 438 | PE/RE/PO | D | S | 2,389 (Germany), 1,620 (Norway) | Cost of PD drugs |
| Noyes et al. [27] | USA | 2006 | 717 | PE/RE | D/OOP | S/P | 18,528 | Cost of illness/drugs/medicare |
| Cordato et al. [28] | Australia | 2006 | 12 | PE/PO | D/I | S | 5,380 | Cost of illness |
| Huse et al. [24] | USA | 2005 | 20,016 | PE/RE | D | CS | 10,037 | Cost of illness |
| Spottke et al. [34] | Germany | 2005 | 145 | PE/PO | D/I/OOP | S/G/P | 22,723 ± 28,297 | Cost of illness |
| Cubo et al. [21] | Spain | 2005 | 23,417 | RE | Int. | G | NA | Years of life lost |
Notes: SPO = semiprospective; PO = prospective; BU = bottom-up; PE = prevalent, MK = Markov; RE = retrospective; D = direct cost; I = indirect cost; OOP = out-of-pocket; Int. = intangible; S = society; CH = human capital; CS = insurance companies; G = government; NA = not applicable; P = patient; PDINST = patients with PD institutionalized; Medicare = USA health care.
Practically, all articles used the general costs of the disease, without naming the type of economic evaluation. Only one article [21] referred to the burden of disease as DALY (disability-adjusted life in years), suggesting the use of cost-utility.
Because there is no way of implementing measures so as to reduce new cases, the most appropriate model for PD costs may be developed from prevalence studies. They are conducted when diagnosis has already been established, obtaining ample results, and are to conduct than incidence ones, which demand rigorous criteria for diagnosis. In this review, we identified 20 papers that followed this line of research (see Table 4).
The use of questionnaires, suggesting a bottom-up approach, is common practice found in the research reviewed here, although not all of them described the design. The unit value of the inputs used is more easily acquired than full reports obtained from large databases in top-down approaches, although at least six papers suggest the use of this approach for studying large samples [22–27].
Since it is a disease with long survival, retrospective studies are the most common for PD, despite the bias of memory that can be generated depending on the retroactive period. In reviewed articles, 12 authors (see Table 4) opted for a prospective study with patient monitoring. Despite being lengthy and expensive, some studies used Markov models [19] or econometric studies [28, 29] with cohorts, for example.
The aforementioned chosen variables are related to the purpose of the study, but, in general, we observed that 20 (see Table 4) out of 30 papers opted for the total cost of the disease, including direct, indirect, and/or personal costs. In one of the studies [20], however, indirect costs were only considered, from the perspective of insurance companies and human capital, whereas, in another [21], the aim was to evaluate intangible costs alone through lost years of life. Similarly, three studies concentrated only on medication costs [25, 30, 31].
Among direct costs, the most common variables analyzed in most studies were medication, hospitalizations, outpatient visits, auxiliary treatments, home care, transport, and special equipment. Not all studies divided the direct costs into medical and nonmedical (Table 3). In one of the studies, even dental care provided to patients was assessed [29].
Regarding indirect costs, most studies are related to the patient and/or the caregiver in terms of loss of productivity, early retirement, and sick leaves (medical certificates). As for personal costs, they consider informal care, copayment treatments, drugs, and equipment.
The society costs are the most studied, the society being the most affected regarding allocation of resources. Only four studies assessed this prospect from the patient's point of view [27, 32–34].
Clarity is needed in the way data and/or results are expressed, which may generate uncertainty or confusion in the conclusion of a study. For instance, one of the studies [35] does not provide a clear cost of PD, and groups are very stratified and only the differences between them are highlighted. Moreover, albeit the many variables analyzed, important components were not assessed, such as auxiliary treatments. On the other hand, another study [20] does not enlist which direct cost components were used. As such, statistical analyses must be well established, so that other studies can be replicated if necessary. In this review, some studies did not provide that [25, 31, 36].
Many authors have chosen to direct certain types of costs towards one category, which reinforces the uniqueness of each study. One author [37] argued that informal care should be placed with indirect costs, but with direct nonmedical ones, based on the fact that if home care is not provided by the family, professional care would be needed.
The fact that the same cost component is classified in different categories can have a strong influence on the final results, not considering the values set by the inputs in each country. In a study conducted in Russia [37], for example, direct costs accounted for 67% of total costs, while indirect ones accounted for 33%. Besides, in a study in Singapore [38], direct costs were 38.5% and indirect ones 61.5% of the total cost. Another author states [39] that costs were distributed as 35.7% direct, 29.4% as direct nonmedical costs and 34.9% as indirect. In this study, we have verified that the value of consultation with a specialist in the Hungarian public health care system costs around $7, while in the UK [33] this value is approximately $225.
Issues related to health insurance also influence the comparison of studies greatly and must be considered. For example, in India, a study [36] revealed that only 7.4% of patients are covered by health insurance, and, unlike most studies reviewed, the cost of PD treatment is very low, at around $707 per year, since most of the expenses are covered by the patients and their family. Conversely, in the UK [33], maintaining virtually the same research approach, a final value of around $20,000 a year was calculated. In Japan, on the other hand [40], a study found a value of around $6,000 per PD patient, where the health insurance covers 100% of the population. Depending on the patient's income or age, he/she contributes 10% to 30% to medical costs.
There are some other factors that certainly affect the results obtained: samples ranged from small cohorts (n = 12) [28] to large populations (n = 630,000) [22], some studies [41, 42] have excluded from their samples patients with advanced PD (Hoehn & Yahr 5), and others [19, 43] assessed not only PD, but also its complications and/or comorbidities.
Finally, with regard to the revised articles of this manuscript, we could suggest an instrument as a guideline to determine PD-related costs even though several methodologies and different variables could be taken into account for each particular scenario. Therefore, prospective studies would be the ideal methodology, but cross-sectional, retrospective ones, with a bottom-up approach from the perspective of society, could be more feasible. The questionnaire to obtain data could be divided into the following parts:
Clinical, social, demographic, and economic issues of the patient;
Medical and nonmedical direct costs;
Indirect costs;
Personal costs (including caregivers).
The most common variables found in the literature used to determine the costs of PD, depending on the scope of the study, are shown in Table 6.
Table 6.
Most common variables found in the cost studies of Parkinson's disease.
| Patient/disease | Direct medical cost | Direct nonmedical cost | Indirect cost | Out-of-pockets |
|---|---|---|---|---|
| Age | Hospitalization | Ancillary therapy/rehabilitation | Retirement | Transportation∗ |
| Gender | Pharmacotherapy (PD and comorbidities) |
Home Care∗ | Retirement premature | Special food |
| Marital status | Outpatient visit | Transportation∗ | Sick leave | Laundry |
| Instruction | Diagnostics | Special equipment∗ | Working days loss of the patient | Home Care∗ |
| Working status | Nursing home | Home modification∗ | Working days loss of the caregivers | Caregivers |
| Duration of PD | Copayments∗ | Productivity loss | Special equipment∗ | |
| Comorbidities | Loss of leisure time | Home modification∗ | ||
| H & Y stage1 | Private health plans | |||
| UPDRS2 | Copayments∗ | |||
| PQD-393 | ||||
| MMSE4 |
1Hoehn & Yahr scale of disability/2Unified Parkinson's Disease Rating Scale/3Parkinson's Disease Questionnaire–39 (quality of life)/4Mini-Mental State Examination.
∗Variables that may be in more than one cost type.
4. Conclusion
The concepts mentioned in this review do not aim to finalize the discussion on health economics tackling the costs of neurodegenerative diseases, such as PD, but only to allow access to introductory concepts of these assessments, so that the reader can contextualize the articles analyzed here.
The very definition of studies on costs of disease suggests limitations, as the articles here reviewed display methodological heterogeneity regarding PD costs, and this variation is an important factor that should receive more attention in literature. Unlike Alzheimer's disease, which has a validated instrument to determine the costs of illness [49], PD presents considerable problems in its analysis, since evaluations and comparisons are made between individual studies. If there were a standardized and validated instrument, the data costs would be more reliable and transparent and there would be rational allocation of resources and better collection of data for cost analysis and efficacy.
We observed that there is no standardization of terminology used for the definition of costs, or even unanimity in the identification of categories because a variable can be found in different classifications, depending on the criterion used by the researcher, which may underestimate the total cost of the disease.
On the other hand, there is no one perfect research methodology covering a single answer for all solutions. Sometimes, certain types of studies are more appropriate than others. There are several limitations that must be discussed and related to, such as the methodological problems, and the validity of their assumptions can differ because they may introduce bias in analysis in favor of a variable, lack of interest in its assessment, or even lack of information. Therefore, researchers must be careful with the source of their data and the method used for performing the calculations, so that their research can be replicated and validated, since there is no specific instrument for the assessment of PD costs. A limitation also deals with the funding sources of studies, which may be from government sources, insurance companies, or pharmaceutical industries, for example, generating important biases that also need to be addressed. It is necessary to define useful metrics for public health and private ones for managers of health, employers, insurance companies, and even patients themselves, because, without this agreement, the work of researchers and the funds invested shall remain uncertain and inconsistent.
Nevertheless, if the evidence obtained is of good quality in terms of transparency, there is quality and credibility in the data completeness of the documentation. Overall, if they are relevant to health care [50], these studies contribute to a better allocation of resources that are not related to savings but evaluate the efficiency, effectiveness, and safety of interventions.
The age group that the PD affects, if well attended to, can experience “healthy aging,” with a good quality of life and preserve its autonomy for longer, thus reducing its cost to the state and society.
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Catalá-López F., García-Altés A., Alvarez-Martín E., Gènova-Maleras R., Morant-Ginestar C., Parada A. Burden of disease and economic evaluation of healthcare interventions: are we investigating what really matters? BMC health services research. 2011;11, article 75 doi: 10.1186/1472-6963-11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu S.-Y., Wu J.-J., Zhao J., et al. Onset-related subtypes of Parkinson's disease differ in the patterns of striatal dopaminergic dysfunction: a positron emission tomography study. Parkinsonism and Related Disorders. 2015;21(12):1448–1453. doi: 10.1016/j.parkreldis.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 3.Guo X., Song W., Chen K., et al. Gender and onset age-related features of non-motor symptoms of patients with Parkinson's disease—a study from Southwest China. Parkinsonism & Related Disorders. 2013;19(11):961–965. doi: 10.1016/j.parkreldis.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Nero C. R. D. What is health economics? In: Piola S. F., Vianna S. M., editors. Health Economics: Concepts and Contributions to Health Management. chapter I. Brasília, Brazil: Ipea; 2002. pp. 5–23. [Google Scholar]
- 5.Vanni T., Luz P. M., Ribeiro R. A., Novaes H. M. D., Polanczyk C. A. Economic evaluation in health: applications in infectious diseases. Cadernos de saúde pública. 2009;25(12) doi: 10.1590/s0102-311x2009001200002. [DOI] [PubMed] [Google Scholar]
- 6.Brazil Ministry of Health. Methodological Guidelines: Economic Evaluation of Health Technologies. Brasília, Brazil: Brazil Ministry of Health; 2009. http://bvsms.saude.gov.br/bvs/publicacoes/avaliacao_economica_tecnologias_saude_2009.pdf. [Google Scholar]
- 7.Moraes E., Campos G. M., Figlie N. B., Laranjeira R. R., Ferraz M. B. Introductory concepts of health economics and the social impact of alcohol abuse. Revista Brasileira de Psiquiatria. 2006;28(4):321–325. doi: 10.1590/s1516-44462006000700014. [DOI] [PubMed] [Google Scholar]
- 8.Secoli S. R., Nita M. E., Ono-Nita S. K., Nobre M. Health technology assessment. II. Cost effectiveness analysis. Arquivos de Gastroenterologia. 2010;47(4):329–333. doi: 10.1590/S0004-28032010000400002. [DOI] [PubMed] [Google Scholar]
- 9.Tarride J.-E., Blackhouse G., Bischof M., et al. Approaches for economic evaluations of health care technologies. Journal of the American College of Radiology. 2009;6(5):307–316. doi: 10.1016/j.jacr.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Jo C. Cost-of-illness studies: concepts, scopes, and methods. Clinical and Molecular Hepatology. 2014;20(4):327–337. doi: 10.3350/cmh.2014.20.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rice D. P. Cost of illness studies: what is good about them? Injury Prevention. 6(3):177–179. doi: 10.1136/ip.6.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tarricone R. Cost-of-illness analysis. What room in health economics? Health Policy. 2006;77(1):51–63. doi: 10.1016/j.healthpol.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 13.Gustavsson A., Svensson M., Jacobi F., et al. Cost of disorders of the brain in Europe 2010. European Neuropsychopharmacology. 2011;21(10):718–779. doi: 10.1016/j.euroneuro.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Organização Mundial da Saúde. Financing Health Systems: The Road to Universal Coverage. Genebra, Switzerland: Organização Mundial da Saúde; 2010. http://www.who.int/eportuguese/publications/WHR2010.pdf?ua=1. [Google Scholar]
- 15.Dorsey E. R., Constantinescu R., Thompson J. P., et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68(5):384–386. doi: 10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- 16.Morris M. E., Watts J. J., Iansek R., et al. Quantifying the profile and progression of impairments, activity, participation, and quality of life in people with Parkinson disease: protocol for a prospective cohort study. BMC Geriatrics. 2009;9(1, article no. 2) doi: 10.1186/1471-2318-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbosa M. T., Caramelli P., Maia D. P., et al. Parkinsonism and Parkinson's disease in the elderly: a community-based survey in Brazil (the Bambuí Study) Movement Disorders. 2006;21(6):800–808. doi: 10.1002/mds.20806. [DOI] [PubMed] [Google Scholar]
- 18.Andlin-Sobocki P., Jönsson B., Wittchen H. U., Olesen J. Cost of disorders of the brain in Europe. European Journal of Neurology. 2005;12(supplement 1):1–27. doi: 10.1111/j.1468-1331.2005.01202.x. [DOI] [PubMed] [Google Scholar]
- 19.Zhao Y. J., Tan L. C. S., Au W. L., et al. Estimating the lifetime economic burden of Parkinson's disease in Singapore. European Journal of Neurology. 2013;20(2):368–374. doi: 10.1111/j.1468-1331.2012.03868.x. [DOI] [PubMed] [Google Scholar]
- 20.Johnson S., Davis M., Kaltenboeck A., et al. Early retirement and income loss in patients with early and advanced Parkinsons disease. Applied Health Economics and Health Policy. 2011;9(6):367–376. doi: 10.2165/11596900-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 21.Cubo E., Alvarez E., Morant C., et al. Burden of disease related to Parkinson's disease in Spain in the year 2000. Movement Disorders. 2005;20(11):1481–1487. doi: 10.1002/mds.20622. [DOI] [PubMed] [Google Scholar]
- 22.Kowal S. L., Dall T. M., Chakrabarti R., Storm M. V., Jain A. The current and projected economic burden of Parkinson's disease in the United States. Movement Disorders. 2013;28(3):311–318. doi: 10.1002/mds.25292. [DOI] [PubMed] [Google Scholar]
- 23.Kaltenboeck A., Johnson S. J., Davis M. R., et al. Direct costs and survival of medicare beneficiaries with early and advanced parkinson's disease. Parkinsonism and Related Disorders. 2012;18(4):321–326. doi: 10.1016/j.parkreldis.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 24.Huse D. M., Schulman K., Orsini L., Castelli-Haley J., Kennedy S., Lenhart G. Burden of illness in Parkinson's disease. Movement Disorders. 2005;20(11):1449–1454. doi: 10.1002/mds.20609. [DOI] [PubMed] [Google Scholar]
- 25.Lökk J., Borg S., Svensson J., Persson U., Ljunggren G. Drug and treatment costs in Parkinson's disease patients in Sweden. Acta Neurologica Scandinavica. 2012;125(2):142–147. doi: 10.1111/j.1600-0404.2011.01517.x. [DOI] [PubMed] [Google Scholar]
- 26.Jennum P., Zoetmulder M., Korbo L., Kjellberg J. The health-related, social, and economic consequences of parkinsonism: A Controlled National Study. Journal of Neurology. 2011;258(8):1497–1506. doi: 10.1007/s00415-011-5969-1. [DOI] [PubMed] [Google Scholar]
- 27.Noyes K., Liu H., Li Y., Holloway R., Dick A. W. Economic burden associated with Parkinson's disease on elderly Medicare beneficiaries. Movement Disorders. 2006;21(3):362–372. doi: 10.1002/mds.20727. [DOI] [PubMed] [Google Scholar]
- 28.Cordato D. J., Schwartz R., Abbott E., Saunders R., Morfis L. A comparison of health-care costs involved in treating people with and without Parkinson's disease in Southern Sydney, New South Wales, Australia. Journal of Clinical Neuroscience. 2006;13(6):655–658. doi: 10.1016/j.jocn.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 29.Bhattacharjee S., Sambamoorthi U. Co-occurring chronic conditions and healthcare expenditures associated with Parkinson's disease: a propensity score matched analysis. Parkinsonism and Related Disorders. 2013;19(8):746–750. doi: 10.1016/j.parkreldis.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winter Y., Von Campenhausen S., Reese J. P., et al. Costs of Parkinson's disease and antiparkinsonian pharmacotherapy: an Italian cohort study. Neurodegenerative Diseases. 2010;7(6):365–372. doi: 10.1159/000302644. [DOI] [PubMed] [Google Scholar]
- 31.Vossius C., Gjerstad M., Baas H., Larsen J. P. Drug costs for patients with Parkinson's disease in two different European countries. Acta Neurologica Scandinavica. 2006;113(4):228–232. doi: 10.1111/j.1600-0404.2005.00574.x. [DOI] [PubMed] [Google Scholar]
- 32.Winter Y., von Campenhausen S., Brozova H., et al. Costs of Parkinson's disease in Eastern Europe: a Czech cohort study. Parkinsonism and Related Disorders. 2010;16(1):51–56. doi: 10.1016/j.parkreldis.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 33.McCrone P., Allcock L. M., Burn D. J. Predicting the cost of Parkinson's disease. Movement Disorders. 2007;22(6):804–812. doi: 10.1002/mds.21360. [DOI] [PubMed] [Google Scholar]
- 34.Spottke A. E., Reuter M., Machat O., et al. Cost of illness and its predictors for Parkinson's disease in Germany. PharmacoEconomics. 2005;23(8):817–836. doi: 10.2165/00019053-200523080-00007. [DOI] [PubMed] [Google Scholar]
- 35.Leibson C. L., Hall Long K., Maraganore D. M., et al. Direct medical costs associated with Parkinson's disease: a population-based study. Movement Disorders. 2006;21(11):1864–1871. doi: 10.1002/mds.21075. [DOI] [PubMed] [Google Scholar]
- 36.Ragothaman M., Govindappa S. T., Rattihalli R., Subbakrishna D. K., Muthane U. B. Direct costs of managing Parkinson's disease in India: concerns in a developing country. Movement Disorders. 2006;21(10):1755–1758. doi: 10.1002/mds.21035. [DOI] [PubMed] [Google Scholar]
- 37.Winter Y., Campenhausen S. V., Popov G., et al. Costs of illness in a Russian cohort of patients with parkinsons disease. PharmacoEconomics. 2009;27(7):571–584. doi: 10.2165/11310160-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 38.Zhao Y. J., Tan L. C. S., Li S. C., et al. Economic burden of Parkinson's disease in Singapore. European Journal of Neurology. 2011;18(3):519–526. doi: 10.1111/j.1468-1331.2010.03210.x. [DOI] [PubMed] [Google Scholar]
- 39.Tamás G., Gulácsi L., Bereczki D., et al. Quality of life and costs in Parkinson's disease: a cross sectional study in Hungary. PLoS ONE. 2014;9(9) doi: 10.1371/journal.pone.0107704.e107704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoritaka A., Fukae J., Hatano T., Oda E., Hattori N. The direct cost of Parkinson disease at Juntendo medical university hospital, Japan. Internal Medicine. 2016;55(2):113–119. doi: 10.2169/internalmedicine.55.4484. [DOI] [PubMed] [Google Scholar]
- 41.Johnson S. J., Kaltenboeck A., Diener M., et al. Costs of parkinson's disease in a privately insured population. PharmacoEconomics. 2013;31(9):799–806. doi: 10.1007/s40273-013-0075-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vargas A. P., Carod-Artal F. J., Nunes S. S., Melo M. Disability and use of healthcare resources in Brazilian patients with Parkinson's disease. Disability and Rehabilitation. 2008;30(14):1055–1062. doi: 10.1080/17483100701456079. [DOI] [PubMed] [Google Scholar]
- 43.Bach J.-P., Riedel O., Klotsche J., Spottke A., Dodel R., Wittchen H.-U. Impact of complications and comorbidities on treatment costs and health-related quality of life of patients with Parkinson's disease. Journal of the Neurological Sciences. 2012;314(1-2):41–47. doi: 10.1016/j.jns.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 44.Martínez-Martin P., Rodriguez-Blazquez C., Paz S., et al. Parkinson symptoms and health related quality of life as predictors of costs: A Longitudinal Observational Study with Linear Mixed Model Analysis. PLOS ONE. 2015;10(12) doi: 10.1371/journal.pone.0145310.e0145310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.von Campenhausen S., Winter Y., Rodrigues e Silva A., et al. Costs of illness and care in Parkinson's disease: an evaluation in six countries. European Neuropsychopharmacology. 2011;21(2):180–191. doi: 10.1016/j.euroneuro.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 46.Winter Y., Balzer-Geldsetzer M., Spottke A., et al. Longitudinal study of the socioeconomic burden of Parkinson's disease in Germany. European Journal of Neurology. 2010;17(9):1156–1163. doi: 10.1111/j.1468-1331.2010.02984.x. [DOI] [PubMed] [Google Scholar]
- 47.Winter Y., Balzer-Geldsetzer M., von Campenhausen S., et al. Trends in resource utilization for Parkinson's disease in Germany. Journal of the Neurological Sciences. 2010;294(1-2):18–22. doi: 10.1016/j.jns.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 48.Wang G., Cheng Q., Zheng R., et al. Economic burden of Parkinson's disease in a developing country: a retrospective cost analysis in Shanghai, China. Movement Disorders. 2006;21(9):1439–1443. doi: 10.1002/mds.20999. [DOI] [PubMed] [Google Scholar]
- 49.Dodel R., Jönsson B., Reese J. P., et al. Measurement of costs and scales for outcome evaluation in health economic studies of Parkinson's disease. Movement Disorders. 2014;29(2):169–176. doi: 10.1002/mds.25571. [DOI] [PubMed] [Google Scholar]
- 50.Langer A. A framework for assessing Health Economic Evaluation (HEE) quality appraisal instruments. BMC health services research. 2012;12, article no. 253 doi: 10.1186/1472-6963-12-253. [DOI] [PMC free article] [PubMed] [Google Scholar]


