Abstract
Background
The ratio of high amyloid-β peptide40 (Aβ40) and low Aβ42 in plasma predicts the risk of Alzheimer’s disease (AD) and is associated with episodic recall in depression. We thus examined the relationship between plasma Aβ levels and brain volumes.
Methods
Homebound elders (N = 352) who had undergone brain MRI were used. Plasma Aβ1-40 and Aβ1-42 were measured by ELISA. Volumes of medial temporal regions, including the amygdala and hippocampus, were manually measured.
Results
Amygdala volume was associated with log10 of plasma Aβ1-42 (β = + 0.19, SE = 0.07, p = 0.005) after adjusting for AD, infarcts, white matter hyperintensities and demographics. In the absence of dementia, decreasing quartiles of plasma Aβ1-42 (Mean + SD ml: Q4 = 4.1±0.8; Q3 = 3.9±0.7; Q2 = 3.6 ± 0.8 and Q1 = 3.7 ± 0.8, p = 0.01) and increasing quartiles of plasma Aβ1-40/l-42 ratio were associated with smaller amygdala volume. Those depressed subjects with a high plasma Aβ1-40/1–42 ratio had smaller amygdala (Mean + SD ml: 3.3 ±0.8 vs. 3.6 ±0.8, p = 0.04) and total brain volume (Mean + SD liter: 0.95 ±0.07 vs. 1.04 ± 0.12, p = 0.005), and had a higher rate of MCI (67 vs. 36%, p = 0.02) than those with a low plasma Aβ1-40/l-42 ratio.
Conclusions
The combination of low plasma Aβ1-42 concentration and atrophy of the medial temporal lobe structures, which regulates mood and cognition, may represent a biomarker for a prodromal stage of AD.
Keywords: amyloid-β peptide, amygdala, depression and Alzheimer’s disease, hippocampus
Introduction
Alzheimer’s disease (AD) is a gradual pathological process that evolves from silent AD pathology formation in the brain to a preclinical stage of mild cognitive impairment (MCI) (Petersen et al., 1999) and depression (Ownby et al., 2006), and then to full blown, clinically diagnosed AD. Three large epidemiology studies, Rotterdam, Mayo Clinic and Washington Heights cohorts, have shown that a combination of low Amyloid-β peptide42 (Aβ42) and high Aβ40 in plasma predicts an increased risk of developing AD (Graff-Radford et al., 2003; van Dijk et al., 2004; Schupf et al., 2008). Animal and human studies suggest that plasma Aβ42 levels are initially high and then decline leading to a higher Aβ40/Aβ42 ratio in plasma. Theses changes probably precede the Aβ42 aggregation and deposition in the brain that is the defining pathology of AD (Kawarabayashi et al., 2001; Schupf et al., 2008). Low levels of plasma Aβ42 are correlated with low levels of plasma profibrillar Aβ (Schupf et al., 2008), a key element of AD pathology. Interestingly, plasma Aβ42 levels are correlated with cerebrospinal fluid (CSF) Aβ42 levels when cognition is normal, but this relationship is not found in AD (Strazielle et al., 2000; Giedraitis et al., 2007). Plasma Aβ has the potential to be developed into an AD-related bio-marker that can help identify at-risk individuals, in the same way that cholesterol levels are used to identify those at risk for cardiovascular disease. The challenge of using plasma Aβ level as a biomarker is that the plasma Aβ level is only 1–10% of the CSF Aβ level; an Aβ antibody test that is high in both specificity and sensitivity is necessary before plasma Aβ can be considered a potential biomarker. For these reasons, there has been much less research and slower progress on the relationship between plasma Aβ and AD compared to that of CSF Aβ and AD.
While high plasma Aβ40 levels are associated with cerebrovascular pathology (Gurol et al., 2006), the relationship between plasma Aβ42 and brain structures at a preclinical stage of AD is unclear. Using quantitative magnetic resonance imaging (MRI), it is found that in elderly with MCI atrophy begins in the amygdala and the anterior portion of the hippocampus and later in the disease extends to include the entire hippocampus (Whitwell et al., 2007). Baseline atrophy of medial temporal lobe structures, i.e. the hippocampus and amygdala, predicts AD development in an elderly population (den Heijer et al., 2006). Atrophy of the amygdala but not the hippocampus is associated with depression and agitation at an early preclinical stage of AD (Smith et al., 1999), suggesting that amygdala atrophy may be the underlying pathology of prodromal depression of AD.
The presence of depression is associated with an increase in amyloid plaques and neurofibrillary tangles, the hallmark of AD pathology, in the medial temporal lobe (Rapp et al., 2006). Our previous study found that in nondemented elderly, low levels of plasma Aβ42 resulting in a high Aβ40/42 ratio are associated with severity of depression (Qiu et al., 2007). Depression with a high Aβ40/42 ratio in plasma is associated with poor mean episodic recall, the defining abnormality of MCI (Sun et al., 2008). We thus hypothesize that plasma Aβ42 levels would be associated with brain structures that regulate mood and cognition, i.e. the amygdala and hippocampus, in nondemented elderly persons. Subjects who underwent diagnostic evaluation by physicians and brain MRI were used in this study. The relationships between plasma Aβ levels and brain volumes, especially structures of the medial temporal lobe, were examined.
Methods
Study population and recruitment
We studied the subgroup of 352 subjects, who underwent clinical evaluation by physicians, including brain MRI, from a population-based study, the Nutrition, Aging and Memory in the Elderly (NAME) study. The NAME study was based on the clients of four homecare agencies for the city of Boston. Anyone receiving homecare services is registered with one of these agencies if he/she lives in the city of Boston, has an annual income <$ 18,890 and needs homecare service. All homebound elders aged 60 and older receiving services from the four agencies were invited to participate in the study. Of all eligible subjects, 66% enrolled in the study, and gave informed consent approved by the Institutional Review Board of Tufts University New England Medical Center (Qiu et al., 2007). Those with Mini-Mental State Examination (MMSE) < 10 or verbal IQ < 75 were not eligible to continue in the study. 1262 subjects completed the neuropsychological evaluation during the home visits and were asked whether they would be willing to participate in the second phase of the study, which was to undergo brain MRI. Of this number 352 agreed and were recruited.
Diagnoses of dementia and mild cognitive impairment (MCI)
All subjects, who consented to participate in the second phase of the study, came to the hospital to be evaluated by a psychiatrist and a neurologist, and undergo a brain MRI. During a consensus diagnosis meeting attended by the psychiatrist, neurologist, neuropsychologist, and radiologist the clinical data, neuropsychological scores and imaging studies were reviewed in order to generate a consensus diagnosis for each subject. Evidence of vascular disease on imaging studies was required to make the diagnoses of vascular dementia and stroke.
Diagnosis of dementia
The diagnosis of dementia was based on the DSM-IV criteria. NINCDS-ADRDA guidelines (McKhann et al., 1984) were used to determine whether criteria were met for a diagnosis of possible or probable AD.
Diagnosis of MCI
The diagnostic criteria for MCI were based on Petersen et al., 1999 guidelines with some modifications to broaden the concept of MCI. The diagnosis of MCI was made according to the following criteria with some modification: (1) no dementia; (2) self-reported forgetfulness in daily activities or for recent events; (3) normal general cognitive functioning as assessed by the MMSE, that is, a score less than 1 SD below the mean of an age- and education matched sample after exclusion of prevalent (What is “prevalent” dementia?) dementia at entry; (4) objective memory impairment or impairment in other cognitive domains as assessed by performance on neuropsychological tests not more than 1.5 SD of the mean of an age- and education matched sample; (5) ability to independently perform basic activities of daily living. The neuropsychological battery included WMS-III Word List Learning, WMS-III Logical Memory, verbal fluency, WAIS-III Block Design, WAIS-III Digit Span, and Trails A and Trails B. Those with memory impairment only or cognitive impairment including memory and other domains were considered to have amnestic MCI; those without forgetfulness and with impairments in other cognitive domains, such as executive and visuospatial dysfunction, were considered to have non-amnestic MCI.
Definition of the controls
Subjects were considered cognitively intact if they were not demented and scored no more than 1 SD below the mean of age-and education-defined strata on MMSE and no more than 1.5 SD below the mean of age- and education-defined strata on the neuropsychological tests.
Evaluation of depression
Severity of depressive symptoms was assessed by the Center for Epidemiological Studies Depression scale (CES-D) (Radloff, 1977). A CES-D score of > 16 was used as the cut-off point for clinical depression (Fuhrer and Rouillon, 1989).
Plasma Aβ40 and Aβ42 measurements
Blood samples were centrifuged immediately after the blood was drawn. The sandwich Aβ ELISA was used as described previously (Qiu et al., 2007). Plates were coated with 2G3 (anti-Aβ40) and 21F12 (anti-Aβ42) antibodies overnight at 4 °C. Samples were then loaded and incubated overnight at 4 °C followed by incubation with a biotinylated monoclonal anti-N terminus Aβ antibody (3D6B) for 2 h. Finally, streptavidin-con-jugated alkaline phosphatase (Promega, USA) was added and incubated, and the signal was amplified by adding alkaline phosphatase fluorescent substrate (Promega, USA), which was then measured. Each sample assay was duplicated for each data point. The lowest detection for both Aβ peptides was 1.6 pg/ml in the standard curves with %CV between 1.1 and 7.2. For both Aβ1-40 and 1–42 we used 3.1 pg/ml as the low cut-off point. The samples with higher levels than the standard curve were repeated with dilutions for measurement. The intra-correlations with two other laboratories that have published results of Aβ measurement (Fukumoto et al. 2003) (Perez et al., 1999), showed R = 0.63 and 0.84 for Aβ40 and R = 0.90 and 0.96 for Aβ42.
Brain MRI and volumetric measurements
MRI scans were performed on a 1.5 Tesla magnet (Siemens’ Symphony; Islin, NJ). All subjects had the following imaging protocol: (1) Intermediate (TE = 20 ms) and T2-weighted (TE = 80 ms) conventional spin-echo axial images (TR = 3000 ms), (2) fluid attenuation inversion recovery (FLAIR) axial images, and (3) magnetization prepared rapid acquisition gradient echo (MPRAGE) coronal 3D images with section thickness of 1.5 mm (Scott et al., 2004).
The study radiologist evaluated images for the presence of brain infarcts including large and small vessel infarcts. Small vessel infarcts were defined as a focal brain lesion hyperintense on T2-weighted images with a minimum diameter of 3 mm and a maximum diameter of 1.5 cm. Large vessel infarcts were defined as infarcts larger than 1.5 cm in size in a major vascular territory (Scott et al., 2006). The infarct variable was the number of large and small vessel infarcts combined.
All images were analyzed using analyze image analysis software (AnalyzeDirect, Inc. Overland, KS) by trained readers/analysts who were blinded to the subjects’ clinical status and under the supervision of a board-certified neuroradiologist (R.B.) (Scott et al., 2006). The following measurements were made: (1) total Intra-cranial volume, (2) total brain volume and (3) white matter hyperintensity volume (WMHI) (4) amygdala and hippocampus volumes. Conventional spin-echo intermediate and T2-weighted images were used to perform histogram segmentation to derive whole total intra-cranial, brain, and white matter hyperintensities (WMHI) volumes in cubic millimeter (DeCarli et al., 1992). Good inter-rater reliability was established and was periodically re-assessed (Scott et al., 2006)
The margins of hippocampus and amygdala were traced by a mouse-driven cursor on consecutive images of MPRAGE 3D sequence on which these structures were seen. For volume measurement, ANALYZE software was used to count the number of voxels within each slice and multiplied by voxel volume to derive a numeric value in cubic millimeters. Measurements were made on both sides. The boundaries of amygdala were defined by gray/white matter borders, by cerebral spinal fluid (CSF) in the uncal cistern or by uncal recess of the temporal horn or the alveus covering the hippocampal head as appropriate. The anterior boundaries of the hippocampus were defined by the dentate gyrus and the subiculum, and was separated from the amygdala by visualizing either the shape of hippocampal digitations and the uncal recess of the temporal horn or by the high signal-intensity generated by the white matter of alveus. The posterior boundary of the hippocampus was chosen at the level where both cruces of the fo mix were seen (Scott et al., 2004). The volumes (left + right sides) of amygdala or hippocampus were normalized by the total intracranial volume to compensate for variations in total brain size (Scott et al., 2004). The measurements of hippocampal and amygdala volumes had an inter-reader correlation of 0.92 (p< 0.001).
Other measurements
A 244 bp of the ApoE gene, which included the two polymorphic sites was amplified by PCR using a robotic Thermal Cycler (ABI 877, Perkin-Elmer/ Applied Biosystems). The PCR products were digested with 5 units of Hha I, and the fragments were separated by electrophoresis. The allelic fragments were: E2; E3; and E4. ApoE4 was defined by E4/4, E3/4, or E2/4.
Statistical analysis
Statistical analysis was performed using SAS (version 9.1, SAS Institute Inc., Cary, North Carolina). Multivariate linear regression was performed to evaluate the association between the normalized volume of amygdala, hippocampus or total brain as an outcome and plasma Aβ peptides after adjusting for potential confounding by age, gender, ethnicity, education, ApoE4, AD, WMHI, and infarcts. To compare brain volumes and other key variables among different subgroups, mean + SD and t-test or ANOVA were used for the variables with a normal distribution, and median (Q1, Q3) and Wilcoxon rank sum test or Kruskal–Kallis test were used for the variables with a skewed distribution. Chi-square was applied to evaluate frequencies. Pearson correlation was applied to examine the relationship between brain volumes and plasma Aβ peptides. Both Aβ40 and Aβ42 were transformed to log10 for multivariate regression due to their skewed distribution. For all analyses, level of significance was α = 0.05.
Results
Three hundred and fifty-two homebound elders recruited from a population-based study, the NAME study, came to the hospital to be examined by physicians and had a brain MRI. The subset was comparable to the parent population in age, education level, status of depression, and general cognition; however, their scores in activities of daily life (ADL) were lower than the parent population (Scott et al., 2006). The average age of this study sample was 73.3 (SD = 8.3) years old, and 73% were females (Table 1). The sample was multi-ethnic with 62% white, 35% African American, and 3% other ethnicities. 76% of the subjects completed a high school education or above, and 25% were ApoE4 carriers. Among them, 35% had depression, 24% had dementia, and 32% had MCI. Of the 85 demented subjects, 46 subjects (54%) had probable or possible AD and 39 (46%) had other types of dementia, including four cases of mixed dementia and 22 cases of vascular dementia. Of the 113 subjects with MCI, 16 subjects (13%) had amnestic MCI and 98 subjects (87%) had non-amnestic MCI.
Table 1.
General information of the study sample
| N = 352 | |
|---|---|
| Age, year, Mean±SD | 73.3 ±8.3 |
| Female, n/total (%) | 256/352 (73%) |
| African American, n/total (%) | 123/351 (35%) |
| High School and above, years, n/total (%) | 267/350 (76%) |
| ApoE4, n/total (%) | 87/345 (25%) |
| Depression, n/total (%) | 118/342 (35%) |
| Dementia, n/total (%) | 85/351 (24%) |
| AD, n/total (%) | 46/85 (54%) |
| Other dementia, n/total (%) | 39/85 (46%) |
| MCI, n/total (%) | 113/351 (32%) |
| Amnestic MCI, n/total (%) | 16/113 (13%) |
| Non-Amnestic MCI, n/total (%) | 98/113 (87%) |
| Plasma Aβ | |
| Aβ1-40, pg/ml, Median, (Q1, Q3) | 130.7 (99.3,168.9) |
| Aβ1-42, pg/ml, Median, (Q1, Q3) | 16.0(11.4, 26.3) |
| Aβ1-40/Aβ1-42, Median, (Q1, Q3) | 8.1 (5.8, 11.2) |
| Brain structures | |
| Total brain volume, Liter, mean±SD | 1.0±0.1 |
| Infarcts, number, mean±SD | 0.6±1.1 |
| WMHI, ml, mean±SD | 0.006 ±0.007 |
| Hippocampus volume, ml, mean±SD | 5.1 ±0.8 |
| Amygdala volume, ml, mean±SD | 3.7±0.8 |
Distributions of plasma Aβ peptides were skewed: Aβ1-40 (median: 130.7 pg/ml; minimum: 6.9 pg/ml and maximum: 769.0 pg/ml) and Aβ1-42 (median: 16.0 pg/ml; minimum: 3.1 pg/ml and maximum: 405.0 pg/ml), so Aβ levels were presented with median, Q1 (25%) and Q3 (75%).
Univariate analyses showed that log10 of plasma Aβ1-42 was correlated with amygdala volume (r = +0.18, p = 0.002) but not with other brain volumes. We used multivariate linear regression analysis to examine the relationship between plasma Aβ and the brain volumes as outcomes after adjusting for confounders (Table 2). Because plasma Aβ peptides have been demonstrated to predict the development of AD (Graff-Radford et al., 2003; van Dijk et al., 2004; Schupf et al., 2008) but are not associated with the disease after AD is diagnosed (Iwatsubo, 1998), we chose to adjust for AD in the multivariate linear regression analysis. As shown in Table 2, log10 of plasma Aβ1-42, but not Aβ1-40, remained to be positively associated with the normalized volume of amygdala (β = +0.19, SE = 0.07, p = 0.005) after adjusting for AD and other brain pathologies including infarcts and WMHI, ApoE4, and the demographic variables including age, gender, race, and education. The hippocampus or total brain volume as an outcome was not found to be associated with plasma Aβ1-42 levels in the same model. In this model, AD was consistently associated with smaller volumes of amygdala (β = −0.42, SE = 0.10, p < 0.0001) and hippocampus (β = −030, SE = 0.10, p = 0.003), and tended to be associated with smaller total brain volume in this sample; brain infarcts were associated with smaller total brain volume (β = −0.03, SE = 0.01, p< 0.0001) (Table 2). As expected, age was negatively associated with total brain volume and tended to be negatively associated with hippocampus volume, but not with amygdala volume. Higher education was positively associated with all three measurements, the amygdala, the hippocampus, and the total brain volume, while female gender was negatively associated with these measurements.
Table 2.
General linear models with volume of amygdale, hippocampus or total brain as an outcome of multiple factors
| Amygdala (ml) N = 299
|
Hippocampus (ml) N = 299
|
Total brain (L) N = 302
|
||||
|---|---|---|---|---|---|---|
| β Estimate (SE) | p-value | β Estimate (SE) | p-value | β Estimate (SE) | p-alue | |
| LgB42 | +0.19(0.07) | 0.005 | +0.08 (0.07) | 0.27 | +0.01 (0.01) | 0.26 |
| LgB40 | −0.01 (0.09) | 0.91 | −0.06 (0.10) | 0.54 | +0.01 (0.01) | 0.48 |
| Age, year | −0.003 (0.006) | 0.58 | −0.01 (0.01) | 0.10 | −0.002 (0.001) | 0.03 |
| Female | −0.36(0.11) | 0.001 | −0.35(0.11) | 0.001 | −0.10(0.01) | <0.0001 |
| School, year | +0.03 (0.02) | 0.05 | +0.03 (0.02) | 0.04 | +0.01 (0.02) | <0.0001 |
| ApoE4 | +0.07(0.11) | 0.52 | +0.23(0.11) | 0.03 | −0.01 (0.01) | 0.48 |
| WMHI, ml | −3.94 (7.31) | 0.59 | +1.55 (7.40) | 0.83 | +2.13(0.98) | 0.03 |
| Infarcts, number | −0.05 (0.04) | 0.26 | −0.05 (0.04) | 0.25 | −0.03 (0.01) | <0.0001 |
| Alzheimer’s disease | −0.42 (0.10) | <0.0001 | −0.30 (0.10) | 0.003 | −0.02 (0.01) | 0.18 |
Aβ40 (LgB40) and Aβ42 (LgB42) were transformed to log10 due to their skewed distribution.
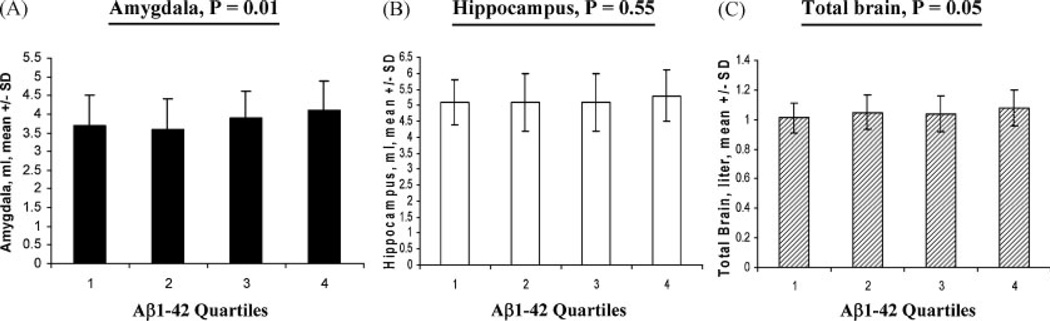
Upon removing the subjects with dementia from the study sample, decreasing quartiles of plasma Aβ1-42 were found to be associated with smaller amygdala volumes (Mean + SD ml: Q4 = 4.1 + 0.8; Q3 = 3.9 + 0.7; Q2 = 3.6 + 0.8 and Q1 = 3.7 + 0.8, p = 0.01) and marginally with smaller total brain volumes (Mean + SD liter: Q4= 1.08 + 0.12; Q3 = 1.04 + 0.12; Q2=1.05 + 0.12 and Q1 = 1.01+ 0.10, p = 0.05), but were not associated with the hippocampus volumes (Figure 1). Plasma Aβ1-40/1–42 ratio was more determined by low plasma Aβ1-42 (r = −0.81, p< 0.0001) than by high plasma Aβ1-40 (r = +0.24, p< 0.0001). Increasing quartiles of plasma Aβ1-40/1–42 ratio, like decreasing plasma Aβ1-42 quartiles, were consistently associated with smaller amygdala volumes but not with volumes of the hippocampus or total brain (data not shown). There were no differences in race, gender, education, and ApoE4 status across four quartiles. The average ages among the quartiles of plasma Aβ1-42 were slightly different (mean + SD: Q1 = 72.5 + 7.8; Q2 = 72.7 + 7.8; Q3 = 70.1 + 7.8 and Q4 = 73.6 + 7.4, p = 0.03).
Figure 1.
Brain volumes and plasma Aβ1-42: Volumes of amygdala (A), hippocampus (B) and total brain (C) in the nondemented subjects (MCI ± Controls) according to plasma Aβ1-42 quartiles were illustrated. Mean±SD in each quartile with ANOVA and p values were presented.
We further divided nondemented, depressed subjects into those with high vs. low plasma Aβ1-40/1-42 ratio. When the median of plasma Aβ1-40/1-42 ratio (>8.0) was used, those with a high plasma Aβ1-40/1-42 ratio had smaller total brain volume (mean + SD liter: 0.98 + 0.09 vs. 1.05 + 0.13, p = 0.03) when compared to those with a low plasma Aβ1-40/1-42 ratio. When the Q4 (top 25%) of plasma Aβ1-40/1-42 ratio (>11.2) was used, those depressed subjects with a high plasma Aβ1-40/1-42 ratio had both smaller amygdala (mean + SD ml: 3.3 + 0.8 vs. 3.8 + 0.8, p = 0.04) and smaller total brain volumes (mean + SD liter: 0.95 + 0.07 vs. 1.04 + 0.12, p = 0.005) compared to those with a low plasma Aβ1-40/1-42 ratio, and the number of subjects with MCI was higher in those with high plasma Aβ1-40/1-42 ratio than those with low plasma Aβ1-40/1-42 ratio (67 vs. 36%, p = 0.02) (Table 3). These relationships persisted when the depression diagnosis using DSM-IV instead of CES-D > 16 criteria was used (data not shown).
Table 3.
Characterization of depression and plasma Aβ1-40/1-42 ratio
| Depressed subjects | High plasma Aβ1-40/ 1-42 ratio N = 23 |
Low plasma Aβ1-40/ 1-42 ratio N = 64 |
p-values |
|---|---|---|---|
| Age, year, Mean + SD | 71.9 ±7.6 | 69.7±7.5 | 0.22 |
| ApoE4, n/total (%) | 7/23 (29%) | 11/63(17%) | 0.25 |
| MCI, n/total (%) | 16/23(67%) | 23/64 (36%) | 0.02 |
| Amygdala volume, ml, mean±SD | 3.3±0.8 | 3.8±0.8 | 0.04 |
| Hippocampus volume, ml, mean±SD | 5.0±0.7 | 5.1 ±0.9 | 0.88 |
| Total brain volume, Liter, mean±SD | 0.95 ±0.07 | 1.04±0.12 | 0.005 |
Depressed elderly subjects were used for the analysis and further divided into two subgroups according to plasma Aβ1-40/1-42 ratio. The Q4 (top 25%) of plasma Aβ1-40/1-42 ratio (>11.2) was used, as a high and the rest Q1-Q3 were used as a low levels of plasma Aβ1-40/1-42 ratio. Mean ± SD with t-test and n/ total (%) with chi-square were presented. p-values for statistical significance are shown.
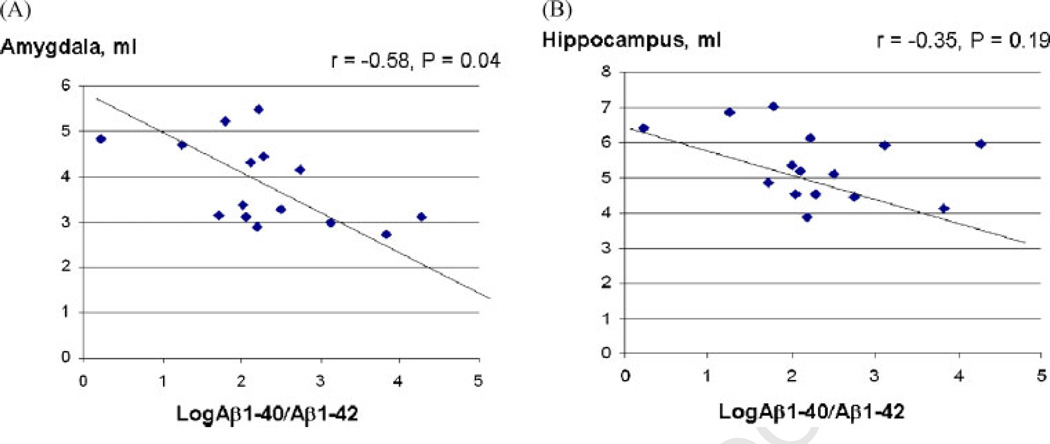
In this study sample there were only 16 subjects with amnestic MCI (Table 1). We examined the relationship between plasma Aβ1-40/1-42 ratio and brain structure volumes in this subgroup. Using pearson correlation in those with amnestic MCI, log10 of plasma Aβ1-40/1-42 ratio correlated with amygdala volume (r = −0.58, p = 0.04), and tended to correlate with hippocampus volume (r = −0.35, p = 0.19) (Figure 2), but did not correlate with WMHI, infarcts or total brain volume (data not shown). This relationship was not observed among those with non-amnestic MCI.
Figure 2.
Correlations between plasma Aβ1-40/1-42 and the volume of amygdala or hippocampus in amnestic MCI: The log10 of plasma Aβ1-40/1-42 ratio (x-axis) and its correlation with either amygdala volume (A) or hippocampus volume (B) (y-axis) are shown for each individual subject with amnestic MCI. Correlation coefficient factor, r, and statistical significance, p-value, are indicated.
Discussion
Our previous study showed that depression associated with a high plasma Aβ1-40/1-42 ratio is linked with poor memory (Sun et al., 2008), the major symptom of the preclinical stage of AD. In this study, we extended our previous findings and found that in the same population low plasma Aβ1-42 levels were associated with smaller amygdala volume (Tables 2 and 3), a brain structure that regulates mood and cognition. Moreover, depressed subjects with a high plasma Aβ1-40/1-42 ratio had smaller amygdala and total brain volumes as well as a higher rate of MCI compared to those with a low plasma Aβ1-40/1-42 ratio (Table 4). There are different subtypes of late-life depression with different pathologies and etiologies including (1) post-stroke depression (Robinson et al., 1987); (2) vascular depression (Alexopoulos et al., 1997); and (3) preclinical depression or co-morbid depression in AD. We propose that depression with high plasma Aβ1-40/1-42 ratio is a unique depression subtype related to AD, pathology, which we term amyloid-associated depression (Sun et al., 2008). The decline of plasma Aβ1-42 levels results in a high plasma Aβ1-40/1-42 ratio that predicts both the risk of AD (Graff-Radford et al., 2003; van Dijk et al., 2004; Schupf et al., 2008) and amygdala atrophy. Amygdala atrophy itself is associated with an increased risk of AD (den Heijer et al., 2006; Whitwell et al., 2007). We therefore hypothesize that during a prodromal stage of AD decline of plasma Aβ1-42 levels may be an early biomarker of impending amygdala atrophy and depression.
A low level of plasma Aβ1-42 is the major determining factor for a high plasma Aβ1-40/1-42 ratio. A study by Schupf et al. demonstrated that prior to AD onset precipitous declines in plasma Aβ42 correlate with low levels of plasma Aβ oligomers, the major toxic Aβ species in AD pathogenesis (Schupf et al., 2008). It is shown that plasma Aβ42 declines before cognitive symptoms of AD appear (Mayeux et al., 2003; Pomara and Murali Doraiswamy, 2003; Irizarry, 2004; Solfrizzi et al., 2006). As a potential prognostic biomarker of AD, plasma Aβ42 levels correlate with CSF Aβ42 levels only when cognition is normal but this equilibrium is disrupted when AD is diagnosed (Strazielle et al., 2000; Giedraitis et al., 2007) explaining why plasma Aβ42 levels cannot be used as a diagnostic biomarker for AD (Zetterberg and Blennow, 2006).
Our study showed that low Aβ1-42 and high Aβ1-40/1-42 ratio as well in plasma were associated with a smaller volume of amygdala (Tables 2 and 3). The amygdala is a structure of the medial temporal lobe that mediates emotion and mood (Leppanen, 2006). Interestingly, through its connection with the hippocampus the amygdala mediates emotional memory (Olsson and Ochsner, 2008; Phelps, 2006), a cognitive function that is impaired at a preclinical stage of AD (Herlitz et al., 1995; Petersen et al., 1999). Studies suggest that amygdala atrophy precedes hippocampus involvement in the AD pathological process and that the amygdala is at least as marked as the hippocampus (Lehericy et al., 1994; Mori et al., 1997; Krasuski et al., 1998; Mizuno et al., 2000; Basso et al., 2006;). Compared to controls, patients with very mild AD have smaller amygdala volume, not hippocampus volume (Ishii et al., 2006). It has been shown that the pattern of gray matter loss in MCI 3 years before the development of AD is most pronounced in the anterior medial temporal lobe, including the amygdala, with relative sparing of the posterior hippocampus; gray matter loss through the whole extent of the hippocampus is observed at a later stage of AD (Whitwell et al., 2007). Elders with AD at a mild or moderate stage (MMSE score from 23 to 27) have evident amygdala atrophy, but have no hippocampal atrophy or ventricular enlargement (Basso et al., 2006); in contrast, the AD elderly with severe cognitive impairment (MMSE less than 21) have similar degrees of atrophy of amygdala and hippocampus, as well as enlarged ventricles (Lehericy et al., 1994; Basso et al., 2006). Plasma Aβ1-42 is not correlated with the diagnosis of AD (Iwatsubo, 1998) when atrophy of both hippocampus and amygdala is significant, but may be a useful biomarker for a preclinical stage of AD (van Oijen et al., 2006; Graff-Radford et al., 2007) when atrophy of amygdala begins (Whitwell et al., 2007). AD only tended to be associated with total brain volume in this study sample (Table 2) probably due to that severe AD cases (MMSE score < 10), which usually presents with total brain atrophy, were excluded.
Pre-clinical depression of AD heralds the primary decline in memory followed by more global cognitive impairment characteristic of AD (Ownby et al., 2006; Amieva et al., 2008). There are different subtypes of depression in the elderly and not all depression subtypes are expected to be related to plasma Aβ1-42 levels. In this study using the top 25% highest plasma Aβ1-40/1-42 ratio as the cut-off level, amyloid-associated depression was associated with smaller amygdala and total brain volumes in addition to a higher MCI rate than those with non-amyloid depression (Table 3). Lower plasma Aβ1-42 levels are associated with depression severity when vascular depression is removed from the study sample (Qiu et al., 2007). That the combination of low Aβ42 and high Aβ40 plasma levels predict an increased risk of AD in the elderly (van Oijen et al., 2006; Graff-Radford et al., 2007; Schupf et al., 2008) may explain why amyloid-associated depression and non-amyloid depression have different cognitive profiles and brain pathology if amyloid-associated depression is truly a subtype for prodromal AD.
MCI is associated with depression (Apostolova and Cummings, 2008). We also found that the plasma Aβ1-40/1-42 ratio was negatively correlated with amygdala volume and tended to be negatively correlated with the hippocampus volume in those with amnestic MCI (Figure 2). A study by Teng et al. showed that elderly with concomitant MCI and depression are more likely to go on to develop AD than those with MCI alone (Teng et al., 2007), suggesting that MCI complicated by depression represents a more imminent prodromal stage of AD. Hippocampus and amygdala atrophy are key components of structural change in AD (Braak and Braak, 1991; Scott et al., 1992; Mori et al., 1997). In the early stages of AD amygdala volume is correlated with memory scores only, the earliest sign of MCI (Petersen et al., 1999), but not with deficits in other cognitive domains, such as language and executive function (Mori et al., 1997; Mizuno et al., 2000; Basso et al., 2006). These latter cognitive deficits are not seen until later in the disease. We found that amyloid-associated depression, but not non-amyloid depression, is significantly associated with poor memory (Sun et al., 2008).
There were limitations in this study. This study is cross-sectional in design and longitudinal studies are needed to determine the decline of plasma Aβ1-42 or the change of Aβ1-40/1-42 ratio that defines amyloidassociated depression for a given individual instead of group basis. It is unclear why the majority of elderly with MCI in the homebound elderly population had the nonamnestic type. One possible explanation is that we studied an elderly population employing homecare service agencies and elderly with nonamnestic MCI who have visuospatial and executive dysfunction might be more likely to receive such services than those with primarily memory problems. That we examined relatively few subjects with amnestic MCI might explain why we found only a tendency for a relationship between the plasma Aβ1-40/1-42 ratio and hippocampal volume. Since those who came to the hospital to undergo brain MRI had higher scores on ADL function than those with home visits only (Scott et al., 2006), it is possible that those excluded subjects with poor ADL function had poorer memory, defining amnestic MCI. A limitation to using plasma Aβ42 as a biomarker now is the under characterized relationship between peripheral Aβ42 levels and CSF Aβ42 levels (Oprisiu et al., 2006; Zetterberg and Blennow, 2006). ‘ Another issue in using plasma Aβ as a biomarker is that it lacks standardization across research laboratories to determine which Aβ species to measure to directly relate to the AD pathogenesis (Okereke et al., 2008).
Nevertheless, this study and others (Graff-Radford et al., 2003; van Dijk et al., 2004) suggest that plasma Aβ1-42 has potential as a prognostic biomarker of AD, especially if longitudinal measurements of plasma Aβ1-42 (Schupf et al., 2008) are conducted to follow its decline and when combined with neuroimaging of the medial temporal lobe. A recent study published by Butters et al. shows that concomitant MCI and depression is associated with amyloid deposits in the brain detected by Pittsburge Compound-B (PiB) PET scan (Butters et al., 2008). Keeping in mind that our study needs to be replicated in other populations, studies examining the relationship between specific biomarkers, such as CSF Aβ or PiB PET scan, and easily accessible biomarkers, such as plasma Aβ in prodromal depression of AD, are needed to further characterize this depression subtype.
Acknowledgments
The authors are grateful to Dr. Dennis J. Selkoe for his support and collaboration. We thank the NAME study staff and the Boston homecare agencies for their hard work and acquisition of subjects. This work was supported by grants from the NIA, AG-022476 for W.Q.Q and AG-21790 for M.F.F. Support was also provided through the General Clinical Research Center funded by the National Center for Research Resources of the NIH under grant no. MO1-RR00054.
Footnotes
Conflict of interest
None known.
References
- Alexopoulos GS, Meyers BS, Young RC, et al. Clinically defined vascular depression. Am J Psychiatry. 1997;154:562–565. doi: 10.1176/ajp.154.4.562. [DOI] [PubMed] [Google Scholar]
- Amieva H, Le Goff M, Millet X, et al. Prodromal Alzheimer’s disease: successive emergence of the clinical symptoms. Ann Neurol. 2008;64:492–498. doi: 10.1002/ana.21509. [DOI] [PubMed] [Google Scholar]
- Apostolova LG, Cummings JL. Neuropsychiatric manifestations in mild cognitive impairment: a systematic review of the literature. Dement Geriatr Cogn Disord. 2008;25:115–126. doi: 10.1159/000112509. [DOI] [PubMed] [Google Scholar]
- Basso M, Yang J, Warren L, et al. Volumetry of amygdala and hippocampus and memory performance in Alzheimer’s disease. Psychiatry Res. 2006;146:251–261. doi: 10.1016/j.pscychresns.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Demonstration of amyloid deposits and neurofibrillary changes in whole brain sections. Brain Pathol. 1991;1:213–216. doi: 10.1111/j.1750-3639.1991.tb00661.x. [DOI] [PubMed] [Google Scholar]
- Butters MA, Klunk WE, Mathis CA, et al. Imaging Alzheimer pathology in late-life depression with PET and Pittsburgh Compound-B. Alzheimer Dis Assoc Disord. 2008;22:261–268. doi: 10.1097/WAD.0b013e31816c92bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarli C, Maisog J, Murphy DG, et al. Method for quantification of brain, ventricular, and subarachnoid CSF volumes from MR images. J Comput Assist Tomogr. 1992;16:274–284. doi: 10.1097/00004728-199203000-00018. [DOI] [PubMed] [Google Scholar]
- den Heijer T, Geerlings MI, Hoebeek FE, et al. Use of hippocampal and amygdalar volumes on magnetic resonance imaging to predict dementia in cognitively intact elderly people. Arch Gen Psychiatry. 2006;63:57–62. doi: 10.1001/archpsyc.63.1.57. [DOI] [PubMed] [Google Scholar]
- Fuhrer R, Rouillon F. French version of CES-D scale: description and translation of self evaluation. Psychiatry Psyhology. 1989;4:163–166. [Google Scholar]
- Giedraitis V, Sundelof J, Irizarry MC, et al. The normal equilibrium between CSF and plasma amyloid beta levels is disrupted in Alzheimer’s disease. Neurosci Lett. 2007;427:127–131. doi: 10.1016/j.neulet.2007.09.023. [DOI] [PubMed] [Google Scholar]
- Graff-Radford N, Lucas J, Younkin L, Younkin S. Longitudinal analysis of plasma Ab42 in subjects progressing from normal through mild cognitive impairment to Alzheimer’s disease. Neurology. 2003;60:A245. [Google Scholar]
- Graff-Radford NR, Crook JE, Lucas J, et al. Association of low plasma Abeta42/Abeta40 ratios with increased imminent risk for mild cognitive impairment and Alzheimer disease. Arch Neurol. 2007;64:354–362. doi: 10.1001/archneur.64.3.354. [DOI] [PubMed] [Google Scholar]
- Gurol ME, Irizarry MC, Smith EE, et al. Plasma beta-amyloid and white matter lesions in AD, MCI, and cerebral amyloid angiopathy. Neurology. 2006;66:23–29. doi: 10.1212/01.wnl.0000191403.95453.6a. [DOI] [PubMed] [Google Scholar]
- Herlitz A, Hill RD, Fratiglioni L, Backman L. Episodic memory and visuospatial ability in detecting and staging dementia in a community-based sample of very old adults. J Gerontol A Biol Sci Med Sci. 1995;50:M107–M113. doi: 10.1093/gerona/50a.2.m107. [DOI] [PubMed] [Google Scholar]
- Irizarry MC. Biomarkers of Alzheimer disease in plasma. NeuroRx. 2004;1:226–234. doi: 10.1602/neurorx.1.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii H, Meguro K, Yamaguchi S, et al. Different MRI findings for normal elderly and very mild Alzheimer’s disease in a community: implications for clinical practice the Tajiri Project. Arch Gerontol Geriatr. 2006;42:59–71. doi: 10.1016/j.archger.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Iwatsubo T. Amyloid beta protein in plasma as a diagnostic marker for Alzheimer’s disease. Neurobiol Aging. 1998;19:161–163. doi: 10.1016/s0197-4580(98)00015-3. [DOI] [PubMed] [Google Scholar]
- Kawarabayashi T, Younkin LH, Saido TC, et al. Age-dependent changes in brain, CSF, and plasma amyloid (beta) protein in the Tg2576 transgenic mouse model of Alzheimer’s disease. J Neurosci. 2001;21:372–381. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasuski JS, Alexander GE, Horwitz B, et al. Volumes of medial temporal lobe structures in patients with Alzheimer’s disease and mild cognitive impairment (and in healthy controls) Biol Psychiatry. 1998;43:60–68. doi: 10.1016/s0006-3223(97)00013-9. [DOI] [PubMed] [Google Scholar]
- Lehericy S, Baulac M, Chiras J, et al. Amygdalohippocampal MR volume measurements in the early stages of Alzheimer disease. AJNR Am J Neuroradiol. 1994;15:929–937. [PMC free article] [PubMed] [Google Scholar]
- Leppanen JM. Emotional information processing in mood disorders: a review of behavioral and neuroimaging findings. Curr Opin Psychiatry. 2006;19:34–39. doi: 10.1097/01.yco.0000191500.46411.00. [DOI] [PubMed] [Google Scholar]
- Mayeux R, Honig LS, Tang MX, et al. Plasma A[beta]40 and A[beta]42 and Alzheimer’s disease: relation to age, mortality, and risk. Neurology. 2003;61:1185–1190. doi: 10.1212/01.wnl.0000091890.32140.8f. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Wakai M, Takeda A, Sobue G. Medial temporal atrophy and memory impairment in early stage of Alzheimer’s disease: an MRI volumetric and memory assessment study. J Neurol Sci. 2000;173:18–24. doi: 10.1016/s0022-510x(99)00289-0. [DOI] [PubMed] [Google Scholar]
- Mori E, Yoneda Y, Yamashita H, et al. Medial temporal structures relate to memory impairment in Alzheimer’s disease: an MRI volumetric study. J Neurol Neurosurg Psychiatry. 1997;63:214–221. doi: 10.1136/jnnp.63.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okereke O, Xia W, Irizarry MC, et al. Performance Characteristics of Plasma Amyloid-Beta 40 and 42 Assays. J Alzheimer’s Dis. 2008 doi: 10.3233/JAD-2009-0948. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson A, Ochsner KN. The role of social cognition in emotion. Trends Cogn Sci. 2008;12:65–71. doi: 10.1016/j.tics.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Oprisiu R, Serot JM, Godefroy O, Black SE, Fournier A. Plasma amyloid-beta concentrations in Alzheimer’s disease: an alternative hypothesis. Lancet Neurol. 2006;5:1001–1002. doi: 10.1016/S1474-4422(06)70612-3. [DOI] [PubMed] [Google Scholar]
- Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry. 2006;63:530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez RG, Soriano S, Hayes JD, et al. Mutagenesis identifies new signals for beta-amyloid precursor protein endocytosis, turnover, and the generation of secreted fragments, including Abeta42. J Biol Chem. 1999;274:18851–18856. doi: 10.1074/jbc.274.27.18851. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annu Rev Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Pomara N, Murali Doraiswamy P. Does increased platelet release of Abeta peptide contribute to brain abnormalities in individuals with depression? Med Hypotheses. 2003;60:640–643. doi: 10.1016/s0306-9877(02)00380-8. [DOI] [PubMed] [Google Scholar]
- Qiu WQ, Sun X, Selkoe DJ, et al. Depression is associated with low plasma Abeta42 independently of cardiovascular disease in the home-bound elderly. Int J Geriatr Psychiatry. 2007;22:536–542. doi: 10.1002/gps.1710. [DOI] [PubMed] [Google Scholar]
- Radloff L. The CES-D scale: a self report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- Rapp MA, Schnaider-Beeri M, Grossman HT, et al. Increased hippocampal plaques and tangles in patients with Alzheimer disease with a lifetime history of major depression. Arch Gen Psychiatry. 2006;63:161–167. doi: 10.1001/archpsyc.63.2.161. [DOI] [PubMed] [Google Scholar]
- Robinson RG, Bolduc PL, Price TR. Two-year longitudinal study of poststroke mood disorders: diagnosis and outcome at one and two years. Stroke. 1987;18:837–843. doi: 10.1161/01.str.18.5.837. [DOI] [PubMed] [Google Scholar]
- Schupf N, Tang MX, Fukuyama H, et al. Peripheral Abeta subspecies as risk biomarkers of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2008;105:14052–14057. doi: 10.1073/pnas.0805902105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SA, DeKosky ST, Sparks DL, Knox CA, Scheff SW. Amygdala cell loss and atrophy in Alzheimer’s disease. Ann Neurol. 1992;32:555–563. doi: 10.1002/ana.410320412. [DOI] [PubMed] [Google Scholar]
- Scott TM, Peter I, Tucker KL, et al. The Nutrition, Aging, and Memory in Elders (NAME) study: design and methods for a study of micronutrients and cognitive function in a homebound elderly population. Int J Geriatr Psychiatry. 2006;21:519–528. doi: 10.1002/gps.1503. [DOI] [PubMed] [Google Scholar]
- Scott TM, Tucker KL, Bhadelia A, et al. Homocysteine and B vitamins relate to brain volume and white-matter changes in geriatric patients with psychiatric disorders. Am J Geriatr Psychiatry. 2004;12:631–638. doi: 10.1176/appi.ajgp.12.6.631. [DOI] [PubMed] [Google Scholar]
- Smith CD, Malcein M, Meurer K, et al. MRI temporal lobe volume measures and neuropsychologic function in Alzheimer’s disease. J Neuroimaging. 1999;9:2–9. doi: 10.1111/jon1999912. [DOI] [PubMed] [Google Scholar]
- Solfrizzi VDIA, Colacicco AM, Capurso C, et al. Circulating biomarkers of cognitive decline and dementia. Clin Chim Acta. 2006;364:91–112. doi: 10.1016/j.cca.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Strazielle N, Ghersi-Egea JF, Ghiso J, et al. In vitro evidence that beta-amyloid peptide 1–40 diffuses across the blood-brain barrier and affects its permeability. J Neuropathol Exp Neurol. 2000;59:29–38. doi: 10.1093/jnen/59.1.29. [DOI] [PubMed] [Google Scholar]
- Sun X, Steffens DC, Au R, et al. Amyloid-associated depression: a prodromal depression of Alzheimer disease? Arch Gen Psychiatry. 2008;65:542–550. doi: 10.1001/archpsyc.65.5.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng E, Lu PH, Cummings JL. Neuropsychiatric symptoms are associated with progression from mild cognitive impairment to Alzheimer’s disease. Dement Geriatr Cogn Disord. 2007;24:253–259. doi: 10.1159/000107100. [DOI] [PubMed] [Google Scholar]
- van Dijk EJ, Prins ND, Vermeer SE, et al. Plasma amyloid beta, apolipoprotein E, lacunar infarcts, and white matter lesions. Ann Neurol. 2004;55:570–575. doi: 10.1002/ana.20050. [DOI] [PubMed] [Google Scholar]
- van Oijen M, Hofman A, Soares HD, Koudstaal PJ, Breteler MM. Plasma Abeta(1–40) and Abeta(1–42) and the risk of dementia: a prospective case-cohort study. Lancet Neurol. 2006;5:655–660. doi: 10.1016/S1474-4422(06)70501-4. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Przybelski SA, Weigand SD, et al. 3D maps from multiple MRI illustrate changing atrophy patterns as subjects progress from mild cognitive impairment to Alzheimer’s disease. Brain. 2007;130:1777–1786. doi: 10.1093/brain/awm112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterberg H, Blennow K. Plasma Abeta in Alzheimer’s disease-up or down? Lancet Neurol. 2006;5:638–639. doi: 10.1016/S1474-4422(06)70503-8. [DOI] [PubMed] [Google Scholar]