TO THE EDITOR
As epithelial appendages of the skin, hair follicle/sebaceous glands (HF/SGs) and eyelid meibomian glands (MGs) are developmentally similar and share common characteristics including holocrine differentiation, lipid secretion, and the need for long-term proliferative capacity and self-renewal (Knop et al., 2011). Although the capacity for self-renewal has been linked to the presence of a quiescent adult stem cell pool in the epidermis (Tumbar et al., 2004), cornea (Cotsarelis et al., 1989), and intestine (Pinto and Clevers, 2005), the location and characterization of MG and SG stem cells remain controversial. To address this problem, we used a novel three-dimensional (3D) reconstruction technique, immunofluorescence tomography, to identify label-retaining cells (LRCs) using the H2B-GFP/K5tTA ‘tet-off’ mouse model (Tumbar et al., 2004) and further characterize LRCs based on expression of SOX9 and BLIMP1.
Eyelids from three mice killed at each chase time point (n = 9) were embedded in butyl-methyl methacrylate (BMMA), serially sectioned at 2 μm, and 3D reconstructions generated according to previous protocol (Parfitt et al., 2012). Animals were treated according to the ARVO statement on the use of animals in vision research, and experiments were approved by the IACUC of the University of California, Irvine (P.I. Jester, protocol no. 2,011–3,002, approved 8 September 2011). After imaging GFP, sections were sequentially immunolabeled with CK6 (Abcam–Cambridge, UK, no. ab24646), SOX9 (Millipore–Billerica, MA, no. Ab5535), and BLIMP1 (Abcam no.ab96479) and reimaged. Semi-automated alignment of serial montage images and 3D reconstruction was then carried out using Amira software (Visage Imaging, San Diego, CA). Finally, the total GFP+ LRC count was quantified in three high-resolution 3D reconstructions of MGs and HF/SG at 28 (MG =25±4; HF/SG =32±6), 42 (MG =12±2; HF/ SG =29±7), and 56-day (MG =9±3; HF/SG =28±6) chase through physical counting and ImageJ software (National Institutes of Health, http://rsb.info.nih.gov/ij/).
In Figure 1a, MG (encircled white) and HF/SG (encircled yellow) skin appendages of the eyelid are shown in histological section. After 28-day doxy-cycline chase, very few epithelial cells retain GFP in the H2B-GFP/K5tTA mouse MG (Figure 1b—red arrowhead) compared with the HF (Figure 1b—white arrowhead). In 3D (Figure 1c and Supplementary Movie S1 online), LRCs were observed at the transition between CK6+ duct and acini epithelium of the MG. In the HF/SG 3D reconstruction (Figure 1D and Supplementary Movie S2 online), LRCs are restricted to the bulge region of the HF, and no LRCs were detected in the SG basal layer.
Figure 1. Immunofluorescence tomography of the H2B-GFP/K5tTA mouse eyelid after doxycycline chase.
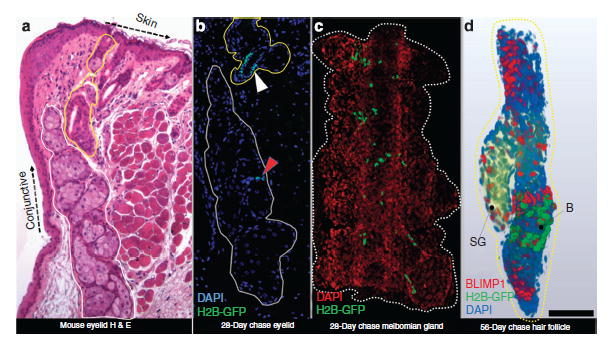
(a) Hematoxylin and eosin (H & E) staining of BMMA-embedded mouse eyelid showing the meibomian gland (encircled white) and hair follicle/sebaceous gland (encircled yellow). Both the meibomian gland (red arrowhead) and the hair follicle (white arrowhead) exhibit populations of slow-cycling epithelial cells after 28-day doxycycline chase. (c) In the meibomian gland, the label-retaining cells (LRCs) are located at the ductule or ductal epithelium terminus where the acinus forms. (d) BLIMP1 is expressed in differentiated hair follicle and sebaceous gland (SG) and is unlikely to be a marker of sebocyte progenitors. The hair follicle LRCs (green) are restricted to the bulge region (b) and are absent in the SG. Scale bar =50 μm.
Quantification of total cells (Figure 2a) and LRCs (Figure 2b) expressing BLIMP1, SOX9, or CK6 in the MG and HF was performed on eyelids (n =3) from mice euthanized at 56-day doxycycline chase. SOX9 and BLIMP1 were found to be expressed in the majority of MG and HF/SG epithelial cells, whereas LRCs occupy only a small population in these tissues. There was a mean of 9±3 LRCs out of 4356±497 total cells per MG and a mean of 28±6 LRCs out of 573±95 total cells per HF (Figure 2a). LRCs in the MG were exclusively SOX9+ and BLIMP1−, whereas HF LRCs exhibited a variable expression of SOX9 and BLIMP1 (Figure 2b). Slow-cycling MG cells express SOX9 as do terminally differentiated meibocytes (Figure 2c) and, in the HF, SOX9 is present in differentiated and quiescent cells (Figure 2d). In MGs, the putative sebocyte progenitor marker, BLIMP1, is absent in LRCs but present in differentiated epithelial cells (Figure 2e). BLIMP1 was found expressed in differentiated HF cells, bulge LRCs, and terminally differentiated sebocytes (Figure 2f). MG LRCs were localized to the ductal epithelium terminus where it transitions to basal acinar epithelium (Figure 2g) and infrequently express CK6, whereas HF LRCs are exclusively CK6− (Figure 2h).
Figure 2. Quantification of GFP+ label–retaining cells (LRCs) and the expression of BLIMP1, SOX9, and CK6 in the meibomian gland and hair follicle/sebaceous gland after 56-day doxycycline chase.
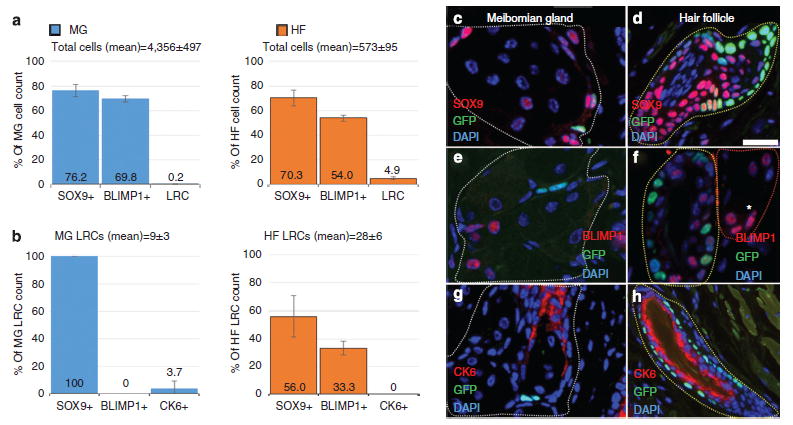
(a) The average total cell and (b) LRCs that express either SOX9, BLIMP1, or CK6 in the meibomian gland (blue) or hair follicle (orange). (c–h) Immuno-staining of meibomian gland (c, e, and g—encircled white), hair follicle (d, f, and h—encircled yellow), and sebaceous gland (f*—encircled red) showing co-localization of quiescent epithelial LRCs with (c and d) SOX9, (e and f), BLIMP1, and (g and h) CK6 in the H2B-GFP/K5tTA mouse after 56-day doxycycline chase. Scale bar =50 μm.
Through immunofluorescence tomography, we have localized and quantified a slow-cycling population of the mouse MG found at the CK6+ ductule that interconnects each acini with the central duct. We hypothesize that turn-over of the MG epithelia may be directed from these quiescent epithelial cells. However, additional functional studies are required to validate the potency of these cells. In the HF, LRCs were restricted to the bulge zone and none were found in the SG basal layer in a total of 6 glands evaluated. After localizing LRCs, we immuno-stained for SOX9, a putative stem cell marker (Formeister et al., 2009; Scott et al., 2010) considered an essential adult stem cell gene (Nowak et al., 2008). In the HF and MG of the eyelid, quiescent and differentiated cells express SOX9, and therefore our data indicate that SOX9 is not a good marker of quiescent epithelial cells, although it still may regulate stem cell quiescence as previously suggested (Nowak et al., 2008; Kadaja et al., 2014).
Previous research also suggests that the zinc-finger transcriptional repressor BLIMP1 is a marker of a unipotent progenitor population that directs SG turnover and sebocyte differentiation (Horsley et al., 2006). However, our high-resolution 3D data reveal that BLIMP1 is expressed throughout the HF in both quiescent and differentiated HF cells and sebocytes. Furthermore, BLIMP1+ cells located near or in the SG were not found to be LRCs. This finding supports a more recent study, which found BLIMP1 in mature sebocytes, (Sellheyer and Krahl, 2010), raising important questions about the role of BLIMP1 and the true identity of sebocyte progenitor cells. The discovery of a slow-cycling population offers unprecedented insights into MG cell turnover and may shed light on MG age–related atrophy and dry eye disease.
Supplementary Material
Acknowledgments
This work was supported in part by NEI EY021510, the Skirball Program in Molecular Ophthalmology, Research to Prevent Blindness, and Vistakon/ ARVO Foundation.
Abbreviations
- HF
hair follicle
- LRC
label-retaining cells
- MG
meibomian glands
- SG
sebaceous glands
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
References
- Cotsarelis G, Cheng SZ, Dong G, et al. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201–9. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- Formeister EJ, Sionas AL, Lorance DK, et al. Distinct SOX9 levels differentially mark stem/ progenitor populations and enteroendocrine cells of the small intestine epithelium. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1108–18. doi: 10.1152/ajpgi.00004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsley V, O’Carroll D, Tooze R, et al. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell. 2006;126:597–609. doi: 10.1016/j.cell.2006.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadaja M, Keyes BE, Lin M, et al. SOX9: a stem cell transcriptional regulator of secreted niche signaling factors. Genes Dev. 2014;28:328–41. doi: 10.1101/gad.233247.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop E, Knop N, Millar T, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci. 2011;52:1938–78. doi: 10.1167/iovs.10-6997c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak JA, Polak L, Pasolli HA, et al. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell. 2008;3:33–43. doi: 10.1016/j.stem.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfitt GJ, Xie Y, Reid KM, et al. A novel immunofluorescent computed tomography (ICT) method to localise and quantify multiple antigens in large tissue volumes at high resolution. PLoS One. 2012;7:e53245. doi: 10.1371/journal.pone.0053245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto D, Clevers H. Wnt, stem cells and cancer in the intestine. Biol Cell. 2005;97:185–96. doi: 10.1042/BC20040094. [DOI] [PubMed] [Google Scholar]
- Scott CE, Wynn SL, Sesay A, et al. SOX9 induces and maintains neural stem cells. Nat Neurosci. 2010;13:1181–9. doi: 10.1038/nn.2646. [DOI] [PubMed] [Google Scholar]
- Sellheyer K, Krahl D. Blimp-1: a marker of terminal differentiation but not of sebocytic progenitor cells. J Cutan Pathol. 2010;37:362–70. doi: 10.1111/j.1600-0560.2009.01434.x. [DOI] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–63. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


