Abstract
Little is known about the effect of community versus health facility-based interventions to improve and sustain antiretroviral therapy (ART) adherence, virologic suppression, and retention in care among HIV-infected individuals in low-and middle-income countries (LMICs). We systematically searched four electronic databases for all available randomized controlled trials (RCTs) and comparative cohort studies in LMICs comparing community versus health facility-based interventions. Relative risks (RRs) for pre-defined adherence, treatment engagement (linkage and retention in care), and relevant clinical outcomes were pooled using random effect models. Eleven cohort studies and eleven RCTs (N = 97,657) were included. Meta-analysis of the included RCTs comparing community- versus health facility-based interventions found comparable outcomes in terms of ART adherence (RR = 1.02, 95 % CI 0.99 to 1.04), virologic suppression (RR = 1.00, 95 % CI 0.98 to 1.03), and all-cause mortality (RR = 0.93, 95 % CI 0.73 to 1.18). The result of pooled analysis from the RCTs (RR = 1.03, 95 % CI 1.01 to 1.06) and cohort studies (RR = 1.09, 95 % CI 1.03 to 1.15) found that participants assigned to community-based interventions had statistically significantly higher rates of treatment engagement. Two studies found community-based ART delivery model either cost-saving or cost-effective. Community- versus facility-based models of ART delivery resulted in at least comparable outcomes for clinically stable HIV-infected patients on treatment in LMICs and are likely to be cost-effective.
Keywords: Community, Interventions, ART, Adherence, Retention, LMIC
Introduction
The number of people living with HIV (PLWH) who have started life-saving antiretroviral therapy (ART) has markedly increased in low- and middle-income countries (LMICs) [1]. This impressive development, however, has led to overcrowding in health care facilities, longer waiting times during visits, and reduced time for counseling and clinical care of newly enrolled patients. In most public sector clinics in LMICs, it has also restricted the workforce’s capacity to provide ongoing adherence support and monitor patients who do not remain engaged in care (treatment engagement) to ensure optimal ART-related benefits on patient health and community HIV prevention [2]. Further, in July 2014, UNAIDS called for a global scale-up of treatment as prevention and efforts to meet the following “90-90-90” targets by 2030: (1) 90 % of all people living with HIV should know their HIV status (90 % diagnosed); (2) 90 % of all people diagnosed with HIV infection should receive ART (90 % on treatment); and (3) 90 % of all people receiving ART should achieve viral suppression [3]. This ambitious target for 73 % of all PLWH with documented undetectable viral load is expected to be associated with a significant decrease in HIV-related morbidity and mortality, lowest risk of sexual transmission, and decrease in HIV incidence at population level as well as supported by the latest World Health Organization (WHO) guidelines recommending treating all PLWH irrespective of immune status [4•]. It implies that an additional 21 million people are in need for treatment as of 2015 and underscores the importance of strengthening health systems’ capacity to meet the growing health needs within communities [4•].
Emerging data from both resource rich and limited settings have demonstrated that a substantial reduction in patient retention in clinical care occurs between each stage of the HIV treatment continuum from diagnosis and linkage to care, assessment of ART readiness to acceptability, receipt of initial ART, adherence and long-term retention in care, and treatment success as reflected by virologic suppression [5, 6, 7•, 8]. A systematic review reported that retention of PLWH on ART at 36 months in LMICs averages only 65 to 70 % [9•]. This proportion is markedly lower in patients who present to hospitals with advanced HIV. Success along the HIV treatment cascade is even worse in key populations, namely, pregnant women, children and adolescents, sex workers, people who inject drugs, and men who have sex with men, and they are at high risk of acquiring as well as transmitting HIV to others, thus experiencing poor clinical and public health outcomes [10–15]. Against this background, it is critical to determine how effective interventions are at every level of the treatment cascade to prevent new infections and promote health outcomes to achieve the goal of an AIDS-free generation [7•].
In LMICs, selected approaches to reducing loss at every stage of the HIV treatment cascade include decentralization of services and task-shifting aspects of care to nurses and to nonclinical staff, including lay counselors who may be patients themselves. These approaches have been found to be feasible, effective, and results in good clinical outcomes [16–18]. Task shifting is now recommended and being scaled up in LMICs [16–19]. Such facility-based strategies, however, are reaching their limits as increasing numbers of patients initiate ART. Recently, suggestions have been made to expand accessible and flexible community-based ART service delivery, differentiating the needs of clinically ill patients starting ART or in need of significant adherence counseling from the needs of clinically stable patients with documented optimal ART adherence. This transition from facility- to community-based treatment has been identified as an important strategies for maintaining retention in HIV care and improving ART adherence, and viral suppression, but without reducing quality of care [16].
Community-based programs to promote retention in HIV care and/or ART adherence are now increasingly being recognized as an important and sustainable approach that could contribute significantly toward the UNAIDS 90-90-90 target and ultimately an AIDS-free generation [20–27, 28•, 29–32]. Such approaches are also seen as an essential mechanism of service delivery, including dispensing of ART, and a means of decongesting traditional health services, rather than being purely an adherence adjunct. Furthermore, such interventions are likely to be cost-effective from a societal perspective by offering a shift of certain tasks from overburdened and high-cost health care settings directly into communities and para-professional staff, reducing also transportation costs for patients [33, 34].
We conducted a systematic review and meta-analysis to compare the effect of community-based ART delivery on treatment engagement, ART adherence, virologic suppression, and all-cause mortality among PLWH in LMICs against results obtained from patients treated in traditional health care facilities.
Methods
Protocol
The study background, rationale, and methods were specified in advance and documented in a study protocol registered in the PROSPERO database (CRD42016034114).
Study Inclusion Criteria
In order to be eligible for inclusion in the review, studies had to report on adherence, virologic suppression, and treatment engagement outcomes after initiation of ART. The following selection criteria were used to identify potential studies:
Study design: observational and experimental studies with primary data using cross-sectional, case–control, and cohort (prospective and retrospective) and randomized controlled trial (RCT) designs.
Study population: HIV-infected individuals initiated on ART.
Intervention: community-based ART delivery. Models could include the following: (1) home-based interventions (e.g., friends or family-centered approaches); (2) peer- or HIV patients-led interventions; community ART distribution points (with or without involving primary level formal or informal health facilities); (3) community-based ART adherence clubs (with or without involving primary level formal or informal health facilities); (4) community ART groups (CAGs)
Comparator: traditional health care facility (e.g., hospital or clinics)
Outcomes: primary: (1) proportion of PLH with optimal ART adherence levels* (>80 %); (2) proportion of PLH with virologic suppression (as defined by the studies) at 12 and/or 24 months after ART initiation. Secondary: (1) treatment engagement (combining linkage and retention in HIV care) as proportion of patients retained in care at 12 and/or 24 months post-ART initiation; (2) all-cause mortality; (3) reported stigma; and (4) cost to patient and provider and cost-effectiveness.
Data Sources and Searches
We conducted a systematic literature search using the following databases: Medline (PubMed), Scopus, SCI Web of Science, and Cochrane Central Register of Controlled Trials (CENTRAL) through January 2016. In addition, abstracts from major HIV/AIDS or infectious disease conferences such as the Conference on Retrovirus and Opportunistic Infections (CROI), International AIDS Society (IAS), International AIDS Conference, and Infectious Diseases Society of America (IDSA) were reviewed for inclusion. Our search terms included the following: “community”; “home-based care”; “health facilities”; “hospital”; “clinic”; “adherence”; “virologic suppression”; “adherence club”; “retention in care”; “retention”; “loss to follow up”, “attrition”, “antiretroviral therapy”; “HIV”; “community volunteers”; “treatment supporter”; “DOT”; “DAART”; “cost”; “cost-effectiveness”.
Study Selection and Data Extraction
Two of the authors (JBN and OA) screened the search outputs using titles and abstracts and independently reviewed the full text of all potentially eligible studies to assess whether they met the inclusion criteria. Discrepancies in the choice of included studies between the two authors were resolved through discussion and consensus. For all eligible studies, the same authors reviewed extracted information regarding publication date, study setting, study design, methods, patient population, study intervention, and outcomes.
Risk of Bias (Quality) Assessment
To appraise the risk of bias for included studies, a tool was adapted from the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) (Appendix) [35]. Briefly, the risk of bias was assessed as low risk, unclear risk, or high risk for each of the following domains: selection (sample population), selection (participation rate), performance bias (outcome assessment), performance bias (analytical methods to control for bias), and other forms of bias. We used the Cochrane Collaboration’s tool for assessing the risk of bias for quality assessment of the included studies [36]. The studies were graded based on the following: (i) sequence generation, (ii) blinding of outcome assessor, (iii) incomplete outcome data, (iv) selective outcome reporting, and (v) other sources of bias.
Measures of Treatment Effect and Unit of Analysis
We used relative risks (RR) for the calculation of dichotomous data (such as adherence and retention in care). All results are presented with 95 % confidence intervals (CI).
Data Synthesis
In the absence of statistical heterogeneity, we used a fixed effect model, and we used a random effect model where we detected moderate heterogeneity and it was deemed still reasonable to combine trials. We assessed the presence of statistical heterogeneity in the meta-analyses by visual inspection of the forest plot and applying a Chi-squared test for heterogeneity with a threshold P value of 0.10 to determine statistical significance. Inconsistency was quantified across studies using the I2 value. We used Review Manager 5.3 [37] to conduct analyses and analyzed results for trials and cohort studies separately and also pooled these data. This review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [38].
Results
Study Selection and Characteristics of Included Studies
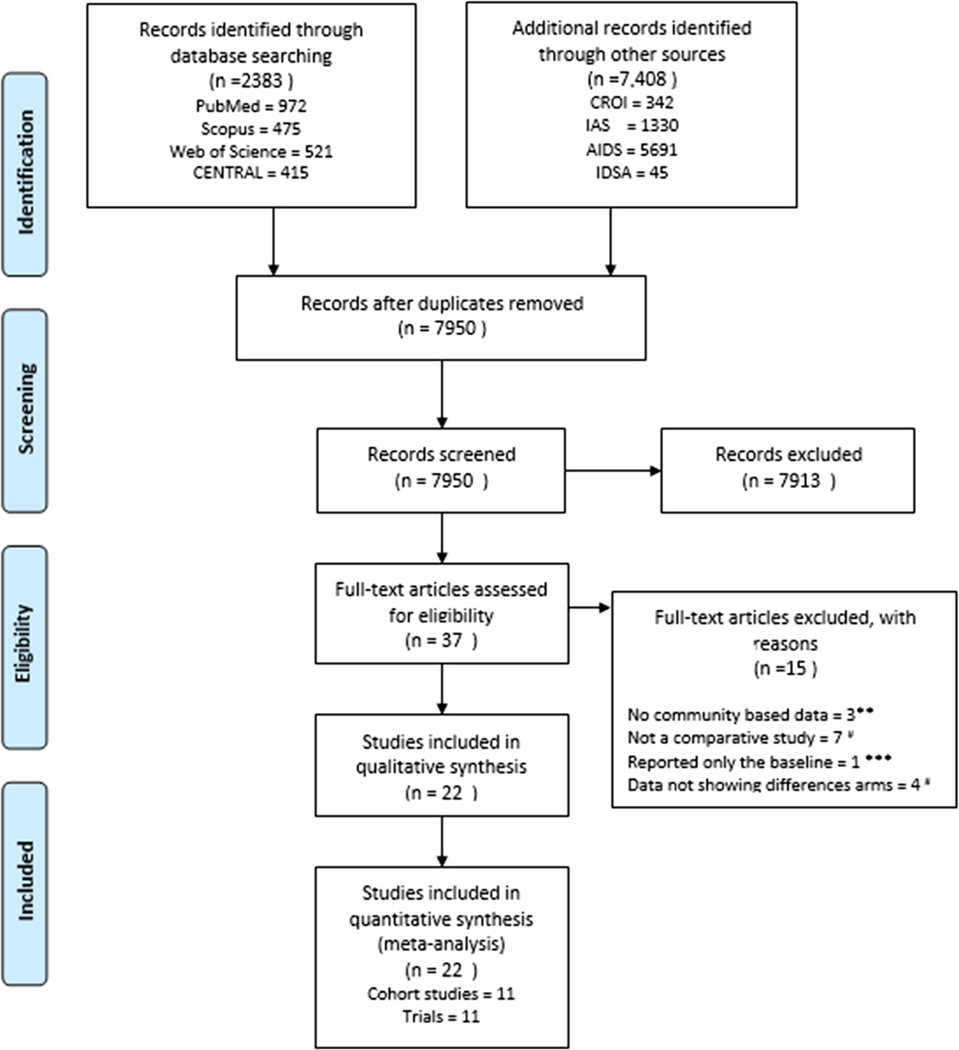
The process of study identification and selection is shown in Fig. 1. The literature search yielded 7950 citations after removing duplicates. After review of title and abstract, 37 full text articles were selected for critical review. A total of 11 RCTs were included [21, 25, 26, 29, 30, 36, 39, 40•, 41–44] and 11 cohort studies [27, 36, 39, 45–48, 49•, 50, 51, 52•, 53, 54] with a total of 5861 and 89,388 participants, respectively. These studies were conducted in eight different sub-Saharan African countries: Botswana, Zimbabwe, South Africa, Nigeria, Uganda, Kenya, Tanzania, and Zambia. Other studies were conducted in other LMICs like Brazil, Haiti, Peru, and Thailand. See additional details in Table 1. We excluded 17 studies [44], three studies were excluded due to non-inclusion of community-based data [55–57] while seven studies were excluded because the studies were non-comparative [19, 58–62]. One study was also excluded because only baseline data were reported [63] while four did not show outcome data for different arms of the studies [17, 64–66].
Fig. 1.
PRISMA flow for study selection
Table 1.
Table of included studies
| Study | Type | Age-median (years) |
Countries included |
Duration of study |
Sample size (n) |
Participants/health care service description |
||
|---|---|---|---|---|---|---|---|---|
| Intervention | Comparator | Intervention | Comparator | |||||
| Chang et al. [21] | Cluster-randomized trial |
35.5 | 34.0 | South Africa | 27 months; May 2006–July 2008 |
1336 | Patients with Community- based peer health workers |
Control group- accessing standard of care |
| Fatti et al. [45] | Cohort | 35.1 | 34.6 | South Africa | 69 months; January 2004– September 2010 |
66,953 | Patients who received community-based adherence support |
Patients without community- based adherence support |
| Fatti et al. [46] | Cohort | 6.3 | 6.6 | South Africa | 69 months; January 2004– September 2010 |
4,853 | Patients who received community-based adherence support |
Patients without community- based adherence support |
| Franke et al. [47] | Prospective cohort | 37.0 | 37.0 | Rwanda | 15 months; June 2007 to August 2008 |
610 | Community-Based Accompaniment |
Clinic-Based Care |
| Grimsrud et al. [48] | Cohort | 33.9 | 33.2 | South Africa | 20 months; May 2012–December 2013 |
8,150 | Community based adherence club |
Community Health Centre |
| Grimwood et al. [49•] | Cohort | 6.8 | 6.2 | South Africa | 57 months; January 2004– September 2009 |
3,563 | Children with patient advocates |
Children without patient advocates |
| Jaffar et al. [25] | Cluster-randomized equivalence trial |
37.0 | 38.0 | Uganda | 48 months; February 2005– January 2009 |
1,453 | Home-based care | Facility based care |
| Johnston et al. [50] | Retrospective cohort | 36.0 | 43.0 | South Africa | 75 months; January 2003– March 2010 |
417 | Community cohort | Workplace cohort |
| Kipp et al. [51] | Cohort | 36.8 | 34.8 | Uganda | 15 months; March 2006–May 2007 |
385 | Centre/Community- based cohort |
Hospital-based cohort |
| Kiweewa et al. [39] | Randomized controlled trial |
27.0 | 27.8 | Uganda | 29 months; May 2007–September 2009 |
92 | Nurse-Peer model | Doctor-Counsellor model |
| Luque-Fernandez et al. [27] | Cohort | Not reported |
Not reported |
South Africa | 40 months; November 2007– February 2011 |
2,834 | Adherence clubs | Traditional clinic-based care |
| Massavon et al. [52•] | Retrospective cohort |
91.0 months | 45.9 months | Uganda | 2003–2010 | 1,623 | Community home- based care approach |
Facility-based family- centred approach |
| Mfinanga et al. [40•] | Open-label, randomized controlled trial |
38.0 35.0 |
37.0 35.0 |
Tanzania Zambia | 19 months; February, 2012–September, 2013 |
1,999 | Clinic plus community support |
Standard care |
| Nachega et al. [29] | Open-label, randomized controlled trial |
35.7 | 36.7 | South Africa | 42 months; February 2005–July 2008 |
274 | Directly observed therapy (DOT-ART) arm |
Self-administered ART (Self-ART arm) |
| Selke et al. [41] | Cluster randomized controlled clinical trial |
38.7 | 37.5 | Kenya | 26 months; March 2006– April 2008. |
208 | Community Care Coordinators arm patients |
Standard of Care arm patients |
| Taiwo et al. [42] | Randomized controlled trial |
Not reported |
Not reported |
Nigeria | 19 months; June 2006– December 2007 |
499 | Treatment partner- assisted ART |
Patient-administered standard of care ART |
| Gross et al. [44] | Randomized controlled trial |
38 | 37 | Botswana, Brazil, Haiti, Peru, South Africa, Uganda, Zambia, Zimbabwe |
30 months; April 2009– September 2011 |
259 | Partner-based modified directly observed therapy |
Standard of care |
| Nakigozi et al. [30] | Randomized controlled trial |
37.6 | 37 | Uganda | 15 months; October 2010– January 2012 |
1,209 | Patient-selected care buddy |
Standard of care |
| Kaihin et al. [53] | Cohort | 18.2 | 19.4 | Thailand | 4 months; April-July 2011 | 46 | Experimental Group (Empowerment Intervention) |
Control |
| Kunutsor et al. [26] | Randomized controlled trial |
39.1 | 39.2 | Uganda | 8 months; March- September 2010 |
174 | Standard adherence intervention package plus treatment supporter intervention |
Standard intervention package |
| Munoz et al. [54] | Cohort | 31.7 | 31.9 | Peru | 17 months; December 2005– April 2007 |
120 | Community-based accompaniment with supervised antiretrovirals |
Control |
| Coker et al. [43]a | Randomized controlled trial |
Not reported |
Not reported |
Nigeria | 18 months; August 2006–January 2008 |
400 | Peer educators arm | Standard of care |
| Coker et al. [43]b | Randomized controlled trial |
Not reported |
Not reported |
Nigeria | 18 months; August 2006–January 2008 |
400 | Home visits and peer educators arm |
Standard of care |
Risk of Bias in Included Studies
The risk of bias of included cohort studies is summarized in Table 2. All the included studies had low risk of bias with respect to the selection of sample population and explaining the rationale for case and control selection while the included cohort studies were at risk of bias for sample selection ambiguity and having samples that were unlikely to be representative. All the studies had a high participation rate (>70–85 %). In terms of outcome assessment, seven of the included studies had objective measures of adherence such as “pill count,” while two had high risk of bias by measuring the outcome using self-reporting format. All the RCTs and cohort studies used one or more analytical methods to control for bias in individual studies. The risk of bias in the included studies was highest from other forms of bias, followed by selection of sample population, and lowest from participation rates and analytical methods to control for bias.
Table 2.
Risk of bias in included studies of cohort studies
| Study | Selection (sample population) |
Selection (participation rate) |
Performance bias (outcome assessment) |
Performance bias (analytical methods to control for bias) |
Other form of bias |
|---|---|---|---|---|---|
| Fatti et al. [45] | High | Low | Unclear | Low | High |
| Fatti et al. [46] | High | Low | Unclear | Low | High |
| Franke et al. [47] | High | Low | Unclear | Low | High |
| Grimsrud et al. [48] | High | Low | Low | Low | High |
| Grimwood et al. [49•] | High | Low | Unclear | Low | High |
| Johnston et al. [50] | High | Low | Unclear | Low | High |
| Kipp et al. [51] | High | Low | Low | Low | High |
| Luque-Fernandez et al. [27] | High | Low | Unclear | Low | High |
| Massavon et al. [52•] | High | Low | Low | Low | High |
| Kaihin et al. [53] | High | Low | Low | Low | High |
| Munoz et al. [54] | High | Low | Low | Low | High |
The risk of bias of included trials is shown in Table 3. Allocation sequence generation was adequate in all the 11 trials. Allocation concealment was adequate in nine trials and unclear in the remaining two trials. Masking of outcome assessors was not clear in all the nine trials. Potential risk of bias due to selective reporting and other bias was low in all 11 trials.
Table 3.
Risk of bias in included studies of randomized controlled trials
| Study | Random sequence generation (selection bias) |
Allocation concealment (selection bias) |
Blinding of outcome assessors (performance bias) |
Incomplete outcome data (attrition bias) |
Selective reporting (reporting bias) |
Other bias |
|---|---|---|---|---|---|---|
| Chang et al. [21] | Low | Unclear | Unclear | Low | Low | Low |
| Jaffar et al. [25] | Low | Low | Unclear | Low | Low | Low |
| Kiweewa et al. [39] | Low | Low | Unclear | Low | Low | Low |
| Mfinanga et al. [40•] | Low | Low | Unclear | Low | Low | Low |
| Nachega et al. [29] | Low | Low | Unclear | Low | Low | Low |
| Selke et al. [41] | Low | Low | Unclear | Low | Low | Low |
| Taiwo et al. [42] | Low | Unclear | Unclear | Low | Low | Low |
| Gross et al. [44] | Low | Low | Low | Low | Low | Low |
| Nakigozi et al. [30] | Low | Low | High | Low | Low | Low |
| Kunutsor et al. [26] | Low | Low | Unclear | Low | Low | Low |
| Coker et al. [43] | Low | Low | Unclear | High | Low | Low |
| Low | Unclear | Unclear | High | Low | High |
Optimal ART Adherence
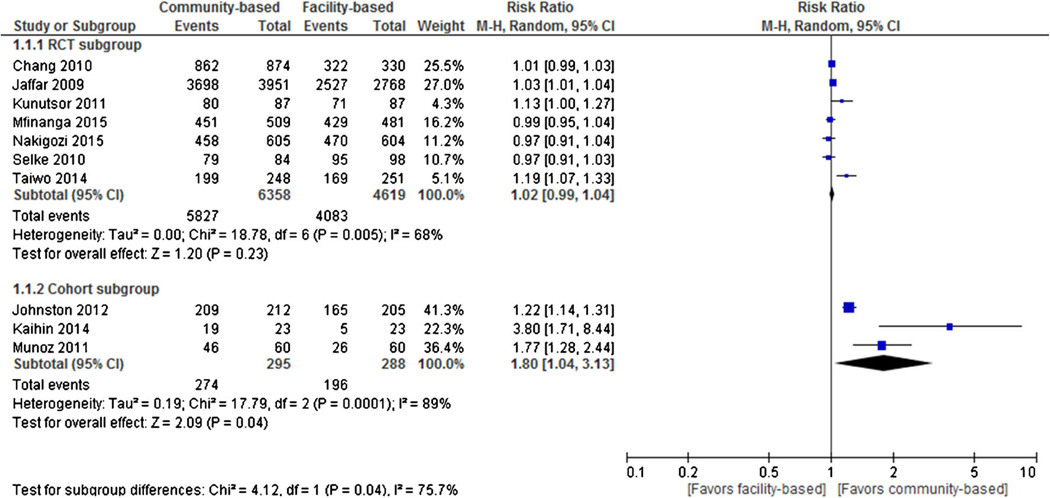
Seven RCTs and three cohort studies reported optimal adherence as an outcome. Individual and pooled RRs for optimal adherence are shown in Fig. 2. The result of pooled analysis from the RCTs showed no statistically significant difference in optimal adherence outcomes between the two treatment groups (pooled RR = 1.02, 95 % CI 0.99 to 1.04, I2 = 68 %), such that among 6358 participants randomized to community-based ART, 5827 (91.7 %) achieved optimal ART adherence compared with 4083 of 4619 in the facility-based ART group (88.4 %). Three cohort studies, however, provided evidence that participants in community-based ART had statistically significant higher optimal adherence outcomes compared to patients in the facility-based ART programs (RR = 1.80, 95 % CI 1.04 to 3.13), such that among 274 participants in the community-based ART, 295 (92.9 %) achieved optimal ART adherence compared with 196 (68.1 %) of 288 in the facility-based ART group.
Fig. 2.
Forest plot of optimal ART adherence comparing community-based ART versus facility-based ART
Virologic Suppression
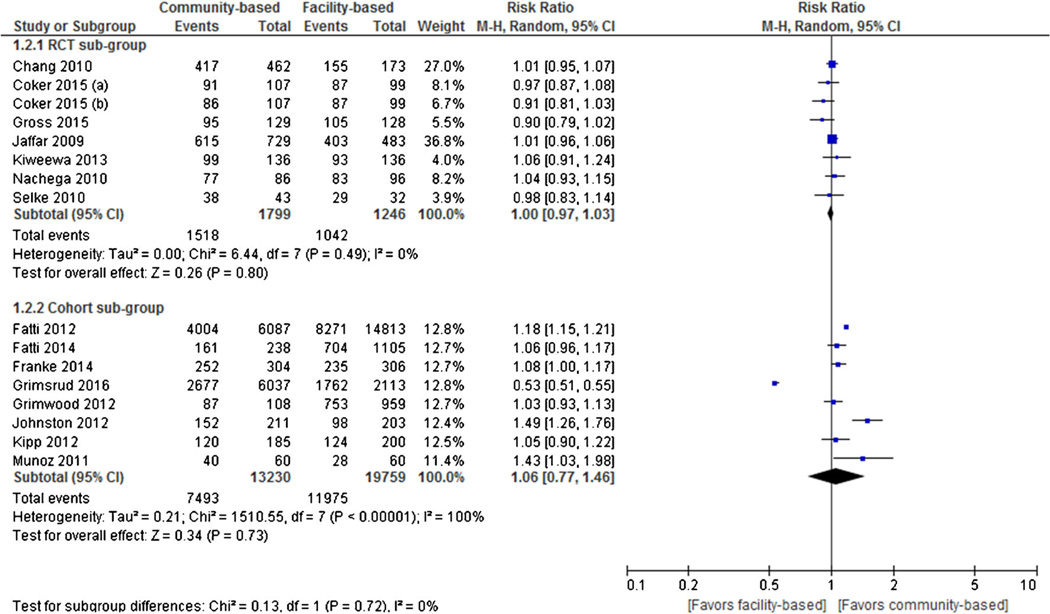
Eight RCTs and eight cohort studies reported virologic suppression as an outcome. Individual and pooled RRs for virologic suppression are shown in Fig. 3. The result of pooled analysis from the RCTs showed no statistically significant difference in virologic suppression rates between the two treatment groups (pooled RR = 1.00, 95 % CI 0.97 to 1.03), with evidence of no statistically significant heterogeneity between studies (I2 = 0 %, p = 0.49). Similarly, the result of pooled analysis from the cohort studies showed no statistically significant difference in virologic suppression rates between the two treatment groups (pooled RR = 1.06, 95 % CI 0.77 to 1.46), with evidence of statistically significant substantial heterogeneity between studies (I2 = 100 %, p < 0.00001).
Fig. 3.
Forest plot of virologic suppression comparing community-based ART versus facility-based ART
Treatment Engagement (Linkage and/or Retention in Care)
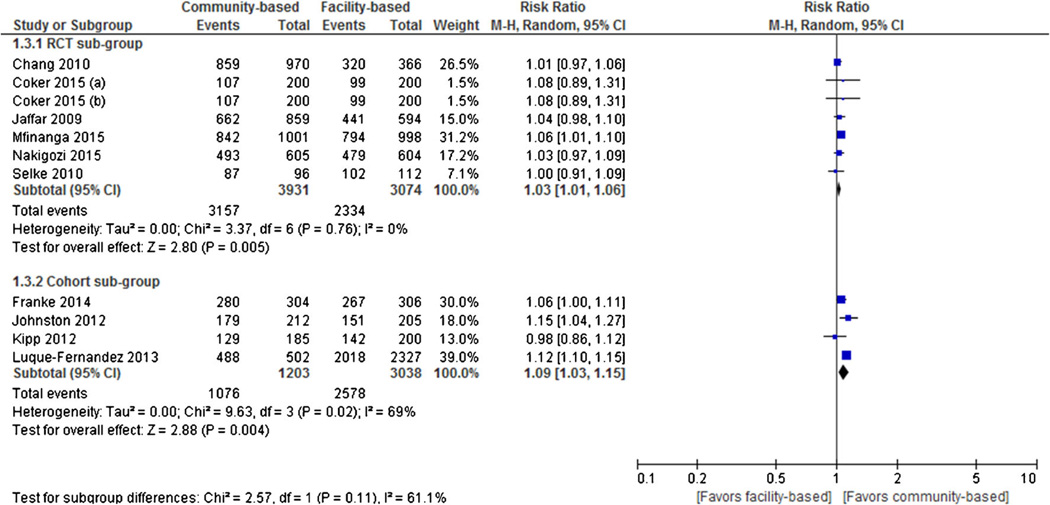
Seven RCTs and four cohort studies reported retention in care as an outcome. Individual and pooled RRs for retention in care are shown in Fig. 4. The result of pooled analysis from the RCTs showed that participants assigned to community-based ART (80.3 % [3157 of 3931]) had statistically significant higher rates of treatment engagement than those in facility-based ART (75.9 % [2334 of 3074]) at the end of the follow-up period (RR = 1.03, 95 % CI 1.01 to 1.06, I2 = 0 %). Similarly, the result of pooled analysis from the cohort studies showed that participants assigned to community-based ART (89.4 % [1074 of 1203]) had statistically significant higher rates of treatment engagement than those in facility-based ART (84.9 % [2578 of 3038]) at the end of the follow-up period (RR = 1.09, 95 % CI 1.03 to 1.15, I2 = 69%)
Fig. 4.
Forest plot of retention in care comparing community-based ART versus facility-based ART
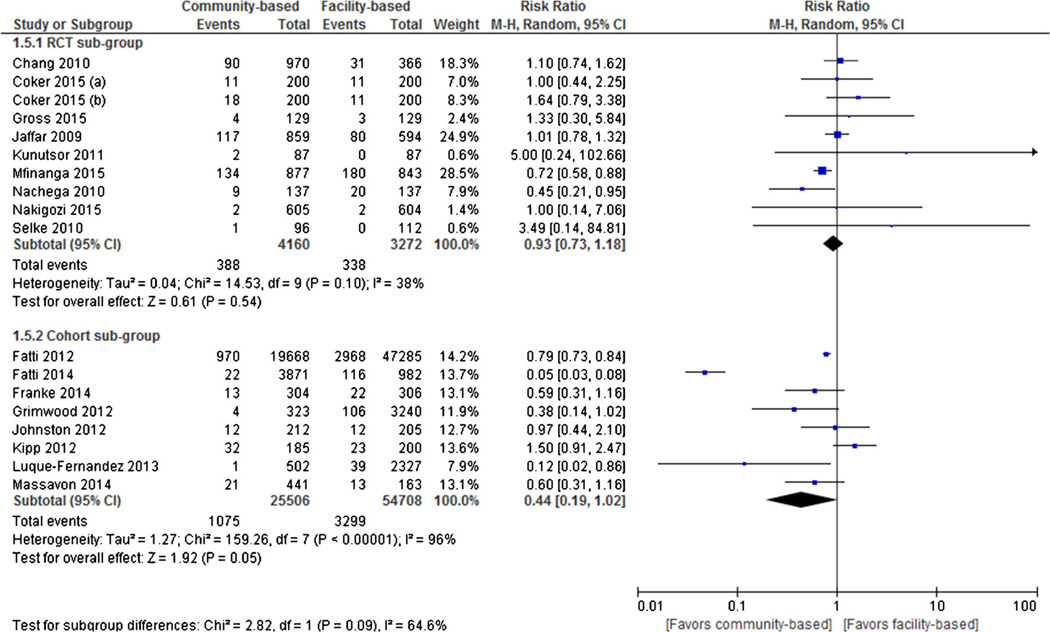
All-Cause Mortality
Ten RCTs and eight cohort studies reported all-cause mortality as an outcome. Individual and pooled RRs for all-cause mortality are shown in Fig. 5. The result of pooled analysis from the RCTs showed that there was no statistically significant difference in rates of all-cause mortality in assigned to community-based ART (9.3 % [388 of 4160]) than to those assigned to facility-based ART (10.3 % [338 of 3272]) at the end of the follow-up period (RR = 0.93, 95 % CI 0.73 to 1.18, I2 = 38 %). Similarly, the result of pooled analysis from the cohort studies showed that there was no statistically significant difference in rates of all-cause mortality in assigned to community-based ART (4.2 % [1075 of 25,506]) than those assigned to facility-based ART (6.0 % [3299 of 54,708]) at the end of the follow-up period (RR = 0.44, 95 % CI 0.19 to 1.02, I2 = 96 %).
Fig. 5.
Forest plot of all-cause mortality comparing community-based ART versus facility-based ART
Costs and Cost-Effectiveness
Jaffar et al. [25] reported costs to access care per patient including transport, lunch, child care costs, and lost work time. The average total cost per patient in the first year was US $29 among the community-based participants compared to the US $60 facility-based patients. In terms of health-service costs, the same study reported average cost per patient per year to be US$793 among the community-based participants compared to US$838 among facility-based patients in Jinja, Uganda. Also, Bango and colleagues reported from South Africa that ART adherence clubs (AAC) were most cost-effective than standard of care (SOC), with a cost per patient year of $296 for AAC versus $374 for SOC. Retention in care at 1 year was 95 % (95 % CI 94.88–95.86) for SOC and 98 % for ACC (95 % CI 97.6–98.3) [67].
Discussion
This review found no statistical difference in optimal ART adherence, virologic suppression, all-cause mortality, and loss to follow-up between those participants assigned to community-based ART and facility-based ART, when the analysis was restricted to RCTs. In the pooled analysis from both RCTs and cohort studies, however, we documented that participants assigned to community-based ART had significantly higher rates of retention in care than those in facility-based ART at the end of the follow-up period.
The above results corroborate the fact that providing patient support and education programs at the community level are equal and certainly not inferior compared to facility-based ones and may in fact be superior when it comes to selected outcomes such as retention in HIV care. Of note, our analysis may be underpowered to show superiority on selected outcomes such as virologic outcomes and all-cause mortality. Of note, ascertainment of selected outcomes such as all-cause mortality is better in the community than in the facility-based settings due to the fact that mortality in facility-based studies may be under-ascertained, which will make mortality in the community looks higher [68]. At the same time, silent health facility-based patient transfer (patients are being seen at other clinics, but the clinic-of-origin staff think that they are disengaged in care) will likely be under-captured, thus making health facility-based retention in care worse [68].
In building decentralized ART delivery, adherence, and retention in care support, community-based ART programs encourage patient autonomy, build social networks, and minimize the structural barriers, such as cost of transport to the clinic, which in turn appear to result in better outcomes [69]. Such community-based interventions are likely to have more impact since they tend to involve trained community health workers, peers, volunteers, or patient’s own social network members (e.g., family and friends) who assist with ART adherence counseling and support. In addition, there is evidence that they may provide material, instrumental, and emotional support, as well as promote other healthy behaviors, such as decreased alcohol and drug use, leading to better health outcomes—including survival [14, 24, 29, 32]. Furthermore, enhancing certain aspects of the patient–supporter relationships—such as trust, supporter availability, communication, reciprocity of support, and medication assistance—in a manner consistent with patients’ expectations may help to optimize the relationship and its positive impact on patient health [14, 24, 70].
Our study complements the findings of a previous review that assessed the effect of home-based interventions on viral outcomes in sub-Saharan Africa; this review found that there were insufficient data to be conclusive [70]. Another recent review summarized the evidence supporting different models of community participation for ART care or community-based ART in resource-limited settings; these community ART programs made treatment readily accessible and affordable [71]. In Uganda and Kenya, community health workers or volunteers delivered ART at home [41, 62], while in Tete, Mozambique, a demonstration project of people living with HIV/AIDS used self-formed community-based ART groups to deliver ART in the community [19]. Also, in South Africa, Médecins Sans Frontières piloted ART adherence clubs with promising results [27]. These clubs may provide some adherence counseling and peer support, as well as enable a “fast track” refill mechanisms. Patients are placed in groups of approximately six patients, and one member of the group (rotating each month) is permitted to obtain refills for all of the patients in his or her group. These approaches decrease the patient burden on health facilities, reduce transportation costs and waiting times for patients, and help overcome structural barriers. They also reduce treatment fatigue and loss to follow-up, increase disclosure and treatment education, and may help patients develop necessary social ties. While supportive of community-based interventions, these evaluations used observational study designs with possibility of selection and observational bias as well as confounding, and most of them did not have a valid comparator and could not be included in our meta-analysis.
We also investigated as secondary outcomes two potential concerns related to community-based ART adherence and retention programs, including reported stigma and low quality of care which could result in an increased all-cause mortality. In terms of stigma, an RCT reported that only 3 % of patients refused to participate in the home-based ART program due to stigma [25]. Furthermore, it has even been suggested that involvement of community-health care workers in HIV care reduced stigma [72] and being part of peer groups has been found to decrease the perception of social stigma [73].
Our results have important clinical and public health implications in the context of reaching the 2030 UNAIDS 90-90-90 targets toward an AIDS-free generation. While this systematic review and meta-analysis did not examine the first step of the HIV treatment cascade, HIV diagnosis, it did examine the next two. Importantly, community-based interventions aim to conveniently deliver a package of essential ART care functions that extend beyond the clinic into the community such as ART refills, monitoring of treatment adherence and outcomes, and detection of sick patients linked to rapid referral to care. This, in turn, frees up capacity within the clinic-based medical workforce to be able to focus on complicated tasks such as clinical care for sick patients, training and supervision of lay health care workers, and management of health care services. Of note, task shifting is somewhat limited in selected LMICs (e.g., Brazil, Argentina, Mexico, and Puerto Rico), because a physician always needs to be present, at the very least to sign off every single prescription. Second, community-based ART delivery and adherence monitoring and support models for clinically stable patients with documented virologic suppression hold the potential of enabling countries to build sustainable, cost-effective, and equitable HIV care for populations in countries with a scarce health care workforce. Indeed, a cost-effectiveness study by Marseille and colleagues concluded that a home-based ART program in rural Africa may be more cost-effective than most previous estimates for facility-based ART programs [74]. Only three cohort studies involved children [46, 49•, 52•]. The outcomes reported by these studies were virologic suppression, mortality, and loss to follow-up, and all of these were not different from what was obtainable in the adult population. These studies were conducted in South Africa and Uganda.
Surprisingly, we found only two eligible studies to inform cost or cost-effectiveness outcomes. Clearly, more research using economic outcomes is needed. Available data suggest that community-based ART services even if they are equivalent to, but not superior to clinic-based programs, may be more cost-effective from a societal perspective because personnel, operational, and utility costs are likely to be lower, and transportation costs for patients will also be lower; these facts, added to the increased effectiveness in terms of retention, are likely to make community-based ART much more cost-effective and sustainable in the long run. In addition, as mentioned, community-based approaches also make use of community health workers, and an overall community health model that will enable a transformation of the health system from the current vertical siloes to a more integrated approach where community-based HIV care may be further combined with care for other chronic conditions, including non-communicable diseases such as cardiovascular and metabolic diseases which are becoming more prevalent in LMICs as these countries experience the epidemiological and demographic transitions.
Our study has several strengths. We performed a comprehensive search of several databases and sources to identify eligible cohorts and RCTs with the latter providing the highest quality of evidence. Two authors independently evaluated each study for inclusion and data extraction. Regarding limitations, inclusion of cohort study designs may bias the overall estimate of effects due to unmeasured confounding not adjusted for in multivariate analyses. Indeed, the fact that we are observing a difference between RCTs and observational studies for the ART adherence outcome may reflect that in many if not all of these community-based interventions, the patients who end up in the intervention, if it is not randomized, are likely to be quite a bit different—selected somehow—for stability even if not measured. However, in the context of implementation science, observational studies often provide strong signals of important direction of effect. Also, as mentioned earlier, facility-based treatment engagement may have been underestimated since such outcome does not account for silent transfers, and which therefore may not completely capture retention in care [68]. Finally, with only 11 RCTs, we may be underpowered to show superiority of either type interventions.
In summary, community- versus facility-based models of ART delivery resulted in at least comparable outcomes for clinically stable HIV-infected patients on treatment in LMICs and are likely to be cost-effective. As ART rollout expands in LMICs, health systems need to continually adjust to accommodate further expansion. Community-based ART delivery for stable patients hold the promise of enabling countries to build sustainable, cost- effective, and equitable HIV care for populations in settings with a scarce health workforce. Further research with well-powered studies may be needed to further explore effectiveness and cost-effectiveness of such community-based ART programs, particularly in under-represented patient groups such as HIV-infected children, adolescents, and pregnant women to sustain optimal outcomes.
Supplementary Material
Acknowledgments
We thank Drs. Badara Samb and Martina Brostrom from the Joint United Nations Programme on HIV/AIDS (UNAIDS), Geneva, Switzerland, for their critical review and advises on this manuscript. Dr. Nachega receives research grant support from the National Institutes of Health/National Institutes for Allergy and Infectious Disease, the AIDS Clinical Trial Group (ACTG)/Stellenbosch University Clinical Trial Unit (2UM1AI069521-08); the US President Emergency Plan for AIDS Relief (PEPFAR; T84HA21652-01 −00) for Medical Education Partnership Initiative; the Stellenbosch University Collaborative Capacity Enhancement through Engagement with Districts (SUCCEED; 1 U2GGH001536-01); and the Wellcome Trust Southern Africa Consortium for Research Excellence (WT087537MA). Dr. Altice is funded by the National Institutes on Drug Abuse for Research (R01-DA 10186; R01-DA 13805; R01-DA 017072). Dr. Uthman acknowledges support from the FAS Marie Curie Internationa] PostDoc (2012-0064).
Opinions expressed in the present manuscript are solely from authors and not from NIH, PEPFAR, WELLCOME TRUST, WHO, or UNAIDS.
Jean B. Nachega receives research grant support from the National Institutes of Health/National Institutes for Allergy and Infectious Disease, the AIDS Clinical Trial Group (ACTG)/Stellenbosch University Clinical Trial Unit (2UM1AI069521-08); the US President Emergency Plan for AIDS Relief (PEPFAR; T84HA21652-01 −00) for Medical Education Partnership Initiative; the Stellenbosch University Collaborative Capacity Enhancement through Engagement with Districts (SUCCEED; 1 U2GGH001536-01); and the Wellcome Trust Southern Africa Consortium for Research Excellence (WT087537MA).
Olalekan A. Uthman acknowledges support from the FAS Marie Curie International PostDoc (2012-0064).
Frederick L. Altice is funded by the National Institutes on Drug Abuse for Research (R01-DA 10186; R01-DA 13805; R01-DA 017072).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/sl 1904-016-0325-9) contains supplementary material, which is available to authorized users.
Authors’ Contributions JBN and EJM conceived the review. JBN, KP, and OA drafted the protocol. OA and JBN conducted eligibility of the searches and researched the data. JBN, OA, OAU, and AWK drafted the manuscript. The paper was revised critically for intellectual content by all the co-authors and gave final approval for publication.
Compliance with Ethical Standards
Conflict of Interest
Olatunji Adetokunboh, Amy W. Knowlton, Mauro Schechter, Omar Gálarraga, Elvin Geng, Karl Peltzer, Larry W. Chang, Gilles Van Cutsem, Shabbar S. Jaffar, Nathan Ford, Claude A. Mellins, Robert H. Remien, and Edward J. Mills declare that they have no conflict of interest
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS) Report on the global AIDS epidemic 2015. Geneva, Switzerland: UNAIDS; 2015. [Google Scholar]
- 2.Ford N, Mills EJ. Simplified ART delivery models are needed for the next phase of scale up. PLoS Med. 2011;8:el001060. doi: 10.1371/journal.pmed.1001060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joint United Nations Programme on HIV/AIDS (UNAIDS) 90-90-90: an ambitious treatment target to help end the aids epidemic. Geneva, Switzerland: UNAIDS; 2014. [Google Scholar]
- 4. World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Geneva, Switzerland: WHO; [Accessed March 3, 2016]. p. 2015. [URL: http://apps.who.int/iris/bitstream/l0665/186275/l/9789241509565_eng.pdf. Latest WHO Consolidated ART Guidelines
- 5.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52:793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Micek MA, Gimbel-Sherr K, Baptista AJ, Matediana E, Montoya P, Pfeiffer J, et al. Loss to follow-up of adults in public HIV care systems in central Mozambique: identifying obstacles to treatment. J Acquir Immune Defic Syndr. 2009;52:397–105. doi: 10.1097/QAI.0b013e3181ab73e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nachega JB, Uthman OA, del Rio C, Mugavero MJ, Rees H, Mills EJ. Addressing the Achilles’ heel in the HIV care continuum for the success of a test-and-treat strategy to achieve an AIDS-free generation. Clin Infect Dis. 2014;(59 Suppl 1):S21–S27. doi: 10.1093/cid/ciu299. Paper addressisng progress, challenges and approaches to address the leaking global HIV treatment cascade including in key populations (youth, pregnancy, men-who-have sex with men, drug users, etc).
- 8.Supervie V, Costagliola D. The spectrum of engagement in HIV care in France: strengths and gaps [Abstract #: 1030]; 20th Conference on Retroviruses and Opportunistic Infections. Edited by; Atlanta, GA. 2013. [Google Scholar]
- 9. Fox MP, Rosen S. Retention of adult patients on antiretroviral therapy in low- and middle-income countries: systematic review and meta-analysis 2008–2013. J Acquir Immune Defic Syndr. 2015;69:98–108. doi: 10.1097/QAI.0000000000000553. State-of-the ART review on retention in HIV care in LMICs.
- 10.Adetokunboh O, Oluwasanu M. Eliminating mother-to-child transmission of the human immunodeficiency virus in sub-Saharan Africa: the journey so far and what remains to be done. J Infect Pub Health. 2016;9(4):396–407. doi: 10.1016/j.jiph.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Brown AE, Gill ON, Delpech VC. HIV treatment as prevention among men who have sex with men in the UK: is transmission controlled by universal access to HIV treatment and care? HIV Med. 2013;14:563–570. doi: 10.1111/hiv.12066. [DOI] [PubMed] [Google Scholar]
- 12.Delpech VC, Brown AE, Conti S, Polavarapu V, Yin Z. Reducing onward transmission: viral suppression among key population groups living with HIV in the United Kingdom [Abstract 018]; 19th Annual Conference of the British HIV Association. Edited by 19th Annual Conference of the British HIV Association; Manchester, UK. 2013. [Google Scholar]
- 13.Kim SH, Gerver SM, Fidler S, Ward H. Adherence to antiretroviral therapy in adolescents living with HIV: systematic review and meta-analysis. Aids. 2014;28:1945–1956. doi: 10.1097/QAD.0000000000000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nachega JB, Hislop M, Nguyen H, Dowdy DW, Chaisson RE, Regensberg L, et al. Antiretroviral therapy adherence, virologic and immunologic outcomes in adolescents compared with adults in southern Africa. J Acquir Immune Defic Syndr. 2009;51:65–71. doi: 10.1097/QAI.0b013e318199072e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nachega JB, Uthman OA, Anderson J, Peltzer K, Wampold S, Cotton MF, et al. Adherence to antiretroviral therapy during and after pregnancy in low-income, middle-income, and high-income countries: a systematic review and meta-analysis. Aids. 2012;26:2039–2052. doi: 10.1097/QAD.0b013e328359590f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bedelu M, Ford N, Hilderbrand K, Reuter H. Implementing antiretroviral therapy in rural communities: the Lusikisiki model of decentralized HIV/AIDS care. J Infect Dis. 2007;(196 Suppl 3):S464–S458. doi: 10.1086/521114. [DOI] [PubMed] [Google Scholar]
- 17.Bemelmans M, van den Akker T, Ford N, Philips M, Zachariah R, Harries A, et al. Providing universal access to antiretroviral therapy in Thyolo, Malawi through task shifting and decentralization of HIV/AIDS care. Trop Med Int Health. 2010;15:1413–1420. doi: 10.1111/j.1365-3156.2010.02649.x. [DOI] [PubMed] [Google Scholar]
- 18.Cohen R, Lynch S, Bygrave H, Eggers E, Vlahakis N, Hilderbrand K, et al. Antiretroviral treatment outcomes from a nurse-driven, community-supported HIV/AIDS treatment programme in rural Lesotho: observational cohort assessment at two years. J Int AIDS Soc. 2009;12:23. doi: 10.1186/1758-2652-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Decroo T, Telfer B, Biot M, Maikere J, Dezembro S, Cumba LI, et al. Distribution of antiretroviral treatment through self-forming groups of patients in Tete Province, Mozambique. J Acquir Immune Defic Syndr. 2011;56:e39–e44. doi: 10.1097/QAI.0b013e3182055138. [DOI] [PubMed] [Google Scholar]
- 20.Chang LW, Alamo S, Guma S, Christopher J, Suntoke T, Omasete R, et al. Two-year virologic outcomes of an alternative AIDS care model: evaluation of a peer health worker and nurse-staffed community-based program in Uganda. J Acquir Immune Defic Syndr. 2009;50:276–282. doi: 10.1097/QAI.0b013e3181988375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang LW, Kagaayi J, Nakigozi G, Ssempijja V, Packer AH, Serwadda D, et al. Effect of peer health workers on AIDS care in Rakai, Uganda: a cluster-randomized trial. PLoS One. 2010;5:e10923. doi: 10.1371/journal.pone.0010923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang LW, Njie-Carr V, Kalenge S, Kelly JF, Bollinger RC, Alamo-Talisuna S. Perceptions and acceptability of mHealth interventions for improving patient care at a community-based HIV/ AIDS clinic in Uganda: a mixed methods study. AIDS Care. 2013;25:874–880. doi: 10.1080/09540121.2013.774315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coetzee D, Boulle A, Hildebrand K, Asselman V, Van Cutsem G, Goemaere E. Promoting adherence to antiretroviral therapy: the experience from a primary care setting in Khayelitsha, South Africa. Aids. 2004;(18 Suppl 3):S27–S31. doi: 10.1097/00002030-200406003-00006. [DOI] [PubMed] [Google Scholar]
- 24.Duwell MM, Knowlton AR, Nachega JB, Efron A, Goliath R, Morroni C, et al. Patient-nominated, community-based HIV treatment supporters: patient perspectives, feasibility, challenges, and factors for success in HIV-infected South African adults. AIDS Patient Care STDS. 2013;27:96–102. doi: 10.1089/apc.2012.0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaffar S, Amuron B, Foster S, Birungi J, Levin J, Namara G, et al. Rates of virological failure in patients treated in a home-based versus a facility-based HIV-care model in Jinja, southeast Uganda: a cluster-randomised equivalence trial. Lancet. 2009;374:2080–2089. doi: 10.1016/S0140-6736(09)61674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kunutsor S, Walley J, Katabira E, Muchuro S, Balidawa H, Namagala E, et al. Improving clinic attendance and adherence to antiretroviral therapy through a treatment supporter intervention in Uganda: a randomized controlled trial. AIDS Behav. 2011;15:1795–1802. doi: 10.1007/s10461-011-9927-9. [DOI] [PubMed] [Google Scholar]
- 27.Luque-Fernandez MA, Van Cutsem G, Goemaere E, Hilderbrand K, Schomaker M, Mantangana N, et al. Effectiveness of patient adherence groups as a model of care for stable patients on antiretroviral therapy in Khayelitsha, Cape Town, South Africa. PLoS One. 2013;8:e56088. doi: 10.1371/journal.pone.0056088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mills EJ, Lester R, Thorlund K, Lorenzi M, Muldoon K, Kanters S, et al. Interventions to promote adherence to antiretroviral therapy in Africa: a network meta-analysis. Lancet HIV. 2014;1:e104–e111. doi: 10.1016/S2352-3018(14)00003-4. State-of-the ART network meta-analysis providing evidence on which ART adherence intervention work in Africa and their relative effectiveness to each others.
- 29.Nachega JB, Chaisson RE, Goliath R, Efron A, Chaudhary MA, Ram M, et al. Randomized controlled trial of trained patient-nominated treatment supporters providing partial directly observed antiretroviral therapy. Aids. 2010;24:1273–1280. doi: 10.1097/QAD.0b013e328339e20e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakigozi G, Makumbi FE, Bwanika JB, Atuyambe L, Reynolds SJ, Kigozi G, et al. Impact of patient-selected care buddies on adherence to hiv care, disease progression, and conduct of daily life among pre-antiretroviral HIV-infected patients in Rakai, Uganda: a randomized controlled trial. J Acquir Immune Defic Syndr. 2015;70:75–82. doi: 10.1097/QAI.0000000000000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Remien RH, Mellins CA, Robbins RN, Kelsey R, Rowe J, Warne P, et al. Masivukeni: development of a multimedia based antiretroviral therapy adherence intervention for counselors and patients in South Africa. AIDS Behav. 2013;17:1979–1991. doi: 10.1007/s10461-013-0438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robbins RN, Mellins CA, Leu CS, Rowe J, Warne P, Abrams EJ, et al. Enhancing lay counselor capacity to improve patient outcomes with multimedia technology. AIDS Behav. 2015;(19 Suppl 2):163–176. doi: 10.1007/s10461-014-0988-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang LW, Kagaayi J, Nakigozi G, Serwada D, Quinn TC, Gray RH, et al. Cost analyses of peer health worker and mHealth support interventions for improving AIDS care in Rakai, Uganda. AIDS Care. 2013;25:652–656. doi: 10.1080/09540121.2012.722600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson MA, Mugavero MJ, Amico KR, Cargill VA, Chang LW, Gross R, et al. Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: evidence-based recommendations from an International Association of Physicians in AIDS Care panel. Ann Intern Med. 2012;156:817–33. doi: 10.7326/0003-4819-156-11-201206050-00419. w-284, w-285, w-286, w-287, w-288, w-289, w-290, w-291, w-292, w-293, w-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vandenbroucke JP, von Elm E, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12:1500–1524. doi: 10.1016/j.ijsu.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 36.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011] London: The Cochrane Collaboration; 2011. [Google Scholar]
- 37.Centre TNC. Review manager (RevMan) [Computer program], version 5.3. Copenhagen: The Cochrane Collaboration; 2014. [Google Scholar]
- 38.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiweewa FM, Wabwire D, Nakibuuka J, Mubiru M, Bagenda D, Musoke P, et al. Noninferiority of a task-shifting HIV care and treatment model using peer counselors and nurses among Ugandan women initiated on ART: evidence from a randomized trial. J Acquir Immune Defic Syndr. 2013;63:e125–e132. doi: 10.1097/QAI.0b013e3182987ce6. [DOI] [PubMed] [Google Scholar]
- 40. Mfinanga S, Chanda D, Kivuyo SL, Guinness L, Bottomley C, Simms V, et al. Cryptococcal meningitis screening and community-based early adherence support in people with advanced HIV infection starting antiretroviral therapy in Tanzania and Zambia: an open-label, randomised controlled trial. Lancet. 2015;385:2173–2182. doi: 10.1016/S0140-6736(15)60164-7. Important paper reporting results of a community-based screening and pre-emptive treatment program for cryptococcal infection combined with a short initial period of adherence support after initiation of ART, substantially reduced mortality in Africa.
- 41.Selke HM, Kimaiyo S, Sidle JE, Vedanthan R, Tierney WM, Shen C, et al. Task-shifting of antiretroviral delivery from health care workers to persons living with HIV/AIDS: clinical outcomes of a community-based program in Kenya. J Acquir Immune Defic Syndr. 2010;55:483–490. doi: 10.1097/QAI.0b013e3181eb5edb. [DOI] [PubMed] [Google Scholar]
- 42.Taiwo BO, Idoko JA, Welty LJ, Otoh I, Job G, Iyaji PG, et al. Assessing the viorologic and adherence benefits of patient-selected HIV treatment partners in a resource-limited setting. J Acquir Immune Defic Syndr. 2010;54:85–92. doi: 10.1097/01.qai.0000371678.25873.1c. [DOI] [PubMed] [Google Scholar]
- 43.Coker M, Etiebet MA, Chang H, Awwal G, Jumare J, Musa BM, et al. Socio-demographic and adherence factors associated with viral load suppression in HIV-infected adults initiating therapy in northern Nigeria: a randomized controlled trial of a peer support intervention. Curr HIV Res. 2015;13:279–285. doi: 10.2174/1570162x13666150407143838. [DOI] [PubMed] [Google Scholar]
- 44.Gross R, Zheng L, La Rosa A, Sun X, Rosenkranz SL, Cardoso SW, et al. Partner-based adherence intervention for second-line antiretroviral therapy (ACTG A5234): a multinational randomised trial. Lancet HIV. 2015;2:e12–e19. doi: 10.1016/S2352-3018(14)00007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fatti G, Meintjes G, Shea J, Eley B, Grimwood A. Improved survival and antiretroviral treatment outcomes in adults receiving community-based adherence support: 5-year results from a multicentre cohort study in South Africa. J Acquir Immune Defic Syndr. 2012;61:e50–e58. doi: 10.1097/QAI.0b013e31826a6aee. [DOI] [PubMed] [Google Scholar]
- 46.Fatti G, Shaikh N, Eley B, Grimwood A. Improved virological suppression in children on antiretroviral treatment receiving community-based adherence support: a multicentre cohort study from South Africa. AIDS Care. 2014;26:448–453. doi: 10.1080/09540121.2013.855699. [DOI] [PubMed] [Google Scholar]
- 47.Franke MF, Kaigamba F, Socci AR, Hakizamungu M, Patel A, Bagiruwigize E, et al. Improved retention associated with community-based accompaniment for antiretroviral therapy delivery in rural Rwanda. Clin Infect Dis. 2013;56:1319–1326. doi: 10.1093/cid/cis1193. [DOI] [PubMed] [Google Scholar]
- 48.Grimsrud A, Lesosky M, Kalombo C, Bekker LG, Myer L. Implementation and operational research: community-based adherence clubs for the management of stable antiretroviral therapy patients in Cape Town, South Africa: a cohort study. J Acquir Immune Defic Syndr. 2016;71:e16–e23. doi: 10.1097/QAI.0000000000000863. [DOI] [PubMed] [Google Scholar]
- 49. Grimwood A, Fatti G, Mothibi E, Malahlela M, Shea J, Eley B. Community adherence support improves programme retention in children on antiretroviral treatment: a multicentre cohort study in South Africa. J Int AIDS Soc. 2012;15:17381. doi: 10.7448/IAS.15.2.17381. Well conducted retrospective cohort study in Cape Town, South Africa showing that community-based adherence clubs were associated with high retention in care compare to facility-based care, even after adjusting for patient and clinics characteristics.
- 50.Johnston V, Fielding K, Charalambous S, Mampho M, Churchyard G, Phillips A, et al. Second-line antiretroviral therapy in a workplace and community-based treatment programme in South Africa: determinants of virological outcome. PLoS One. 2012;7:e36997. doi: 10.1371/journal.pone.0036997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kipp W, Konde-Lule J, Rubaale T, Okech-Ojony J, Alibhai A, Saunders DL. Comparing antiretroviral treatment outcomes between a prospective community-based and hospital-based cohort of HIV patients in rural Uganda. BMC Int Health Hum Rights. 2011;11(Suppl 2):S12. doi: 10.1186/1472-698X-11-S2-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Massavon W, Barlow-Mosha L, Mugenyi L, McFarland W, Gray G, Lundin R, et al. Factors determining survival and retention among hiv-infected children and adolescents in a community home-based care and a facility-based family-centred approach in Kampala, Uganda: a cohort study. Isrn Aids. 2014;2014:852489. doi: 10.1155/2014/852489. Well conducted retrospective cohort study in Uganda showing that retention in care was higher in the community-based care: 94.8 % versus 84.7 % in the facility-based care.
- 53.Kaihin R, Kasatpibal N, Chitreechuer J, Grimes RM. Effect of an empowerment intervention on antiretroviral drug adherence in Thai Youth. Behav Med. 2015;41:186–194. doi: 10.1080/08964289.2014.911717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Munoz M, Bayona J, Sanchez E, Arevalo J, Sebastian JL, Arteaga F, et al. Matching social support to individual needs: a community-based intervention to improve HIV treatment adherence in a resource-poor setting. AIDS Behav. 2011;15:1454–1464. doi: 10.1007/s10461-010-9697-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bussmann H, Wester CW, Thomas A, Novitsky V, Okezie R, Muzenda T, et al. Response to zidovudine/didanosine-containing combination antiretroviral therapy among HIV-1 subtype C-infected adults in Botswana: two-year outcomes from a randomized clinical trial. J Acquir Immune Defic Syndr. 2009;51:37–46. doi: 10.1097/QAI.0b013e31819ff102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Henderson KC, Hindman J, Johnson SC, Valuck RJ, Kiser JJ. Assessing the effectiveness of pharmacy-based adherence interventions on antiretroviral adherence in persons with HIV. AIDS Patient Care STDS. 2011;25:221–228. doi: 10.1089/apc.2010.0324. [DOI] [PubMed] [Google Scholar]
- 57.Peltzer K, Ramlagan S, Jones D, Weiss SM, Fomundam H, Chanetsa L. Efficacy of a lay health worker led group antiretroviral medication adherence training among non-adherent HIV-positive patients in KwaZulu-Natal, South Africa: results from a randomized trial. Sahara J. 2012;9:218–226. doi: 10.1080/17290376.2012.745640. [DOI] [PubMed] [Google Scholar]
- 58.Mermin J, Ekwaru JP, Were W, Degerman R, Bunnell R, Kaharuza F, et al. Utility of routine viral load, CD4 cell count, and clinical monitoring among adults with HIV receiving antiretroviral therapy in Uganda: randomised trial. BMJ. 2011;343:d6792. doi: 10.1136/bmj.d6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mermin J, Were W, Ekwaru JP, Moore D, Downing R, Behumbiize P, et al. Mortality in HIV-infected Ugandan adults receiving antiretroviral treatment and survival of their HIV-uninfected children: a prospective cohort study. Lancet. 2008;371:752–759. doi: 10.1016/S0140-6736(08)60345-1. [DOI] [PubMed] [Google Scholar]
- 60.Moore DM, Yiannoutsos CT, Musick BS, Tappero J, Degerman R, Campbell J, et al. Determinants of early and late mortality among HIV-infected individuals receiving home-based antiretroviral therapy in rural Uganda. J Acquir Immune Defic Syndr. 2011;58:289–296. doi: 10.1097/QAI.0b013e3182303716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weidle PJ, Wamai N, Solberg P, Liechty C, Sendagala S, Were W, et al. Adherence to antiretroviral therapy in a home-based AIDS care programme in rural Uganda. Lancet. 2006;368:1587–1594. doi: 10.1016/S0140-6736(06)69118-6. [DOI] [PubMed] [Google Scholar]
- 62.Wools-Kaloustian KK, Sidle JE, Selke HM, Vedanthan R, Kemboi EK, Boit LJ, et al. A model for extending antiretroviral care beyond the rural health centre. J Int AIDS Soc. 2009;12:22. doi: 10.1186/1758-2652-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Decroo T, Van Damme W, Kegels G, Remartinez D, Rasschaert F. Are expert patients an untapped resource for art provision in Sub-Saharan Africa? AIDS Res Treat. 2012;2012:749718. doi: 10.1155/2012/749718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Achieng L, Musangi H, Ong’uti S, Ombegoh E, Bryant L, Mwiindi J, et al. An observational cohort comparison of facilitators of retention in care and adherence to anti-eetroviral therapy at an HIV treatment center in Kenya. PLoS One. 2012;7:e32727. doi: 10.1371/journal.pone.0032727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Amuron B, Coutinho A, Grosskurth H, Nabiryo C, Birungi J, Namara G, et al. A cluster-randomised trial to compare home-based with health facility-based antiretroviral treatment in Uganda: study design and baseline findings. Open AIDS J. 2007;1:21–27. doi: 10.2174/1874613600701010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bemelmans M, Baert S, Goemaere E, Wilkinson L, Vandendyck M, van Cutsem G, et al. Community-supported models of care for people on HIV treatment in sub-Saharan Africa. Trop Med Int Health. 2014;19:968–977. doi: 10.1111/tmi.12332. [DOI] [PubMed] [Google Scholar]
- 67.Bango F, Ashmore J, Wilkinson L, van Cutsem G, Cleary S. Adherence clubs for long-term provision of antiretroviral therapy: cost-effectiveness and access analysis from Khayelitsha, South Africa. Trop Med Int Health. 2016 Jun 14; doi: 10.1111/tmi.12736. [DOI] [PubMed] [Google Scholar]
- 68.Geng EH, Emenyonu N, Bwana MB, Glidden DV, Martin JN. Sampling-based approach to determining outcomes of patients lost to follow-up in antiretroviral therapy scale-up programs in Africa. JAMA. 2008;300:506–507. doi: 10.1001/jama.300.5.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kredo T, Ford N, Adeniyi FB, Garner P. Decentralising HIV treatment in lower- and middle-income countries. Cochrane Datab Syst Rev. 2013;6:CD009987. doi: 10.1002/14651858.CD009987.pub2. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chishinga N, Godfrey-Faussett P, Fielding K, Ayles H. Effect of home-based interventions on virologic outcomes in adults receiving antiretroviral therapy in Africa: a meta-analysis. BMC Public Health. 2014;14:239. doi: 10.1186/1471-2458-14-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Decroo T, Rasschaert F, Telfer B, Remartinez D, Laga M, Ford N. Community-based antiretroviral therapy programs can overcome barriers to retention of patients and decongest health services in sub-Saharan Africa: a systematic review. Int Health. 2013;5:169–179. doi: 10.1093/inthealth/iht016. [DOI] [PubMed] [Google Scholar]
- 72.Apondi R, Bunnell R, Awor A, Wamai N, Bikaako-Kajura W, Solberg P, et al. Home-based antiretroviral care is associated with positive social outcomes in a prospective cohort in Uganda. J Acquir Immune Defic Syndr. 2007;44:71–76. doi: 10.1097/01.qai.0000243113.29412.dd. [DOI] [PubMed] [Google Scholar]
- 73.Wouters E, Van Damme W, van Rensburg D, Masquillier C, Meulemans H. Impact of community-based support services on antiretroviral treatment programme delivery and outcomes in resource-limited countries: a synthetic review. BMC Health Serv Res. 2012;12:194. doi: 10.1186/1472-6963-12-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marseille E, Kahn JG, Pitter C, Bunnell R, Epalatai W, Jawe E, et al. The cost effectiveness of home-based provision of antiretroviral therapy in rural Uganda. Appl Health Econ Health Policy. 2009;7:229–243. doi: 10.2165/11318740-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.