Abstract
Exposure to xenoestrogens is a probable cause of male infertility in humans. Consumption of high-fat diets and exposure to bisphenol A (BPA) is pervasive in America. Here, we test the hypothesis that gestational exposure to high dietary fats and/or BPA disrupt spermatogenesis in adulthood. Sprague-Dawley rats were fed diets containing 10 kcal% butter fat (AIN), 39 kcal% butter fat (HFB), or 39 kcal% olive oil (HFO), with or without BPA (25 μg/kg body weight/day) during pregnancy. One group of male offspring received testosterone (T)- and estradiol-17β (E2)-filled implants or sham-implants from postnatal day (PND)70-210. Another group was naturally aged to 18 months. We found that adult males with gestational exposure to BPA, HFB, or HFB + BPA, in both the aged group and the T + E2-implanted group, exhibited impairment of spermatogenesis. In contrast, gestational exposure to HFO or HFO + BPA did not affect spermatogenesis. Sham-implanted, gestational exposed groups also had normal spermatogenesis. Loss of ERα expression in round spermatids and premature expression of protamine-1 in diplotene spermatocytes were features associated with impaired spermatogenesis. Compared with the single-treatment groups, the HFB + BPA group experienced more severe effects, including atrophy.
Keywords: Testis, Endocrine disrupting chemicals, High fat butter, High fat olive oil, Bisphenol A
1. Introduction
The adverse health effects of endocrine-disrupting chemicals are extensive. One agent of public health concern is bisphenol A (BPA), a leachable monomer employed as a crosslinker to polymerize polycarbonate plastics. It is found in food can liners, plastic containers, and bottles, and is released over time due to breakdown of chemical bonds [1]. BPA exposure has been found to correlate with increased risk of cardiovascular disease, obesity, diabetes, immune disorders, cancer, and a host of reproductive dysfunctions [2–5]. The action of BPA is in part mediated by epigenetic reprogramming of gene expression in these tissues [6,7].
It has been reported that early life exposure to BPA results in aberrant testicular function in adulthood [8–11]. The reported changes include decreased daily sperm production [2,8], inhibition of testicular steroidogenesis [9], increased testis weights [10], disturbed spermatogenesis [12], and reduced levels of testicular meiotic recombination [11]. BPA exposures h ave also been linked to decreased fertility in wildlife via disrupted spermatogenesis [13]. However, significant disparity in findings and conclusions have occurred in animal studies with fairly similar designs. For example, in some studies, neonatal and maternal BPA exposure was shown to have negative effects on fertility of male rat offspring [12,14]. However, other labs were unable to repeat these results [15,16]. One factor that may contribute to the discrepancy in response to BPA is the amount and type of dietary fat ingested during pregnancy by the dams, a research question that has not been previously explored.
A newly published epidemiological study at an infertility clinic found that a high intake of saturated fat was negatively correlated with sperm concentration [17]. High dietary intake of saturated fat was also found to be associated with reduced semen quality in a Danish study encompassing 701 young Danish men from the general population [18]. However, experimental data supporting a possible influence of maternal dietary fatty acids on spermatogenesis of offspring and the impact of diet on response to toxicants are virtually non-existent.
Aging is associated with a decline in spermatogenesis and coincides with an increase in estradiol-17β (E2) to testosterone (T) ratio in human males [19]. In Sprague-Dawley (SD) rats, the testicular E2 to T ratio is highest at 18 months of age [20], coinciding with declining sperm production. Other contributors to reduced spermatogenesis include exposure to xeno/phyto-estrogens, obesity, inflammatory cytokines, poor dietary choices, and reactive toxins [21–24], all of which contribute to stress, and also increase E2 levels. Here, we have employed two models to investigate the effects of maternal exposure to BPA and high fat diets on spermatogenesis in offspring (1) a hormone treatment model in which male rats are exposed to T + E2 at postnatal day (PND)70-210 to mimic an accelerated aging process and (2) a natural aging model, where rats are sacrificed at 18 months (PND 540).
Our objectives were to determine (a) whether gestational exposure to different types of high dietary fats affects spermatogenesis in adulthood, and (b) if a maternal high-fat diet alters the response to in utero exposure to BPA. We found that adult Taconic outbred SD male rats exposed in utero to just BPA or high-fat butter (HFB) or high-fat olive oil (HFO) plus/minus BPA exhibited qualitatively normal testes, if no T + E2 implants were given in adult life. When hormonally treated, adult males exposed in utero to HFB, BPA, or HFB + BPA exhibited impaired spermatogenesis within the seminiferous tubules (STs) of the testis. Naturally aged animals showed similar impairments. However, gestational HFO or HFO + BPA diet had no adverse effects on spermatogenesis following T + E2 implantation.
2. Materials and methods
2.1. Animals and diets
The animal usage and care protocols were approved by the Institutional Animal Care Committee at the University of Cincinnati, in compliance with NIH guidelines. Female SD rats were housed in a BPA-free environment [6] at the University's animal facility on a 12-h light/12-h dark cycle, as established by Dr. Belcher [25]. Female dams were housed in polycarbonate-free cages with ad libitum access to diet and BPA-free water. Females were fed a modified open standard diet prior to mating, hereafter referred to as AIN (Product #D04042310 AIN 93G, Research Diets, Inc. New Brunswick, NJ, 10 kcal% butter fat), which is certified to contain no phytoestrogens. The test compounds and fats from butter and olive oil were directly incorporated (Research Diets, Inc. New Brunswick, NJ) into the unsupplemented (AIN) pellet diets to give 39 kcal% fat from butter (HFB) or olive oil (HFO), plus or minus BPA or 17α-ethinyl estradiol EE2 (0.5 μg/kg bodyweight per day (Kg bw-d)). Food intake was measured daily in preliminary experiments to allowing the diet to be formulated to deliver the intended exposure. After 1 week on the assigned diet, 8-week-old virgin females were housed with males and were impregnated within 1–7 days. Males were briefly exposed to diets containing BPA during the mating period.
2.2. Dose-response study
This initial pilot study was undertaken to determine the minimal BPA dose that impedes spermatogenesis in the presence of HFB diet. Maternal diets consisting of HFB plus four BPA doses (2500 μg/kg bw-d, 250 μg/kg bw-d, 25 μg/kg bw-d and 2.5 μg/kg bw-d) was designed to fall below the LOAEL (50 mg/kg bw-d). The high doses (2.5 and 250 μg/kg bw-d) are 20 and 10 times below this LOAEL dose, and the other BPA concentrations are below the reference dose (NOAEL 50 μg/kg bw-d) at 2.5 and 25 μg/kg bw-d [26,27]. Diets containing EE2 was estimated to be at the clinically relevant previously reported dose in rat (0.5 μg/kg bw-d) [28,29]. Approximately 1 week prior to mating, SD females were assigned to one of seven diet groups (AIN, HFB, HFB + BPA 2.5, HFB + BPA 25, HFB + BPA 250, HFB + BPA 2500 or EE2) and fed AIN, HFB, EE2, or HFB plus four BPA doses (2500 μg/kg bw-d, 250 μg/kg bw-d, 25 μg/kg bw-d, and 2.5 μg/kg bw-d) before and during pregnancy (Data in Brief [30], Fig. 1A). All diets were switched to AIN after the pups were born. The number of young per litter was restricted to 4 males and 4 females during lactation. Male pups were transferred to the normal (non-BPA-free) environment. At PND 70, prenatally exposed pups from each diet group (Data in Brief [30], Fig. 1A) were treated with T + E2 via Silastic™ implants [6,31] (sex hormone-induced SD rat model) for 20 weeks (PND70-PND210), at which time the testis was fixed, paraffin embedded, stained with hematoxylin and eosin and tubules examined for spermatogenesis. We found that the sham-implanted, gestational exposed groups exhibited normal spermatogenesis on PND210 (100% offspring showed presence of spermatozoa in >14% of STs). Our results further indicated that in the presence of T + E2, the dose-response to BPA is non-monotonic. BPA 25 μg/kg bw-d was the lowest dose that was effective at inducing impaired spermatogenesis, where a significantly high percentage of seminiferous tubules (STs) possessed round spermatids (Data in Brief [30], Fig. 1B, C) as the final differentiated germ cell population. Hence, we used a dietary dose of BPA 25 μg/kg bw-d for the dietary fat and BPA study described below.
Fig. 1.
Scheme of dietary exposure and groups. Mothers were fed the control AIN diet or the alternate diets during mating and gestation. Maternal diets were changed to control AIN diets after the pups were born. n = number of animals per group, one animal per litter.
2.3. Dietary fat and BPA study
The dietary BPA concentration (25 μg/kg bw-d) we have chosen is quite low. We had previously found that dietary exposure of dams before and during pregnancy to 250 μg/kg bw-d BPA resulted in undetectable levels of free BPA and 2–3 ng/ml of total BPA in maternal blood [32]. The presence of HFB did not change these values. To determine the effects of BPA on different HF diet, females were assigned to one of six diet groups (AIN, BPA, HFB, HFB + BPA, HFO, HFO + BPA) and fed the diets for 1 week before and during pregnancy (Fig. 1A). All diets were switched to AIN after the pups were born. The number of young per litter was restricted to 4 males and 4 females during lactation. Male pups were transferred to the normal (non-BPA-free) environment at PND 21 for endpoint studies mentioned below. To avoid litter effects, male pups within each litter were randomly assigned to the diet groups, and the number of animals in each diet group is counted by litter, i.e. one male offspring per litter.
2.4. Hormone treatment SD rat model
14 male pups per group, 2 per litter were employed in the hormone treatment SD rat model. At 10 weeks of age, one male offspring per litter (7 pups per group) was surgically implanted with hormone-filled via Silastic™ capsules packed with T or E2 (Sigma, St. Louis, MO) as described previously [6,31]. Capsules were replaced after 10 weeks. A second group of control rats (one male offspring per litter) were implanted with empty capsules. At the end of a 20-week treatment, all animals were killed by an overdose of isoflurane followed by decapitation. One testis was fixed in 10% neutral-buffered formalin, embedded in paraffin, and cut into 4- to 6-μm sections. The second testis was frozen in Tissue-Tek O.C.T. compound (Sakura Finetek USA, Torrance, CA). The differences in litter numbers for the various treatment groups reflect that some pups were sacrificed due to difficulty healing post-surgery, or which died at earlier time-points due to unknown causes.
2.5. Natural aging model
One male pup from each litter was employed in the natural aging study (five pups for the AIN, BPA25, HFO, HFB groups and eight pups in the HFO + BPA and HFB + BPA groups). Adult rats are sacrificed at 18 months (PND 540), when E2:T ratio was previously been shown to be highest [20]. However, some pups (2 each for HFB + BPA and HFO group and 1 for HFO + BPA group) either died before PND500 (16.6 months) of unknown causes, or were sacrificed early due to distress, as advised by the veterinarians. Organs were collected, fixed and processed as described above.
2.6. Tissue collection and immunohistochemistry
IHC analyses were performed as previously described [33]. Briefly, tissue sections were deparaffinized at 60 °C (1 h) and antigen retrieval was performed by boiling sections in microwave for 2–5 min in 0.01 M citrate buffer, pH 6.0. Following antigen retrieval, the sections were pre-incubated with 5% normal goat serum in PBS for 30 min. Sections were then incubated overnight at 4 °C with the primary antibody diluted in blocking solution. Antibodies for BRDT (1:400), AR (1:250), CYP19 (1:250), PRM1 (1:50), ERα/ESR1(1:100), ERβ/ESR2 (1:100) and WT-1 (1:300), PLZF (1:200) were purchased from either Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), BioGeneX (Fremont, CA) or Abcam (Cambridge, MA). See Table 1 for antibody information. Visualization was with the avidin–biotin–peroxidase complex with biotinylated anti-rabbit or anti-goat secondary antibodies (1:200) (Vector Laboratories, Inc., Burlingame, CA), followed by treatments with Vectastain Elite ABC kit (Vector) and DAB chromagen (Sigma, St. Louis, MO). 100–400 seminiferous tubules were scored per rat.
Table 1.
List of antibodies used in this study.
| Peptide/protein target | Antigen sequence | Name of Antibody | Manufacturer, catalog number | Species raised in; monoclonal or polyclonal | Dilution |
|---|---|---|---|---|---|
| BRDT | 1–100 | an5157 | Abcam: ab5157 | Rabbit polyclonal | 1:400 |
| AR (androgen receptor) | N-terminus of AR | AR (N-20) | Santa cruz biotech. Inc.sc-816 | Rabbit polyclonal | 1:250 |
| CYP19 (aromatase) | 209–503 | H-300 | Santa cruz biotech. Inc.sc-30086 | Rabbit polyclonal | 1:250 |
| Protamine 1 | 1–51 | M-51 | Santa cruz biotech. Inc.sc-30174 | Rabbit polyclonal | 1:50 |
| ESR1/Estrogen Receptor alpha | around residues 104–106 | E115 | Abcam: ab32063 | Rabbit monoclonal | 1:100 |
| ESR2/Estrogen Receptor beta | 17-mer, close to C-terminus | anti-ESR2 | Biogenex, AR385 | Rabbit polyclonal | 1:100 |
| WT-1 | C-terminus peptide | C-19 | Santa cruz biotech. Inc.sc-192 | Rabbit polyclonal | 1:300 |
| PLZF | 101–400 | D-9 | Santa cruz biotech. Inc.sc-28319 | Mouse monoclonal | 1:200 |
CYP19 stained sections were checked and 120 to 200 STs scored for the last step of differentiated germ cell (spermatogenesis) present within the tubules. For apoptosis assessment, terminal deoxynucleotidyl transferase–mediated dUTP nick end labeling (TUNEL) staining was used with the ApopTag peroxidase in situ apoptosis detection kit (Chemicon International, Temecula, CA). We assessed incidence of tubule atrophy in adult male rats from each of the treatment groups by visual analysis of hematoxylin and eosin sections for each animal. Sections were checked for occurrence of spermatogonia and Sertoli cell only tubules. For determining the Sertoli:germ cell ratio, the total number of Sertoli (AR staining) and germ cells (spermatogonia, spermatocyte and round spermatids) was counted in 25 tubules per animal and ratio determined. The cell volumes were not calculated, since in some cases, the cells were highly dispersed.
2.7. Statistical analysis
For Data in Brief [30], Figs. 1 and 2, and Figs. 4 and 5, significance was analyzed with one-way ANOVA and Dunnett's multiple comparison test using the GraphPad Prism software. For Tables 2 and 3, Fisher's exact test (two-tailed) was used to calculate the ODDS ratio using the GraphPad Prism software.
Fig. 2.
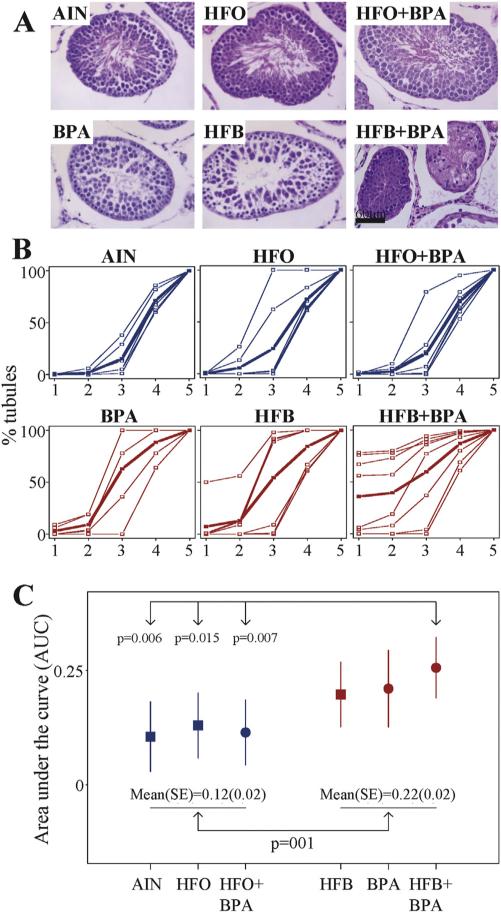
Impaired spermatogenesis in testis of male offspring exposed to various diets in the T + E2 rodent model. (A) Representative tubules illustrating the impaired phenotype observed for each diet group. Offspring prenatally exposed to control AIN diet show all steps of spermatogenesis culminating in spermatozoa. BPA- and HFB-exposed offspring show impaired spermatogenesis, with the last step predominantly consisting of round and elongating spermatids. The HFB + BPA-exposed offspring also show impaired spermatogenesis with atrophic (spermatogonia) tubules. The HFO− and HFO + BPA-exposed offspring show essentially normal spermatogenesis. Bar = 60 μm. (B) Plot of tubules showing cumulative progression of spermatogenesis. STs were scored for spermatogenesis, showing (1) spermatogonia/atrophic tubules (2) spermatocytes (3) round spermatids (4) elongated and condensed spermatids and (5) spermatozoa; as the last differentiated germ cell present within the tubules. Blue and red colored curves are progression of spermatogenesis per subject. The bold curve represents an average among individuals in each group. (C) Comparison of areas under the curve (AUC) as a measure of spermatogenesis among Groups. A solid square and its whiskers represent a mean (95% CI) of AUC in groups without BPA and a solid cycle and its whiskers represent a mean (95% CI) in groups with BPA. The mean of the HFB + BPA group is significantly higher than the AIN, HFO and HFO + BPA groups (* = P<0.05).
Fig. 4.
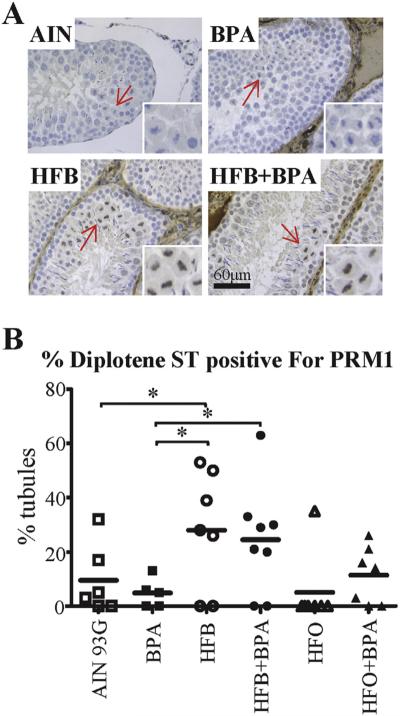
Diplotene spermatocyte (DSp) show expression of PRM1 in HFB- and HFB + BPA-exposed offspring (T + E2 model). (A) Representative pictures illustrating PRM1 expression in the STs of animals exposed in utero to indicated diets. Arrows point to DSp, which are magnified in right bottom of each panel. (B) The testis was scored for number of DSp-positive STs with PRM1 staining in DSps. Each symbol represents a pup from an independent litter. Bar = 60 μm. * p < 0.05, ** p < 0.01, 1-way ANOVA model between groups indicated.
Fig. 5.
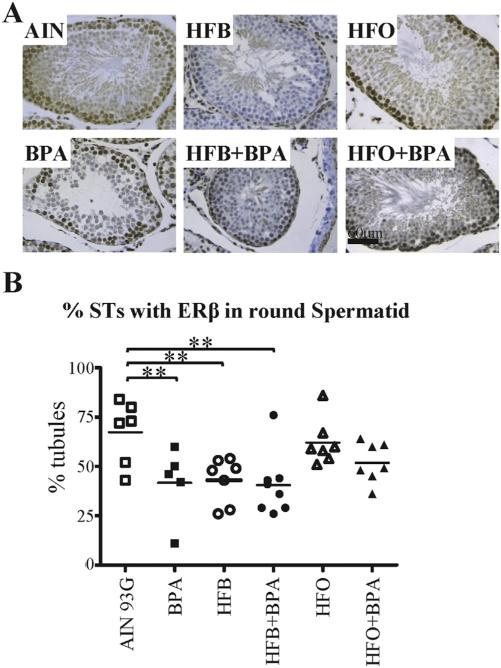
Loss of ERβ expression in round spermatids (RSs) correlates with impaired spermatogenesis in the T + E2 model. (A) Representative pictures illustrating ERβ expression in RSs within STs of animals exposed in utero to indicated diets. (B) The testis was scored for number of STs with ERβ staining in RSs. Each symbol represents a pup from an independent litter. Bar = 60 μm. * p < 0.05, ** p < 0.01, 1-way ANOVA models between groups indicated.
Table 2.
Presence of spermatozoa in ≥14% of STs (T + E2).
| Number animals | % animals (normal) | Odds ratio | P-value | |
|---|---|---|---|---|
| AIN | 6/6 | 100% | ||
| BPA | 2/5 | 40% | 18.2 | 0.061 |
| HFB | 3/7 | 43% | 16.7 | 0.070 |
| HFB + BPA | 3/8 | 37% | 20.4 | 0.031* |
| HFO | 6/7 | 88% | 3 | 1.000 |
| HFO + BPA | 6/7 | 88% | 3 | 1.000 |
p-value from Fisher's exact test (two-tailed) compared with AIN diet.
p < 0.05.
Table 3.
Presence of spermatozoa in ≥20% of STs (aging study).
| Number animals | % animals (normal) | Odds ratio. | P-value | |
|---|---|---|---|---|
| AIN | 5/5 | 100% | ||
| BPA25 | 2/5 | 40% | 15.4 | 0.17 |
| HFB | 2/5 | 40% | 15.4 | 0.17 |
| HFB + BPA | 1/6 | 17% | 40.3 | 0.015* |
| HFO | 2/3 | 67% | 6.6 | 0.375 |
| HFO + BPA | 7/7 | 100% | 1.000 |
p-value from Fisher's exact test compared with AIN diet.
p < 0.05.
For Fig. 2, a spermatogenesis curve was plotted from the scores summed up to specific spermatogenesis progression blocks starting with (1) spermatogonia/atrophic tubules (2) spermatocytes (3) round spermatids (spermatid step 8) (4) condensed spermatids (spermatid step 16) and (5) spermatozoa. The area under the curve (AUC) was considered a measure of overall progression of spermatogenesis. The AUC ranged from 0 to 1 and a higher value indicated a more severe impairment of spermatogenesis.
The numerical measure of overall spermatogenesis was assessed for its associations to the fixed effects of diet (AIN vs. HFB vs. HFO), BPA (no vs. yes) and their interaction using a fixed effect (or two-way ANOVA) model. Post hoc means were estimated from the model and compared between groups. Considering a small sample size was used in each group in this study, such comparisons did not account for any multiple comparison method in analysis. In order to ensure the results were robust and invariant to the statistical methods, several other competing statistical models, such as non-parametric Wilcoxon Rank Sum tests and a cumulative logistical regression model (after using quintiles of the original measure) were used. Since all methods showed similar findings from the analyses, only the results from the primary method of the fixed effect model are presented in this paper. All statistical analyses were computed using SAS 9.4 software (SAS, Cary, NC). P-values < 0.05 were considered statistically significant.
3. Results
3.1. Necropsy and organ weights
There was no significant difference observed in the body weight of male offspring exposed in utero to AIN, BPA, HFB, HFO, HFB + BPA, or HFO + BPA diets (Data in Brief [30], Fig. 2). No statistical significance was observed in testis, epididymis, prostate, spleen, or kidney weights between the groups. Also, no statistical difference was observed between the weights of new borne male pups between these groups (results not shown).
3.2. Prenatal HFB + BPA exposure induces significant spermatogenesis arrest in offspring
SD rats have been shown to be sensitive to prenatal BPA exposure [6,34]. Maternal dams were fed a HFB or HFO diet in the presence and absence of 25 μg/kg bw-d BPA as indicated in Fig. 1. This dose was chosen based on the dose-response study (materials and methods).
The prenatally exposed male offspring from each diet group (one pup per litter) were treated with T + E2 (Fig. 1), a hormonal milieu previously reported to mimic aging [20,35]. We found that while 100% of the offspring exposed to the control AIN diet showed normal spermatogenesis (presence of spermatozoa in >14% of STs), only 37–43% of offspring exposed to 25 μg/kg bw-d BPA and/or HFB had spermatozoa in STs (Table 2). When compared to the AIN diet group, we found that the HFB + BPA group was significantly (p = 0.031) different. The BPA and HFB groups were borderline significant than the control AIN group. Of greater interest, we found that the offspring exposed to HFO behave like AIN controls, as did the offspring exposed to combination diet of HFO + BPA.
We next compared the overall impaired spermatogenesis amongst groups. For this, we plotted a curve reflecting the extent of spermatogenesis within the tubules for each diet. That is, the curve was plotted from the ST scores summed up to specific points where spermatogenesis was blocked culminating with: (1) spermatogonia/atrophic tubules (2) spermatocytes (3) round spermatids (4) condensed spermatids and (5) spermatozoa (Fig. 2A, B). When the curves were compared (bold lines), it was evident that the AIN, HFO and HFO + BPA curves showed a similar pattern in which the slopes of the curves were slight up to the round spermatid step. On the contrary, the average curves of HFB, BPA and HFB + BPA groups showed much higher slopes for these earlier points in spermatogenesis, implying impaired spermatogenesis within these 3 groups. Correspondingly, the means of overall impaired spermatogenesis (or AUC) in groups of HFB, BPA and HFB + BPA were higher than those of the AIN, HFO and HFO + BPA groups (Fig. 2C). Combined, the mean ± SE of the first three groups was 0.22 ± 0.02 statistically higher than that of 0.12 ± 0.02 from the later three groups combined (p = 0.001, Fig. 2C). Also, the AUC of the HFB + BPA group was significantly higher than each of the AIN, HFO and HFO + BPA group, as determined by the fixed effect model (Fig. 2C).
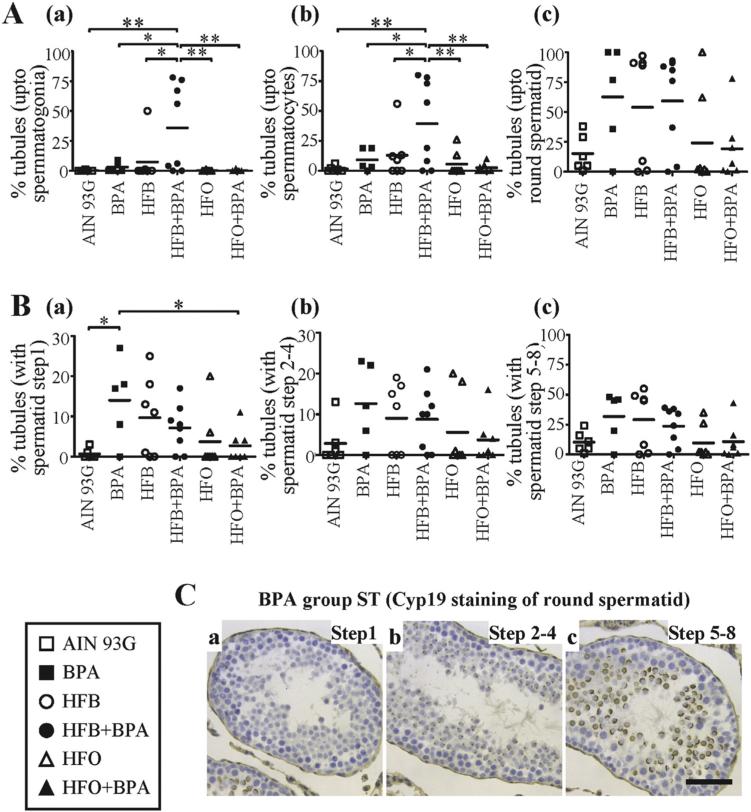
Finally, we examined the groups for the key step(s) at which spermatogenesis was impaired. We found that the HFB + BPA group exhibited significantly higher percent of STs with only spermatogonia and Sertoli cells (Figs 2A, B, 3A), compared to any of the other groups. Additionally, we found that the offspring exposed to BPA (3 out of 5 animals) and HFB (4 out of 7 animals) alone, had a greater number of tubules with round spermatids (Fig. 2B(1–3), A) as the final step in spermiogenesis, than the AIN, HFO and HFO + BPA groups. When the number of tubules containing round spermatid step 1, 2–4 and 5–8 were scored (by CYP19 staining), the BPA group had a significantly greater number of tubules with round spermatid step 1 as the final step of spermiogenesis, than the AIN and HFO + BPA groups (Fig. 3B, C). Our results thus indicate that the HFB, BPA and HFB + BPA groups show different degrees of impaired spermatogenesis, with the HFB + BPA group having the most significant disruptive effect.
Fig. 3.
(A) The number of STs with spermatogenesis upto (a) spermatogonia (b) spermatocyte (c) round spermatid, as the last differentiated germ cell present, were tallied for male offspring exposed to the maternal diets indicated (T + E2 rodent model). (B) The number of STs with spermatogenesis impaired at the round spermatid (a) step1 (b) step 2–4 (c) step 5–8 were tallied for male offspring exposed to the maternal diets indicated. Each symbol represents a pup from an independent litter. (C) Representative CYP19 IHC staining of BPA group testis shows presence of STs with round spermatid at (a) step1 (b) step 2–4 (c) step 5–8. Bar = 40 μm. * p < 0.05, ** p < 0.01, 1-way ANOVA models between groups indicated.
3.3. Prenatal BPA and/or HFB exposure induces spermatogenesis arrest in 18-month-old offspring
Next, we asked whether we would observe similar effects of gestational diet and/or BPA exposures on spermatogenesis in off-spring as they age (PND 540). To address this question, one male offspring per litter exposed in utero to the above diets, without any secondary exposures, was sacrificed at ~18 months of age (equivalent to 45 human years, middle age [36]). It has been previously shown that sperm count and quality begins to decline around age 40 in humans [37,38] and at 18 months of age in rats [20]. We found that while 100% of offspring exposed to the control AIN diet showed normal spermatogenesis (presence of spermatozoa in >20% of STs), only 17% (1 in 6 rats) of offspring exposed to HFB + BPA had significantly normal spermatogenesis (Table 3). The odds ratio suggests that abnormal spermatogenesis is 15 times as likely to occur in the BPA or HFB groups and 40 times as likely to occur in the HFB + BPA group as in the AIN group. Again, we found that spermatogenesis in the HFO and HFO + BPA group is statistically not significant from the control AIN group, with most of the ST containing a full spectrum of developing male germ cells including mature sperm. Hence, using two different model systems, we have found impaired spermatogenesis in the testis of in utero HFB + BPA exposed male offspring.
3.4. Impaired spermatogenesis is not due to changes in Sertoli:germ cell ratio, increase in apoptosis or increase in cell clusters
Since impaired spermatogenesis can be attributed to changes in the number of Sertoli cells [39,40], we scored the number of Sertoli cells per tubule (androgen receptor staining, AR+) and the number of germ cells in the testis of T + E2 treated offspring. We found the Sertoli:germ cell ratio did not significantly change across diets. Since PLZF (promyelocytic leukemia zinc-finger) is required in adult male spermatogonia for stem cell self-renewal [41], we counted the number of PLZF+ cells in tubules of the same shape and size for the AIN, HFB, and HFB + BPA groups. We found that the number of undifferentiated stem cells per tubule did not significantly change with diet. Next, we looked for presence of apoptotic cells in the tubules. We found that while the number of STs with apoptotic cells increased for the BPA and HFB group, significance was not reached due to the high variability in results within groups. However, we found that animals with a higher number of tubules blocked at round spermatids had an increase in the number of STs with apoptotic cells (data not shown). Finally, we examined for presence of clusters of cells which could be indicative of sloughed symplast cells or multinucleated cells. We found that 50–57% of animals in the BPA, HFB, and HFB + BPA groups showed the presence of cell clusters, while only 0–14% of animals in the AIN, HFO, and HFO + BPA groups showed presence of cell clusters (Data in Brief [30], Fig. 3). However, significance was not observed between the groups.
3.5. Expression of chromatin condensing protein PRM1 occurs at an earlier step of spermatogenesis
One of the crucial steps in spermiogenesis is the elongation and condensation step, when the spermatid chromatin undergoes a complex transition in which the acetylated histones are bound by BRDT (bromodomain, testis-specific) and then replaced by protamine (PRM) in a carefully regulated transition [42], including histone modification and replacement of the histones by sperm-specific transition proteins and, finally, by PRM1 and PRM2. The replacement of most histones by PRM1 and PRM2 facilitates a high order of chromatin packaging necessary for normal sperm function [42,43], leading to DNA silencing. It has been shown that PRM1 staining is absent in round spermatid nuclei, and strong in elongating and compacting spermatid nuclei [44]. Since a block to spermiogenesis was observed at the round spermatids, we examined PRM1 expression by IHC staining in the testis of pups from the T + E2 model. To our surprise, we found that in 71–75% of the HFB-exposed (5 out of 7 animals) and HFB + BPA-exposed (6 out of 8 animals) offspring, 20–60% of STs with diplotene spermatocytes showed PRM1 staining (Fig. 4A, B). Hence, in the STs of HFB and HFB + BPA offspring, the expression of PRM1 in some of the tubules is earlier in spermatogenesis than expected. Again, the HFO and HFO + BPA groups were not statistically different than the AIN group for PRM1 staining. We next examined whether expression of BRDT is disrupted during spermatogenesis. We found that BRDT is expressed in the pachytene and diplotene spermatocytes and round spermatids, as previously shown [45,46], with no diet-dependent changes (Data in Brief, Fig. 3).
3.6. Decrease in estrogen receptor beta (ERβ) expression in round spermatids
Germ cells are capable of local estrogen synthesis (via aromatase) and response (via ERα, ERβ), suggesting that estrogens may be important in Sertoli, Leydig, and male germ cell development [47]. ERα, ERβ, and aromatase are found at all stages of testicular development in the rodent [48]. Leydig, Sertoli, and germ cells express the P450 aromatase (CYP19) [49]. While expression of ERα is confined to testicular interstitial Leydig cells [50,51], ERβ expression is also found in Sertoli cell nuclei, type A spermatogonia, pachytene spermatocytes, and round spermatids [52,53]. We hence examined whether exposure to BPA and fats disrupted the localization of these hormone mediators in the rat testis. On IHC staining testis of the T + E2 treated rats, we found no change in expression pattern for ERα (Data in Brief [30], Fig. 4) and CYP19 (Data in Brief [30], Fig. 5) expression between diet groups. However, for ERβ, we found that a significant number of animals showed a decrease or loss of expression of ERβ in the round spermatids of the BPA, HFB, and HFB + BPA groups (Fig. 5A, B) compared to the AIN group.
4. Discussion
The goal of this study was to evaluate the effects of BPA and saturated (butter) and mono-unsaturated (olive oil) fatty acids on male reproductive function. While we did not observe any effects of gestational BPA and/or HFB exposures on spermatogenesis in young adult rats, using two models, the natural aging model and the hormone-treatment model, we found that the combination of HFB + BPA had significantly disrupted spermatogenesis. Our data suggest the adverse BPA effect becomes significant under a HFB background. Furthermore, we uncover an important feature related to high dietary fat intake, i.e., the butter fat, but not the olive oil, in combination with BPA, was detrimental to spermatogenesis in our models.
Although both BPA and high-fat diets have been separately studied for their actions in male infertility, whether the two given together have more adverse action had not previously been investigated. The endocrine-disrupting properties of BPA suggest it may impact developmental plasticity during early life, or the weak estrogenic activity of BPA may predispose individuals to disease. The differential action between butter oil and olive oil has been previously reported. A high saturated fatty acid diet and BPA was reported to increase the levels of corticosteroid, while olive oil decreased the secretion of this hormone [54–56]. We here find a similar scenario in which the intake of high butter fat but not olive oil, appears to exacerbate the risk of spermatogenesis disruption caused by BPA. The underlying mechanism of how the BPA and HFB might interact to increase spermatogenesis defects, bears further investigation.
The last few decades have seen a synchronized increase in the incidence of male reproductive problems, such as genital abnormalities, testicular cancer, reduced semen quality, and subfertility (49–51). The increase in male reproductive problems is too rapid to be explained by genetic factors alone and suggests that environment/lifestyle factors in addition to genetics may play a significant role in their development. The endpoint of our aging study was 18 rat months, which is equivalent to 45 human years [36]. Due to societal demands and expectations, many couples in developed countries are delaying parenthood. For example, in the US, birth rates for men older than 35 years have increased 40% since 1980 [57]. We used outbred Taconic SD rats, which are not selected for growth or reproduction in any manner. With both the 18-month-old rats (PND 540, natural aging) and a model which mimics an aging hormonal milieu, our findings suggest that the ubiquitously present and consumed BPA in the presence of the high-fat Western diet, enriched with butter during pregnancy, may contribute to increased prevalence of male infertility within a few generations.
The dietary BPA intake we chose is lower than the EPA recommended LOAEL of 50 μg/kg bw/d [58]. We previously found that dietary exposure of dams before and during pregnancy to 250 μg/kg bw-d BPA resulted in undetectable levels of free BPA and 2–3 ng/ml of total BPA in maternal blood and the presence of HFB did not change these values [32]. We find that this low level of BPA during pregnancy is sufficient to induce spermatogenesis disruption in offspring. Moreover, the HFB and BPA combination had a worst outcome than singular exposures in disrupting spermatogenesis.
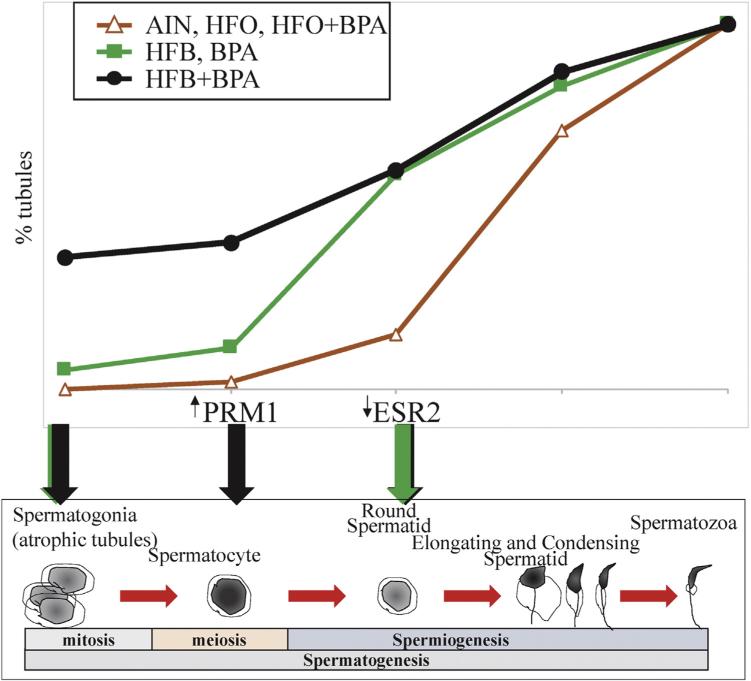
On observing the slopes of the curves, it is clear that in the presence of HFB or BPA diets, there is a higher number (though not statistically significant) of tubules with round spermatids step1, 4 and 8, than in the AIN, HFO and HFO + BPA group. That is, at the transition from diplotene spermatocytes to step 1, 2 spermatids (Figs.3B, 6). The impairment in spermatogenesis in the HFB + BPA diet group appears to be at an earlier point in spermatogenesis progression, resulting in a significantly increased number of STs with spermotogonia (Figs.3A, 6, atrophic tubules). Additionally, the BPA, HFB, and HFB + BPA animals had cell clusters, decreased expression of ERβ in round spermatids, and the presence of PRM1 in diplotene spermatocytes (HFB and HFB + BPA group only), all of which could together contribute to the impaired spermatogenesis observed. Premature translation of PRM1 mRNA in transgenic mice has been found to arrest spermiogenesis in round spermatids, presumably by disrupting the structure of chromatin [59]. One can speculate that the abnormal protamine expression could be due to failings in PRM1 mRNA transcription regulation, mRNA storage, and/or translation. This would need to be investigated in a more systematic manner.
Fig. 6.
Cartoon representing germ cell development in testis and the plot of spermatogenesis. Green arrows (BPA or HFB), and black arrows (BPA + HFB) represent steps where exposure appears to interfere with spermatogenesis and spermiogenesis progression. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
5. Conclusions
Our results suggest that there may be “hidden” effects of gestational BPA and HFB exposures which might not manifest until later in life or when T to E2 ratios change. This has been observed before as a “hidden epigenetic memory” [60,61], which may involve changes in non-coding RNA, DNA methylation, histone modifications, and chromatin modifications [62], all of which could result in alterations in the temporal pattern of transcription and translation regulating spermatogenesis. This is now being examined by our group. Secondly, we found that gestational intake of HFO and HFO + BPA had no effects on spermatogenesis in adult animals. These findings have public health relevance as they provide a strategy for reducing BPA toxicity via diet during the window of gestation.
Acknowledgements
This study was supported in part by grants from the National Institutes of Health (P30ES006096 (SMH, YKL), U01ES019480 (SMH, YKL), and U01ES020988 (SMH, YKL)); the Department of Defense (W81XWH-15-1-0353 (PT)); and the United States Department of Veterans Affairs (I01BX000675 (SMH)). We thank Justin Zhang, Leon Cheong, Rahul Rao, Xuegong Zhu, Neville Tam, and Emma Berry for their technical assistance. We thank Dr. Scott Belcher for guidance in setting up a BPA-free animal housing environment.
Abbreviations
- AIN
AIN 93G diet
- AUC
area under the curve
- BPA
bisphenol A
- BRDT
bromodomain testis associated
- E2
estradiol-17β
- EE2
17α-ethinyl estradiol
- ERα
estrogen receptor α
- ERβ
estrogen receptor β
- HFB
high fat butter
- HFO
high fat olive oil
- IHC
immunohistochemistry
- Kg bw-d
kg body weight/day
- PLZF
promyelocytic leukemia zinc-finger
- PND
postnatal day
- PRM1
protamine1
- SD
Sprague-Dawley
- ST
seminiferous tubules
- T
testosterone
- TUNEL
terminal deoxynucleotidyl transferase–mediated dUTP nick end labeling
- WT-1
Wilms Tumor 1
Footnotes
Disclosure statement
The authors have nothing to disclose.
The authors declare they have no actual or potential competing financial interests.
References
- 1.Kubwabo C, Kosarac I, Stewart B, Gauthier BR, Lalonde K, Lalonde PJ. Migration of bisphenol A from plastic baby bottles, baby bottle liners and reusable polycarbonate drinking bottles, Food Addit. Contam. Part A Chem. Anal. Control Expo Risk Assess. 2009;26:928–937. doi: 10.1080/02652030802706725. [DOI] [PubMed] [Google Scholar]
- 2.Rochester JR. Bisphenol A and human health: a review of the literature. Reprod. Toxicol. 2013;42C:132–155. doi: 10.1016/j.reprotox.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Lee DR, Jacobs DH, Jr, et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr. Rev. 2012;33:378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heindel JJ. Role of exposure to environmental chemicals in the developmental basis of disease and dysfunction. Reprod. Toxicol. 2007;23:257–259. doi: 10.1016/j.reprotox.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Newbold RR. Developmental exposure to endocrine-disrupting chemicals programs for reproductive tract alterations and obesity later in life. Am. J. Clin. Nutr. 2011;94:1939S–1942S. doi: 10.3945/ajcn.110.001057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ho SM, Tang WY, Belmonte de FJ, Prins GS. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006;66:5624–5632. doi: 10.1158/0008-5472.CAN-06-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heindel JJ, Newbold R, Schug TT. Endocrine disruptors and obesity. Nat. Rev. Endocrinol. 2015;11:653–661. doi: 10.1038/nrendo.2015.163. [DOI] [PubMed] [Google Scholar]
- 8.Tinwell H, Haseman J, Lefevre PA, Wallis N, Ashby J. Normal sexual development of two strains of rat exposed in utero to low doses of bisphenol A. Toxicol. Sci. 2002;68:339–348. doi: 10.1093/toxsci/68.2.339. [DOI] [PubMed] [Google Scholar]
- 9.Akingbemi BT, Sottas CM, Koulova AI, Klinefelter GR, Hardy MP. Inhibition of testicular steroidogenesis by the xenoestrogen bisphenol A is associated with reduced pituitary luteinizing hormone secretion and decreased steroidogenic enzyme gene expression in rat Leydig cells. Endocrinology. 2004;145:592–603. doi: 10.1210/en.2003-1174. [DOI] [PubMed] [Google Scholar]
- 10.Atanassova N, McKinnell C, Turner KJ, Walker M, Fisher JS, Morley M, et al. Comparative effects of neonatal exposure of male rats to potent and weak (environmental) estrogens on spermatogenesis at puberty and the relationship to adult testis size and fertility: evidence for stimulatory effects of low estrogen levels. Endocrinology. 2000;141:3898–3907. doi: 10.1210/endo.141.10.7723. [DOI] [PubMed] [Google Scholar]
- 11.Vrooman LA, Oatley JM, Griswold JE, Hassold TJ, Hunt PA. Estrogenic exposure alters the spermatogonial stem cells in the developing testis, permanently reducing crossover levels in the adult. PLoS Genet. 2015;11:e1004949. doi: 10.1371/journal.pgen.1004949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salian S, Doshi T, Vanage G. Perinatal exposure of rats to Bisphenol A affects fertility of male offspring—an overview. Reprod. Toxicol. 2011;31:359–362. doi: 10.1016/j.reprotox.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Oehlmann J, Schulte-Oehlmann U, Kloas W, Jagnytsch O, Lutz I, Kusk KO, et al. A critical analysis of the biological impacts of plasticizers on wildlife. Philos. Trans. R Soc. Lond. B Biol. Sci. 2009;364:2047–2062. doi: 10.1098/rstb.2008.0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vom Saal FS, Welshons WV. Large effects from small exposures. II. The importance of positive controls in low-dose research on bisphenol A. Environ. Res. 2006;100:50–76. doi: 10.1016/j.envres.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Tyl RW, Myers CB, Marr MC, Sloan CS, Castillo NP, Veselica MM, et al. Two-generation reproductive toxicity study of dietary bisphenol A in CD-1 (Swiss) mice. Toxicol. Sci. 2008;104:362–384. doi: 10.1093/toxsci/kfn084. [DOI] [PubMed] [Google Scholar]
- 16.Cagen SZ, Dimond JM, Waechter SS, Jr., Breslin WJ, Butala JH, Jekat FW, et al. Normal reproductive organ development in Wistar rats exposed to bisphenol A in the drinking water. Regul. Toxicol. Pharmacol. 1999;30:130–139. doi: 10.1006/rtph.1999.1340. [DOI] [PubMed] [Google Scholar]
- 17.Attaman JA, Toth TL, Furtado J, Campos H, Hauser R, Chavarro JE. Dietary fat and semen quality among men attending a fertility clinic. Hum. Reprod. 2012;27:1466–1474. doi: 10.1093/humrep/des065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen TK, Heitmann BL, Jensen MB, Halldorsson TI, Andersson AM, Skakkebaek NE, et al. High dietary intake of saturated fat is associated with reduced semen quality among 701 young Danish men from the general population. Am. J. Clin. Nutr. 2013;97:411–418. doi: 10.3945/ajcn.112.042432. [DOI] [PubMed] [Google Scholar]
- 19.Srilatha B, Adaikan PG. Endocrine milieu and erectile dysfunction: is oestradiol-testosterone imbalance, a risk factor in the elderly? Asian J. Androl. 2011;13:569–573. doi: 10.1038/aja.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clarke M, Pearl CA. Alterations in the estrogen environment of the testis contribute to declining sperm production in aging rats. Syst. Biol. Reprod. Med. 2014;60:89–97. doi: 10.3109/19396368.2014.885995. [DOI] [PubMed] [Google Scholar]
- 21.Williams G. Aromatase up-regulation, insulin and raised intracellular oestrogens in men, induce adiposity, metabolic syndrome and prostate disease, via aberrant ER-alpha and GPER signalling. Mol. Cell. Endocrinol. 2012;351:269–278. doi: 10.1016/j.mce.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 22.Sharpe RM, Fisher JS, Millar MM, Jobling S, Sumpter JP. Gestational and lactational exposure of rats to xenoestrogens results in reduced testicular size and sperm production. Environ. Health Perspect. 1995;103:1136–1143. doi: 10.1289/ehp.951031136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharpe RM. Environmental/lifestyle effects on spermatogenesis. Philos. Trans. R Soc. Lond. B Biol. Sci. 2010;365:1697–1712. doi: 10.1098/rstb.2009.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reame V, Pytlowanciv EZ, Ribeiro DL, Pissolato TF, Taboga SR, Goes RM, et al. Obesogenic environment by excess of dietary fats in different phases of development reduces spermatic efficiency of wistar rats at adulthood: correlations with metabolic status. Biol. Reprod. 2014;91:151. doi: 10.1095/biolreprod.114.121962. [DOI] [PubMed] [Google Scholar]
- 25.Kendziorski JA, Kendig EL, Gear RB, Belcher SM. Strain specific induction of pyometra and differences in immune responsiveness in mice exposed to 17alpha-ethinyl estradiol or the endocrine disrupting chemical bisphenol A. Reprod. Toxicol. 2012;34:22–30. doi: 10.1016/j.reprotox.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vom Saal FS, Hughes C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ. Health Perspect. 2005;113:926–933. doi: 10.1289/ehp.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vom Saal FS, Richter CA, Ruhlen RR, Nagel SC, Timms BG, Welshons WV. The importance of appropriate controls, animal feed, and animal models in interpreting results from low-dose studies of bisphenol A. Birth Defects Res. A Clin. Mol. Teratol. 2005;73:140–145. doi: 10.1002/bdra.20120. [DOI] [PubMed] [Google Scholar]
- 28.Thayer KA, Ruhlen RL, Howdeshell KL, Buchanan DL, Cooke PS, Preziosi D, et al. Altered prostate growth and daily sperm production in male mice exposed prenatally to subclinical doses of 17alpha-ethinyl oestradiol. Hum. Reprod. 2001;16:988–996. doi: 10.1093/humrep/16.5.988. [DOI] [PubMed] [Google Scholar]
- 29.Vom Saal FS, Timms BG, Montano MM, Palanza P, Thayer KA, Nagel SC, et al. Prostate enlargement in mice due to fetal exposure to low doses of estradiol or diethylstilbestrol and opposite effects at high doses. Proc. Natl. Acad. Sci. U. S. A. 1997;94:2056–2061. doi: 10.1073/pnas.94.5.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tarapore P, Hennessy M, Song D, Ying J, Ouyang BV, Govindarajah V, et al. Effects of maternal high-fat diets and Bisphenol A on spermatogenesis in rat male offspring Data in Brief. 2016. Submitted. [DOI] [PMC free article] [PubMed]
- 31.Tam NN, Szeto CY, Sartor MA, Medvedovic M, Ho SM. Gene expression profiling identifies lobe-specific and common disruptions of multiple gene networks in testosterone-supported, 17beta-estradiol- or diethylstilbestrol-induced prostate dysplasia in Noble rats. Neoplasia. 2008;10:20–40. doi: 10.1593/neo.07889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez AM, Cheong A, Ying J, Xue J, Kannan K, Leung YK, et al. Effects of high-butterfat diet on embryo implantation in female rats exposed to bisphenol A. Biol. Reprod. 2015 doi: 10.1095/biolreprod.115.131433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tam NN, Zhang X, Xiao H, Song D, Levin L, Meller J, et al. Increased susceptibility of estrogen-induced bladder outlet obstruction in a novel mouse model. Lab. Invest. 2015;95:546–560. doi: 10.1038/labinvest.2015.30. [DOI] [PubMed] [Google Scholar]
- 34.Betancourt AM, Eltoum IA, Desmond RA, Russo J, Lamartiniere CA. In utero exposure to bisphenol A shifts the window of susceptibility for mammary carcinogenesis in the rat. Environ. Health Perspect. 2010;118:1614–1619. doi: 10.1289/ehp.1002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson CJ, Tam NN, Joyce JM, Leav I, Ho SM. Gene expression profiling of testosterone and estradiol-17 beta-induced prostatic dysplasia in Noble rats and response to the antiestrogen ICI 182,780. Endocrinology. 2002;143:2093–2105. doi: 10.1210/endo.143.6.8846. [DOI] [PubMed] [Google Scholar]
- 36.Andreollo NA, Santos EF, Araujo MR, Lopes LR. Rat's age versus human's age: what is the relationship? Arq. Bras. Cir. Dig. 2012;25:49–51. doi: 10.1590/s0102-67202012000100011. [DOI] [PubMed] [Google Scholar]
- 37.Wyrobek AJ, Eskenazi B, Young S, Arnheim N, Tiemann-Boege I, Jabs EW, et al. Advancing age has differential effects on DNA damage, chromatin integrity, gene mutations, and aneuploidies in sperm. Proc. Natl. Acad. Sci. U. S. A. 2006;103:9601–9606. doi: 10.1073/pnas.0506468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zavos PM, Kaskar K, Correa JR, Sikka SC. Seminal characteristics and sexual behavior in men of different age groups: is there an aging effect? Asian J. Androl. 2006;8:337–341. doi: 10.1111/j.1745-7262.2006.00128.x. [DOI] [PubMed] [Google Scholar]
- 39.Auharek SA, de Franca LR, McKinnell C, Jobling MS, Scott HM, Sharpe RM. Prenatal plus postnatal exposure to Di(n-Butyl) phthalate and/or flutamide markedly reduces final sertoli cell number in the rat. Endocrinology. 2010;151:2868–2875. doi: 10.1210/en.2010-0108. [DOI] [PubMed] [Google Scholar]
- 40.Sharpe RM, McKinnell C, Kivlin C, Fisher JS. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction. 2003;125:769–784. doi: 10.1530/rep.0.1250769. [DOI] [PubMed] [Google Scholar]
- 41.Buaas FW, Kirsh AL, Sharma M, McLean DJ, Morris JL, Griswold MD, et al. Plzf is required in adult male germ cells for stem cell self-renewal. Nat. Genet. 2004;36:647–652. doi: 10.1038/ng1366. [DOI] [PubMed] [Google Scholar]
- 42.Aoki VW, Carrell DT. Human protamines and the developing spermatid: their structure, function, expression and relationship with male infertility. Asian J. Androl. 2003;5:315–324. [PubMed] [Google Scholar]
- 43.Carrell DT, Emery BR, Hammoud S. Altered protamine expression and diminished spermatogenesis: what is the link? Hum. Reprod. Update. 2007;13:313–327. doi: 10.1093/humupd/dml057. [DOI] [PubMed] [Google Scholar]
- 44.Zhao M, Shirley CR, Mounsey S, Meistrich ML. Nucleoprotein transitions during spermiogenesis in mice with transition nuclear protein Tnp1 and Tnp2 mutations. Biol. Reprod. 2004;71:1016–1025. doi: 10.1095/biolreprod.104.028191. [DOI] [PubMed] [Google Scholar]
- 45.Shang E, Nickerson HD, Wen D, Wang X, Wolgemuth DJ. The first bromodomain of Brdt, a testis-specific member of the BET sub-family of double-bromodomain-containing proteins, is essential for male germ cell differentiation. Development. 2007;134:3507–3515. doi: 10.1242/dev.004481. [DOI] [PubMed] [Google Scholar]
- 46.Dhar S, Thota A, Rao MR. Insights into role of bromodomain, testis-specific (Brdt) in acetylated histone H4-dependent chromatin remodeling in mammalian spermiogenesis. J. Biol. Chem. 2012;287:6387–6405. doi: 10.1074/jbc.M111.288167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Couse JE, Mahato D, Eddy EM, Korach KS. Molecular mechanism of estrogen action in the male: insights from the estrogen receptor null mice. Reprod. Fertil. Dev. 2001;13:211–219. doi: 10.1071/rd00128. [DOI] [PubMed] [Google Scholar]
- 48.O'Donnell L, Robertson KM, Jones ME, Simpson ER. Estrogen and spermatogenesis. Endocr. Rev. 2001;22:289–318. doi: 10.1210/edrv.22.3.0431. [DOI] [PubMed] [Google Scholar]
- 49.Carreau S, Bourguiba S, Lambard S, Galeraud-Denis I, Genissel C, Bilinska B, et al. Aromatase expression in male germ cells. J. Steroid Biochem. Mol. Biol. 2001;79:203–208. doi: 10.1016/s0960-0760(01)00137-6. [DOI] [PubMed] [Google Scholar]
- 50.Nielsen M, Bjornsdottir S, Hoyer PE, Byskov AG. Ontogeny of oestrogen receptor alpha in gonads and sex ducts of fetal and newborn mice. J. Reprod. Fertil. 2000;118:195–204. doi: 10.1530/jrf.0.1180195. [DOI] [PubMed] [Google Scholar]
- 51.Fisher JS, Millar MR, Majdic G, Saunders PT, Fraser HM, Sharpe RM. Immunolocalisation of oestrogen receptor-alpha within the testis and excurrent ducts of the rat and marmoset monkey from perinatal life to adulthood. J. Endocrinol. 1997;153:485–495. doi: 10.1677/joe.0.1530485. [DOI] [PubMed] [Google Scholar]
- 52.van Pelt AM, de Rooij DG, van der Burg B, van der Saag PT, Gustafsson JA, Kuiper GG. Ontogeny of estrogen receptor-beta expression in rat testis. Endocrinology. 1999;140:478–483. doi: 10.1210/endo.140.1.6438. [DOI] [PubMed] [Google Scholar]
- 53.Saunders PT, Fisher JS, Sharpe RM, Millar MR. Expression of oestrogen receptor beta (ER beta) occurs in multiple cell types, including some germ cells, in the rat testis. J. Endocrinol. 1998;156:R13–R17. doi: 10.1677/joe.0.156r013. [DOI] [PubMed] [Google Scholar]
- 54.Carsia RV, Weber H, McIlroy PJ, Hock CE. Long-term dietary lipid regimen alters adrenocortical function at the cellular level. Horm. Metab. Res. 2008;40:848–853. doi: 10.1055/s-0028-1086025. [DOI] [PubMed] [Google Scholar]
- 55.Poimenova A, Markaki E, Rahiotis C, Kitraki E. Corticosterone-regulated actions in the rat brain are affected by perinatal exposure to low dose of bisphenol A. Neuroscience. 2010;167:741–749. doi: 10.1016/j.neuroscience.2010.02.051. [DOI] [PubMed] [Google Scholar]
- 56.Medwid S, Guan H, Yang K. Prenatal exposure to bisphenol A disrupts adrenal steroidogenesis in adult mouse offspring. Environ. Toxicol. Pharmacol. 2016;43:203–208. doi: 10.1016/j.etap.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 57.Martin JA, Hamilton BE, Ventura SJ, Osterman MJ, Kirmeyer S, Mathews TJ, et al. Births: final data for 2009. Natl. Vital Stat. Rep. 2011;60:1–70. [PubMed] [Google Scholar]
- 58.Bisphenol A. 1988 www epa gov/iris.(CASRN 80-05-7) US-EPA Integrated Risk Information System (IRIS) Substance file
- 59.Lee K, Haugen HS, Clegg CH, Braun RE. Premature translation of protamine 1 mRNA causes precocious nuclear condensation and arrests spermatid differentiation in mice. Proc. Natl. Acad. Sci. U. S. A. 1995;92:12451–12455. doi: 10.1073/pnas.92.26.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tang WY, Newbold R, Mardilovich K, Jefferson W, Cheng RY, Medvedovic M, et al. Persistent hypomethylation in the promoter of nucleosomal binding protein 1 (Nsbp1) correlates with overexpression of Nsbp1 in mouse uteri neonatally exposed to diethylstilbestrol or genistein. Endocrinology. 2008;149:5922–5931. doi: 10.1210/en.2008-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tang WY, Morey LM, Cheung YY, Birch L, Prins GS, Ho SM. Neonatal exposure to estradiol/bisphenol A alters promoter methylation and expression of Nsbp1 and Hpcal1 genes and transcriptional programs of Dnmt3a/b and Mbd2/4 in the rat prostate gland throughout life. Endocrinology. 2012;153:42–55. doi: 10.1210/en.2011-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ho SM, Cheong A, Lam HM, Hu WY, Shi GB, Zhu X, et al. Exposure of human prostaspheres to bisphenol A epigenetically regulates SNORD family noncoding RNAs via histone modification. Endocrinology. 2015;156:3984–3995. doi: 10.1210/en.2015-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]