Abstract
Red blood cells (RBCs) suspended in plasma form multicellular aggregates under low flow conditions, increasing apparent blood viscosity at low shear rates. It has previously been unclear, however, if RBC aggregation affects microvascular perfusion. Here we analyzed the impact of RBC aggregation on perfusion and ‘capillary’ hematocrit in an artificial microvascular network (AMVN) at driving pressures ranging from 5 to 60 cmH2O to determine if aggregation could improve tissue oxygenation. RBCs were suspended at 30% hematocrit in either 46.5 g/L dextran 40 (D40, non-aggregating medium) or 35 g/L dextran 70 (D70, aggregating medium) solutions with equal viscosity. Aggregation was readily observed in the AMVN for RBCs suspended in D70 at driving pressures ≤ 40 cmH2O. The AMVN perfusion rate was the same for RBCs suspended in aggregating and non-aggregating medium, at both ‘venular’ and ‘capillary’ level. Estimated ‘capillary’ hematocrit was higher for D70 suspensions than for D40 suspensions at intermediate driving pressures (5 – 40 cm H2O). We conclude that although RBC aggregation did not affect the AMVN perfusion rate independently of the driving pressure, a higher hematocrit in the ‘capillaries’ of the network for D70 suspensions suggested a better oxygen transport capacity in the presence of RBC aggregation.
Keywords: Artificial microvascular network, Dextran, Hematocrit, Microvascular perfusion, Red blood cell aggregation
Introduction
Red blood cells (RBCs) suspended in plasma tend to aggregate among each other, forming multi-cellular branched or linear aggregates called rouleaux [5]. RBC aggregation occurs under conditions with low shear rates during stagnant or low flow. It is a reversible process, i.e., aggregates are dispersed by higher shear forces and reform within seconds when those forces have ceased. RBC aggregation depends on the concentration of high molecular weight proteins such as fibrinogen in plasma [5].
Increased RBC aggregation has been reported in many different diseases such as bacterial infections [2,26] and sepsis [1], myocardial ischemia and infarction [35,73], cerebral infarction [60], peripheral vascular disease [33], hypertension [19], diabetes mellitus [72], metabolic syndrome [61], hypergammaglobulinemias [34], and inflammatory rheumatological diseases [57,65].
Inflammatory disease is accompanied by a systemic acute phase reaction characterized by an increased production of C-reactive protein, fibrinogen, alpha-2-macroglobulin, and immunoglobulins [23,25,32]. Increased concentration of fibrinogen and other high molecular weight proteins in plasma leads to increased RBC aggregation [9] which becomes apparent through elevated RBC sedimentation rate, a very old and time-honored laboratory test in clinical medicine [21]. It is still a matter of controversy whether increased RBC aggregation is beneficial and contributes to recovery from a disease [30,70], whether it is a harmful process and thus perhaps a mediator of disease [3,62,67], or an irrelevant parameter and only a marker of disease [15,28].
RBC aggregation affects hemorheology by increasing blood viscosity at low shear rates [16]. In tube flow, RBC aggregation occurs in the center axis of the tube, which leads to an increased cell-free layer near the wall [42,59]. Whether RBC aggregation also affects the flow of blood in the microvasculature is still a matter of debate. Current experimental data are confounded by the fact that a change in RBC aggregation is accompanied by a concomitant change in plasma viscosity, which itself is a major determinant of capillary blood flow [4,28]. In the present study we suspended normal human RBCs in two solutions of dextran with equivalent viscosities, but different capacity to cause RBC aggregation, namely dextran 70 as aggregating medium and dextran 40 as non-aggregating medium. We measured the ability of RBCs suspended in these to different media to perfuse an artificial microvascular network (AMVN). We also estimated the hematocrit in the “capillary” and “venular” portions of the AMVN for both suspensions, which in combination with the perfusion rate data, allowed us to test whether RBC aggregation increases or decreases tissue oxygenation capacity.
Material and Methods
Dextran solutions
Dextrans with molecular weights of 70,000 Daltons (dextran 70) and 40,000 Daltons (dextran 40) were used (Tokyo Chemical Industry Co., Tokyo, Japan). Both dextran 70 and dextran 40 were dissolved in saline for measuring RBC aggregation and viscosity, or in GASP buffer (1.3 mM NaH2PO4, 9 mM Na2HPO4, 140 mM NaCl, 5.5 mM glucose, 1% bovine serum albumin; osmolality 290 mOsm/kg; pH 7.4) for measuring AMVN perfusion rates. Dextran 70 was dissolved at a concentration of 35 g/L based on our previous experience studying RBC aggregation [41]. A series of dextran 40 concentrations was prepared and their viscosities were measured (see below). From these viscosity measurements it was calculated that 46.5 g/L dextran 40 solution matched the viscosity of the 35 g/L dextran 70 solution, and therefore these concentrations were used in all experiments.
Blood samples
Samples of whole blood were obtained from healthy consenting volunteers via venipuncture using EDTA as an anticoagulant (4 mL, 7.2 mg K2EDTA, Vacutainer, Becton Dickinson, Franklin Lakes, NJ, USA). The collection of blood samples was approved by the institutional review board (IRB) at the University of Houston (Houston, TX). For measurements of RBC aggregation (n = 5) and suspension viscosity (n = 10), whole blood was centrifuged at 2500 × g for 5 min (Allegra X-15R, Beckman Coulter, Schaumburg IL, USA), and plasma, buffy coat and the uppermost RBC layer were discarded. The pelleted RBCs were washed twice in saline and then resuspended in either dextran 40 or dextran 70 solutions described above with final hematocrit adjusted to 30%.
For measuring AMVN perfusion (n = 7), whole blood was centrifuged at 1300 × g for 5 min, plasma was discarded, and pelleted RBCs were diluted with GASP buffer down to ~5% hematocrit. The diluted RBC suspension was passed through a high-efficiency pediatric leukocyte reduction filter (Purecell NEO, Pall Corp., Port Washington, USA). The filtrate was centrifuged at 3080 × g for 5 min, the supernatant was discarded, and packed RBCs were re-suspended in either dextran 40 or dextran 70 solution described above at 30% hematocrit. All measurements of hematocrit were performed in duplicate on a hematology analyzer (Medonic M-series, Boule Medical AB, Stockholm, Sweden). Samples were kept on a mixer (Labquake, Barnstead Thermolyne, Dubuque, Iowa, USA) until AMVN experiments were performed. Each RBC suspension was tested once then discarded, and all experiments were completed within 4 hours of drawing samples.
Viscosity measurements
A coaxial Couette-type viscometer (Contraves LS 30, ProRheo, Althengstett, Germany) was used to measure solution viscosities. Measurements of viscosity of dextran 40 and dextran 70 solutions were done at a shear rate of 11 s−1. Viscosities of RBC suspensions were measured over a wide range of shear rates in the following order: 69.5, 27.7, 11.0, 3.23, 0.95, 0.28 and 0.11 s−1. Viscosity values were calculated from the plateau value of the stress-strain curve generated by the viscometer. All measurements were performed at room temperature within 4 hours.
Measurements of RBC aggregation
An aggregometer with a cone-plate shearing system and integrated infrared light transmission measurement (Myrenne MA-1, Roetgen, Germany) was used to quantify RBC aggregation. RBC aggregates were dispersed at a shear rate of 600 s−1 in an initial phase, which was followed by an integration of light transmission over 10 s either at stasis (M mode) or at a low shear rate of 3 s−1 (M1 mode). Measurements were performed at room temperature in triplicate.
Measurements of the AMVN perfusion rate
The scientific background, design and fabrication of the artificial microvascular network (AMVN) devices, and the methods used for measuring the AMVN perfusion rate have been described previously [13,24,50,51,54,55]. Each AMVN device contained three identical networks of “capillary” microchannels (widths 5-70 μm) arranged in a pattern inspired by rat mesentery microvasculature, and each having an independent inlet port connected to the “capillary” network via a 70 μm wide channel (“arteriole”) and converging to a common outlet port by a 70 μm wide channel (“venule”) [13]. All microchannels comprising the AMVN were 5 μm deep. AMVN devices were manufactured using polydimethylsiloxane (PDMS; Sylgard 184, Dow Corning Corp., Midland, MI) replicas of a patterned silicon wafer fabricated using soft lithography as described previously [13]. Patterned casts were then sealed to PDMS coated glass slides after 100 second exposure to air plasma. The assembled AMVN devices were treated for 30 min with a 1% solution of mPEG-silane (Laysan Bio, Inc., Arab, AL) in GASP, and then flushed with GASP buffer prior to use.
GASP buffer was removed from the AMVN inlets by drying (Kimwipes, Kimberly-Clark Professional, Roswell, USA) and 25 μL of either dextran 40 or dextran 70 samples were deposited in each inlet. A 2 mm micro-stirring bar was inserted into each inlet, and a magnetic stirrer (Model 1060, Instech Laboratories, Inc., Plymouth Meeting, USA) placed above the inlets was used to actuate micro-stirring bars in the inlets to prevent aggregation and sedimentation of RBCs within the inlets. The driving pressure applied to the system was controlled by a water column connected to the AMVN device outlet. RBCs were allowed to fully perfuse the AMVN device, then the driving pressure was set to 0 cmH2O by adjusting the height of the water column until the RBCs came to a complete stop. Thereafter the system driving pressure was set to 60, 40, 20, 10 and 5 cmH2O for 3 min at each pressure (Note: a driving pressure of 2.5 cmH2O was attempted, however obstruction of microchannels, due to RBC adherence to channel walls – especially with dextran 70, resulted in inconsistent RBC velocities; therefore these data were not included in the final analysis).
Images were captured with a high-speed camera (100 fps, Flea3, Point Grey Research, Inc., Richmond, Canada) and analyzed offline with a custom image analysis algorithm implemented in MATLAB 2014b (The Math Works Inc., Natick, MA, USA) [13,51]. The image analysis algorithm compared subsequent images to determine the change in position of RBCs between images, thereby enabling determination of RBC solution velocity. The AMVN perfusion rate was then calculated by multiplying the solution velocity by the cross-sectional area of the microchannel (5 μm × 70 μm = 350 μm2 for “venules”).
Estimation of hematocrit in microchannels
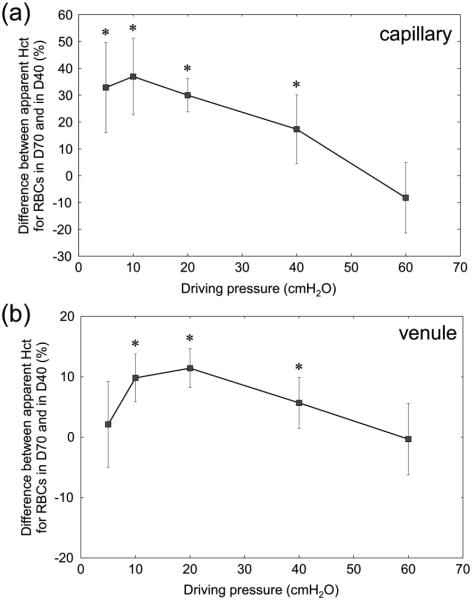
Microscopic observations of the flow through the AMVN suggested that the hematocrit was higher with dextran 70 suspensions than with dextran 40. This prompted a post-hoc analysis as follows. The same images used to measure AMVN perfusion rate were also used to estimate hematocrit in individual microchannels of the AMVN. To improve contrast and simplify the measurements of RBC velocity, we imaged the flow of blood through the AMVN in blue light (RBCs appear dark in blue light) using a band-pass blue-violet filter (394 ± 50 nm, B-390, Hoya Corp. USA, Fremont, CA). We reasoned that the hematocrit within each microchannel, therefore, was proportional to the intensity of light transmitted through the channel, based on the principles of spectrophotometry. The average grayscale color intensity (0 to 255 au) of the interior area within each microchannel and of the device background area around the microchannels was measured using a custom image analysis algorithm implemented in MATLAB (The Math Works Inc.). The average grayscale color intensity of each microchannel was subtracted from the average grayscale color intensity of the device background in order to correct for lighting variations resulting from the thickness of the PDMS device and the microscope settings. The corrected average grayscale color intensities for individual microchannels were then compared to determine the differences in mean estimated hematocrit between different microchannels relative to one another. Importantly, while we were able to determine whether hematocrit in one channel was higher or lower than in another channel using this approach, we could not measure the absolute values of hematocrit in the channels. Therefore all hematocrit data in Figure 6 and Figure 7 is presented as the difference between the two estimated hematocrits (as a percent) and no absolute hematocrit values are given.
Figure 6.
Difference between estimated hematocrit for RBCs suspended in a 46.5 g/L solution of dextran 40 in GASP buffer (D40) and those suspended in a 35.0 g/L solution of dextran 70 in GASP buffer (D70) for (a) “capillaries” and (b) “venules” of the AMVN, for driving pressures ranging from 5 to 60 cm H2O (0.49 to 5.88 kPa). Asterisks denote a statistically significant difference (p-value < 0.01) in estimated hematocrit between RBCs in D40 and D70. Mean values ± standard deviation, n = 7.
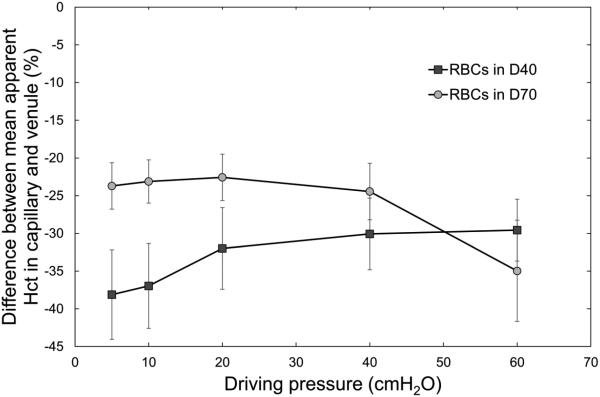
Figure 7.
Difference between mean estimated hematocrit in “capillaries” and “venules” of the AMVN for RBCs suspended in a 46.5 g/L solution of dextran 40 in GASP buffer (D40) and those suspended in a 35.0 g/L solution of dextran 70 in GASP buffer (D70), for driving pressures ranging from 5 to 60 cm H2O (0.49 to 5.88 kPa).
Statistical analysis
Statistical analysis was performed using built-in functions of MATLAB 2014b statistics toolbox. Paired Student’s t-tests were used for comparisons of dextran 40 and dextran 70 data. The results are given as mean values ± standard deviations (SD). A p-value of < 0.01 was considered significant.
Results
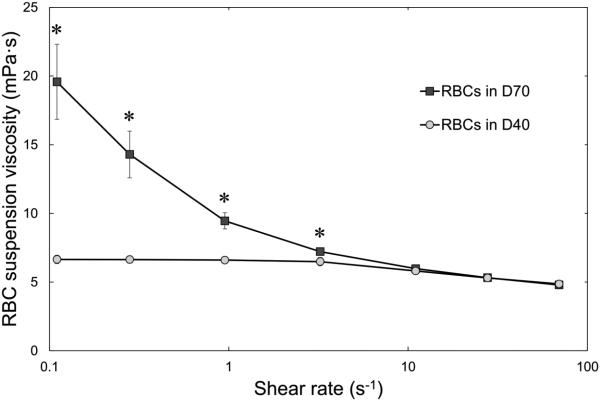
The viscosities of 46.5 g/L solution of dextran 40 and 35 g/L solution of dextran 70 in saline were very similar (Table 1). Aggregometry revealed RBC aggregation in dextran 70 suspensions, but not in dextran 40 suspensions, both at stasis (M mode) and under low shear flow (M1 mode), which is also shown in Table 1. Figure 1 shows viscosities of RBC suspensions in either dextran 40 or dextran 70 with a hematocrit of 30% for a wide range of shear rates. Whereas the viscosity of RBC suspensions in dextran 40 was almost independent of the applied shear rate (so called Newtonian behavior), the viscosity of RBC suspensions in dextran 70 increased exponentially with decreasing shear rates, indicating RBC aggregation.
Table 1.
Viscosities of 46.5 g/L dextran 40 and 35.0 g/L dextran 70 solutions in saline, and their effect on RBC aggregation in suspensions with a hematocrit of 30%. RBC aggregation was measured using Myrenne aggregometer either at stasis (M mode) or at low shear rate (M1 mode). Mean values ± standard deviation, n = 5.
| 46.5 g/L dextran 40 | 35 g/L dextran 70 | |
|---|---|---|
| Solution viscosity (mPa·s) | 2.13 ± 0.24 | 2.08 ± 0.20 |
| RBC aggregation at stasis (M mode) | 7.9 ± 2.6 | 23.9 ± 5.4 |
| RBC aggregation at 3 s−1 (M1 mode) | 15.2 ± 3.8 | 28.4 ± 7.0 |
Figure 1.
Viscosity of 30% hematocrit suspensions of RBCs in a 46.5 g/L solution of dextran 40 in normal saline (D40; circles) or in a 35.0 g/L solution of dextran 70 in normal saline (D70; squares). Measurements were performed at room temperature for shear rates ranging from 0.11 to 69.5 s−1. Asterisks denote a statistically significant difference (p-value < 0.01) in RBC suspension viscosity between RBCs in D40 and D70. Mean values ± standard deviation, n = 10.
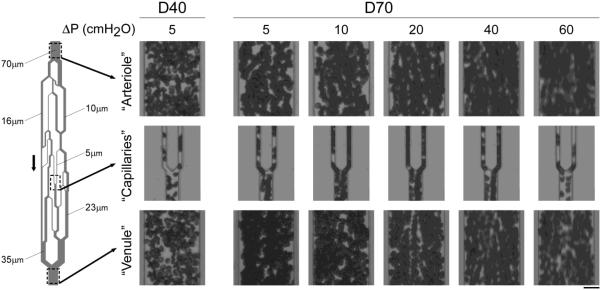
Figure 2 illustrates the microscopic appearance of RBC aggregates in an AMVN at various driving pressures. In contrast to dextran 40, which did not exhibit RBC aggregation even at the lowest driving pressure (far left side Fig. 2), stacks of RBCs without apparent gaps between adjacent cells were seen for dextran 70 suspensions in “arterioles”, “venules” and in “capillaries”, best visible by single files of aggregated RBCs moving like trains into a first order “venule” (e.g., see right “capillary” at driving pressures of 10 – 40 cmH2O in Fig. 2). A driving pressure of 60 cmH2O resulted in such high velocities that we cannot make statements about the occurrence of RBC aggregation. Adhesion of RBCs to the mPEG-silane treated walls of the microchannel was very rarely observed, and was always transient, across all driving pressures evaluated.
Figure 2.
Photographs taken at the level of “arterioles” (top row), “capillaries” (middle row) and “venules” (bottom row) of RBC suspensions flowing through the AMVN at increasing driving pressures ranging from 5 to 60 cm H2O (0.49 to 5.884 kPa). RBCs were suspended in a 46.5 g/L solution of dextran 40 in GASP buffer (D40; far left) or in a 35.0 g/L solution of dextran 70 in GASP buffer (D70; right). Arrow indicates direction of flow. Scale bar is 20 μm.
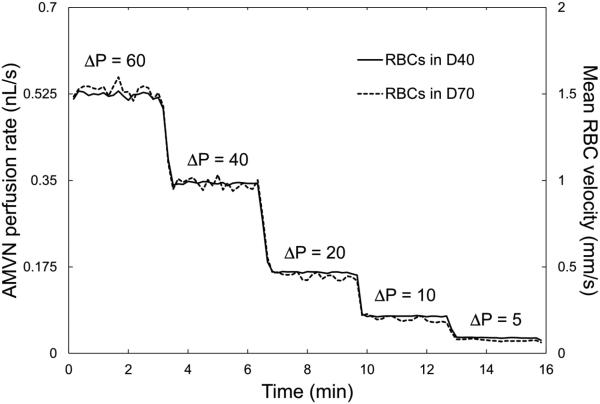
Figure 3 shows characteristic traces of the AMVN perfusion rate, calculated from the mean RBC velocity in “venular” microchannels. The perfusion rate traces for RBCs suspended in dextran 40 (without aggregation) were relatively constant for any given driving pressure, while those for RBCs suspended in dextran 70 (with aggregation) showed a considerable undulating variation over time, which most likely reflected the intermittent passage through the network of large aggregates with aberrant, rotational movements.
Figure 3.
Typical traces of AMVN perfusion rate (left scale) and mean RBC velocity (right scale) obtained at the “venular” level for 30% hematocrit suspensions of RBCs in a 46.5 g/L solution of dextran 40 in GASP buffer (D40; solid line) or in a 35.0 g/L solution of dextran 70 in GASP buffer (D70; dashed line) at decreasing driving pressures (ΔP) ranging from 60 to 5 cm H2O (5.88 to 0.49 kPa).
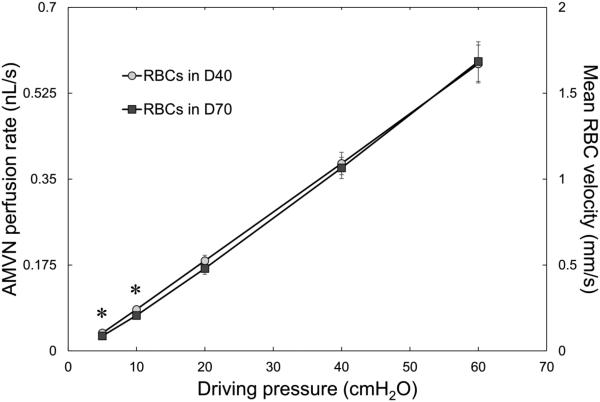
Figure 4 shows the AMVN perfusion rates (calculated using the measured mean RBC velocities in “venular” microchannels) for RBC suspensions in dextran 40 and dextran 70, for a range of driving pressures. There was a linear relationship between the driving pressure applied to the AMVN and the AMVN perfusion rate. The AMVN perfusion rates were similar for RBC suspensions in dextran 70 (with aggregation) and in dextran 40 suspensions (without aggregation) for all driving pressures.
Figure 4.
AMVN perfusion rate and mean RBC velocity measured in the “venular” microchannels for 30% hematocrit suspensions of RBCs in a 46.5 g/L solution of dextran 40 in GASP buffer (D40; circles) or in a 35.0 g/L dextran 70 solution in GASP buffer (D70; squares) for driving pressures ranging from 5 to 60 cm H2O (0.49 to 5.88 kPa). Asterisks denote a statistically significant difference (p-value < 0.01) in AMVN perfusion rate and mean RBC velocity between RBCs in D40 and D70. Mean values ± standard deviation, n = 7.
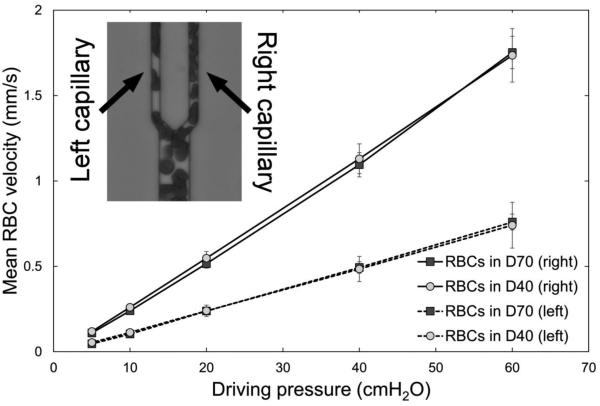
We also measured RBC velocities in two different 5 μm “capillaries”. Figure 5 shows the results of these measurements. The two “capillaries” (Fig. 5 inset) had very different RBC velocities despite having the same dimensions, because the right “capillary” branched from a larger feeding vessel than the left “capillary” (see schematic drawing of an AMVN on the left side of Fig. 2). A linear relationship between driving pressure and RBC velocity was also seen in these two different “capillaries”, and no difference in RBC velocity was observed between RBC suspensions in dextran 70 with aggregation and in dextran 40 suspensions without aggregation, even at low pressures, when linear RBC aggregates were clearly visible within the “capillaries” (see Fig. 2).
Figure 5.
Mean RBC velocity measured in “capillary” microchannels – corresponding to the left capillary (dashed line) and right capillary (solid line) – for RBCs suspended at 30% hematocrit in a 46.5 g/L solution of dextran 40 in GASP buffer (D40; circles) or in a 35.0 g/L solution of dextran 70 solution in GASP buffer (D70; squares) for driving pressures ranging from 5 to 60 cm H2O (0.49 to 5.88 kPa). Asterisks denote a statistically significant difference (p-value < 0.01) in mean RBC velocity between RBCs in D40 and D70 in both the left and right “capillaries”. Mean values ± standard deviation, n = 7.
Figure 6 shows the estimated hematocrits in “capillaries” and “venules” for RBCs suspended in dextran 70 with respect to RBCs suspended in dextran 40, across the same range of driving pressures at which AMVN perfusion rates were evaluated. There was no difference in hematocrit at high driving pressure (60 cmH2O). With decreasing driving pressures down to 10 cmH2O, estimated hematocrits in aggregating suspension medium dextran 70 became gradually higher with respect to non-aggregating dextran 40. This difference in estimated hematocrit was more pronounced in “capillaries” (up to 30 – 40% at driving pressures of 5 – 10 cmH2O) than in “venules” (up to 10% at driving pressures of 10 – 20 cmH2O).
Compared with feeding arterioles or draining venules, capillaries have a lower hematocrit, which is called the Fahraeus effect [19]. We have analyzed the difference in estimated hematocrit between “capillaries” and draining “venules” of the AMVN (Figure 7). For RBCs in dextran 40, i.e. without RBC aggregation, “capillary” hematocrit was 30 – 40% below “venular” hematocrit and showed a tendency to become more pronounced with decreasing driving pressure. In contrast, aggregation-inducing dextran 70 reduced the “capillary” hematocrit to only 20 – 25% of the “venular” hematocrit at driving pressures of 5 – 40 cmH2O, where RBC aggregation was visually observed (Fig. 2).
Discussion
The key finding of this study is that RBC aggregation did not affect the RBC flow velocity through microchannels of the AMVN. Blood flow is determined by plasma viscosity, hematocrit, RBC deformability and RBC aggregation [58]. In our in vitro experiments, we kept every parameter except RBC aggregation constant, which allowed an appraisal of the influence of RBC aggregation by itself without confounding factors: 1) the viscosities of 46.5 g/L dextran 40 and 35 g/L dextran 70 solutions were equivalent, 2) the hematocrit was 30% for both suspensions, and 3) RBC deformability is not known to be affected by either version of dextran. The only difference between the two types of suspensions was presence of absence of RBC aggregation (Table 1), as documented by light transmission aggregometry either at stasis or at low flow (at a shear rate of 3 s−1) as well as by the increased viscosity at low shear rates (Fig. 1). In contrast to RBC suspensions in dextran 40, strong RBC aggregation was found in dextran 70, which confirms the results of the many previous studies done with dextran 70 [5,18,41,47,48,56,68].
As expected, RBC aggregation affected suspension viscosities at shear rates <10 s−1; in contrast to RBC suspensions in dextran 40, RBC suspensions in dextran 70 behaved as non-Newtonian fluids characterized by a progressive increase of viscosity with decreasing shear rate. At shear rates ≥10 s−1, suspension viscosities were similar for RBC suspensions in dextran 70 and dextran 40, indicating that RBC aggregates were disrupted at these higher shear rates. The shear rate threshold around 10 s−1 for RBC aggregation in a rotational viscometer is in agreement with earlier observations [12,16,58].
So far it has not been clear whether RBC aggregation occurs at all in the microcirculation in vivo, which is known to be a high shear environment. We have calculated pseudo-shear rates at capillary walls for our AMVN as the quotient of mean RBC velocity in the channel and channel diameter [31]. For RBC suspensions flowing through the faster capillary (shown on the right in Fig. 5 inset), pseudo-shear rates of 22, 48, 104, 220, and 350 s−1 correspond to driving pressures of 5, 10, 20, 40 and 60 cmH2O respectively. We observed RBC aggregates even at these high wall shear rates (Fig. 2). This observation could be explained by the fact that shear forces act differently on RBC aggregates in the relatively large gaps of rotational viscometers (500 μm) than in 5 μm microchannels. Shear forces disrupt aggregates only when they produce velocity gradients between neighboring cells, e.g., when one plane of flow is moved relative to the adjacent plane, as is the case in rotational viscometers. In the case of single-file flow through 5 μm microchannels, the velocity gradient between adjacent RBCs is virtually zero and hence shear forces are negligible. This could potentially explain how RBC aggregates may form during flow through the microcirculation [31]. The occurrence of RBC aggregation in microcirculatory flow under physiologically relevant flow rates agrees with observations in vitro in microfluidic channels with a diameter of 4.5 μm and in capillaries in vivo [12,38].
RBC aggregation induced by dextran 70 did not affect RBC flow velocity in “capillaries” and “venules” of the AMVN, and thus had no influence on AMVN perfusion rates at any driving pressure. This observation is in agreement with the work of others [6,11,15,17,30,66,69,70], but contradicts many studies, which suggested a negative influence of RBC aggregation on microvascular perfusion [3,7,10,63,67]. The difference could be due to the difference in experimental approaches. In the discordant studies, RBC aggregation was changed by the addition of an aggregating substance, which concomitantly also increased the viscosity of plasma or other suspending medium. Since the viscosity of the suspending medium is a major determinant of microvascular perfusion, these studies could not separate between the influence of RBC aggregation alone and viscosity of the suspending medium [4,28,49]. Carefully matching viscosity of both aggregating and non-suspending media in our experiments allowed us to draw the conclusion that RBC aggregation induced by dextran 70 per se did not affect AMVN perfusion.
The microscopic impression of a lower hematocrit in AMVN microchannels for dextran 40 suspensions compared with dextran 70 prompted further investigations. To this end we estimated changes in hematocrit based on differences in color density in “capillaries” and “venules” of the AMVN. It was confirmed that the hematocrit was lower, for RBCs suspended in dextran 40 (i.e., without aggregation) as compared to aggregating dextran 70 suspensions, in “capillaries”, and to a lesser degree also in “venules”. RBC aggregation increases sedimentation rate and could theoretically explain higher hematocrit values with dextran 70 in AMVNs. RBC sedimentation is, however, characterized by a lag phase of several minutes (usually 10 – 20 min) before it becomes visible [21]. In addition, we prevented RBC sedimentation in the inlets of the AMVN devices with magnetically actuated micro-stirring bars. Furthermore, the fact that the changes in hematocrit were dissimilar in “capillaries” and “venules” (up to a 4-fold difference) make an artefact rather unlikely. These observations and data presented in Fig. 7 suggest that RBC aggregation may blunt to some extent the Fahraeus effect (i.e., the lowering of hematocrit during the passage of capillaries [22,53]).
The AMVN experimental setup has inherent limitations. It is an in vitro study on an idealized, artificial microvasculature comprised of rectangular microchannels 5 to 70 μm wide, but only 5 μm high. Thus, our device simulates the in vivo dimensions of arterioles and venules less well, allowing only the formation of two-dimensional, but not 3-dimensional aggregates, which occur in such vessels [8]. Furthermore, the AMVN lacks endothelial lining. In vivo, endothelial cells, coated by a glycocalix, interact with flowing RBCs and produce the vasodilatory active nitric oxide (NO). It has been recently shown that RBC aggregation reduces NO production [17]. Additionally, the AMVN device is constructed of PDMS, which is stiff and inert, unlike microvessels in vivo which are capable of dynamically altering vessel diameter in order to modulate blood flow [13,24]. However, spontaneous self-sustained oscillations of capillary blood flow, resulting solely from the non-linear rheological behavior of blood at the microscale, have previously been demonstrated in the AMVN system despite the lack of active regulatory input, such as vasomotion [24]. Last but not least, we studied only samples with a single standardized hematocrit and suspending medium viscosity. Our data, therefore, should only be used to draw conclusions about the hemodynamic effects of RBC aggregation induced by dextran 70 in vitro. They should not be directly and uncritically extrapolated to the microcirculation in vivo since dextran solutions, although studied intensively, do not represent physiological suspensions. Nevertheless, the AMVN has been rigorously validated in several studies [13,14,44,50,51,54,55] and it is arguably the best available model system for measuring the influence of various properties of blood on microvascular perfusion in vivo.
Limitations notwithstanding, our data are not in favor of therapeutic reduction of RBC aggregation in patients, which has been attempted by lowering fibrinogen with the snake venom ancrod in acute stroke [27,36], by infusing low molecular weight dextran (dextran 40) in critically ill patients [37], hydroxyethyl starch (6% HAES 130/0.4) in severe sepsis [20,43] and during cardiopulmonary bypass operations [39]. It is noteworthy that the latter study showed that reduced RBC aggregation induced by HAES 130/0.4 resulted in an undesirable activation of endothelial cells [39]. One of these clinical studies on patients receiving either HAES 130/0.4 or saline also analyzed sublingual microcirculation and found an increased capillary flow index, percentage of perfused capillaries and perfused capillary density after HAES 130/0.4 [20]. However, these therapeutic approaches have not been consistently successful, and are no longer recommended [29,40,46,52,64,71]. Our data show that the negative results of these clinical trials could be explained, at least in part, by RBC aggregation having no negative influence on tissue perfusion, and therefore lowering of RBC aggregation could not be expected – from this purely hemorheological point of view – to improve the clinical outcome of a disease.
RBC oxygen transport capacity, and thus tissue oxygenation, depends on both capillary hematocrit and blood flow velocity [45]. Our data of a higher “capillary” hematocrit and unchanged flow velocity for dextran 70 suspensions suggests that increased RBC aggregation during an acute phase reaction could be beneficial for tissue oxygenation. This is an intriguing finding, which, if confirmed, could contribute significantly to a better understanding of pathophysiological mechanisms.
Acknowledgements
None.
Grants
This work has been supported by a 2012 NIH Director's Transformative Research Award (NHLBI R01HL117329, PI: Shevkoplyas).
Footnotes
Disclosures
All authors declare no potential conflicts of interest.
Data and materials availability
All data and described custom-written scripts are available from the authors upon reasonable request.
References
- 1.Alt E, Amann-Vesti BR, Madl C, Funk G, Koppensteiner R. Platelet aggregation and blood rheology in severe sepsis/septic shock: relation to the Sepsis-related Organ Failure Assessment (SOFA) score. Clinical hemorheology and microcirculation. 2004;30:107–115. [PubMed] [Google Scholar]
- 2.Ami RB, Barshtein G, Zeltser D, Goldberg Y, Shapira I, Roth A, Keren G, Miller H, Prochorov V, Eldor A, Berliner S, Yedgar S. Parameters of red blood cell aggregation as correlates of the inflammatory state. American journal of physiology Heart and circulatory physiology. 2001;280:H1982–1988. doi: 10.1152/ajpheart.2001.280.5.H1982. [DOI] [PubMed] [Google Scholar]
- 3.Arbel Y, Banai S, Benhorin J, Finkelstein A, Herz I, Halkin A, Keren G, Yedgar S, Barashtein G, Berliner S. Erythrocyte aggregation as a cause of slow flow in patients of acute coronary syndromes. Int J Cardiol. 2012;154:322–327. doi: 10.1016/j.ijcard.2011.06.116. [DOI] [PubMed] [Google Scholar]
- 4.Barras JP. Blood rheology - general review. Bibl Haematol. 1969;33:277–297. doi: 10.1159/000384849. [DOI] [PubMed] [Google Scholar]
- 5.Baskurt OK. Handbook of Hemorheology and Hemodynamics. IOS Press; 2007. [Google Scholar]
- 6.Baskurt OK, Bor-Kucukatay M, Yalcin O. The effect of red blood cell aggregation on blood flow resistance. Biorheology. 1999;36:447–452. [PubMed] [Google Scholar]
- 7.Baskurt OK, Meiselman HJ. RBC aggregation: more important than RBC adhesion to endothelial cells as a determinant of in vivo blood flow in health and disease. Microcirculation. 2008;15:585–590. doi: 10.1080/10739680802107447. [DOI] [PubMed] [Google Scholar]
- 8.Baskurt OK, Meiselman HJ. Erythrocyte aggregation: basic aspects and clinical importance. Clinical hemorheology and microcirculation. 2013;53:23–37. doi: 10.3233/CH-2012-1573. [DOI] [PubMed] [Google Scholar]
- 9.Baskurt OK, Temiz A, Meiselman HJ. Red blood cell aggregation in experimental sepsis. J Lab Clin Med. 1997;130:183–190. doi: 10.1016/s0022-2143(97)90094-9. [DOI] [PubMed] [Google Scholar]
- 10.Bishop JJ, Nance PR, Popel AS, Intaglietta M, Johnson PC. Effect of erythrocyte aggregation on velocity profiles in venules. American journal of physiology Heart and circulatory physiology. 2001;280:H222–236. doi: 10.1152/ajpheart.2001.280.1.H222. [DOI] [PubMed] [Google Scholar]
- 11.Braasch D. The missing negative effect of red cell aggregation upon blood flow in small capillaries at low shear forces. Biorheology Suppl. 1984;1:227–230. doi: 10.3233/bir-1984-23s139. [DOI] [PubMed] [Google Scholar]
- 12.Brust M, Aouane O, Thiebaud M, Flormann D, Verdier C, Kaestner L, Laschke MW, Selmi H, Benyoussef A, Podgorski T, Coupier G, Misbah C, Wagner C. The plasma protein fibrinogen stabilizes clusters of red blood cells in microcapillary flows. Sci Rep. 2014;4:4348. doi: 10.1038/srep04348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burns JM, Yang X, Forouzan O, Sosa JM, Shevkoplyas SS. Artificial microvascular network: a new tool for measuring rheologic properties of stored red blood cells. Transfusion. 2012;52:1010–1023. doi: 10.1111/j.1537-2995.2011.03418.x. [DOI] [PubMed] [Google Scholar]
- 14.Burns JM, Yoshida T, Dumont LJ, Yang X, Piety NZ, Shevkoplyas SS. Deterioration of red blood cell mechanical properties is reduced in anaerobic storage. Blood transfusion = Trasfusione del sangue. 2016;14:80–88. doi: 10.2450/2015.0241-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charansonney O, Mouren S, Dufaux J, Duvelleroy M, Vicaut E. Red blood cell aggregation and blood viscosity in an isolated heart preparation. Biorheology. 1993;30:75–84. [PubMed] [Google Scholar]
- 16.Chien S, Usami S, Dellenback RJ, Gregersen MI, Nanninga LB, Guest MM. Blood viscosity: influence of erythrocyte aggregation. Science. 1967;157:829–831. doi: 10.1126/science.157.3790.829. [DOI] [PubMed] [Google Scholar]
- 17.Cho S, Namgung B, Kim HS, Leo HL, Kim S. Effect of erythrocyte aggregation at pathological levels on NO/O2 transport in small arterioles. Clinical hemorheology and microcirculation. 2015;59:163–175. doi: 10.3233/CH-141837. [DOI] [PubMed] [Google Scholar]
- 18.Chong-Martinez B, Buchanan TA, Wenby RB, Meiselman HJ. Decreased red blood cell aggregation subsequent to improved glycaemic control in Type 2 diabetes mellitus. Diabet Med. 2003;20:301–306. doi: 10.1046/j.1464-5491.2003.00926.x. [DOI] [PubMed] [Google Scholar]
- 19.Cicco G, Pirrelli A. Red blood cell (RBC) deformability, RBC aggregability and tissue oxygenation in hypertension. Clinical hemorheology and microcirculation. 1999;21:169–177. [PubMed] [Google Scholar]
- 20.Dubin A, Pozo MO, Casabella CA, Murias G, Palizas F, Jr., Moseinco MC, Kanoore Edul VS, Palizas F, Estenssoro E, Ince C. Comparison of 6% hydroxyethyl starch 130/0.4 and saline solution for resuscitation of the microcirculation during the early goal-directed therapy of septic patients. J Crit Care. 2010;25:659. doi: 10.1016/j.jcrc.2010.04.007. e651-658. [DOI] [PubMed] [Google Scholar]
- 21.Fabry TL. Mechanism of erythrocyte aggregation and sedimentation. Blood. 1987;70:1572–1576. [PubMed] [Google Scholar]
- 22.Fahraeus R, Lindqvist T. The viscosity of blood in narrow capillary tubes. American Journal of Physiology. 1931;96:562–568. [Google Scholar]
- 23.Flormann D, Kuder E, Lipp P, Wagner C, Kaestner L. Is there a role of C-reactive protein in red blood cell aggregation? Int J Lab Hematol. 2015;37:474–482. doi: 10.1111/ijlh.12313. [DOI] [PubMed] [Google Scholar]
- 24.Forouzan O, Yang X, Sosa JM, Burns JM, Shevkoplyas SS. Spontaneous oscillations of capillary blood flow in artificial microvascular networks. Microvascular research. 2012;84:123–132. doi: 10.1016/j.mvr.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 26.Goldin Y, Tulshinski T, Arbel Y, Rogowski O, Ami RB, Serov J, Halperin P, Shapira I, Berliner S. Rheological consequences of acute infections: the rheodifference between viral and bacterial infections. Clinical hemorheology and microcirculation. 2007;36:111–119. [PubMed] [Google Scholar]
- 27.Hennerici MG, Kay R, Bogousslavsky J, Lenzi GL, Verstraete M, Orgogozo JM, investigators E. Intravenous ancrod for acute ischaemic stroke in the European Stroke Treatment with Ancrod Trial: a randomised controlled trial. Lancet. 2006;368:1871–1878. doi: 10.1016/S0140-6736(06)69776-6. [DOI] [PubMed] [Google Scholar]
- 28.Jung F, Mrowietz C, Hiebl B, Franke RP, Pindur G, Sternitzky R. Influence of rheological parameters on the velocity of erythrocytes passing nailfold capillaries in humans. Clinical hemorheology and microcirculation. 2011;48:129–139. doi: 10.3233/CH-2011-1392. [DOI] [PubMed] [Google Scholar]
- 29.Karakala N, Raghunathan K, Shaw AD. Intravenous fluids in sepsis: what to use and what to avoid. Curr Opin Crit Care. 2013;19:537–543. doi: 10.1097/MCC.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 30.Katanov D, Gompper G, Fedosov DA. Microvascular blood flow resistance: Role of red blood cell migration and dispersion. Microvascular research. 2015;99:57–66. doi: 10.1016/j.mvr.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Kim S, Popel AS, Intaglietta M, Johnson PC. Aggregate formation of erythrocytes in postcapillary venules. American journal of physiology Heart and circulatory physiology. 2005;288:H584–590. doi: 10.1152/ajpheart.00690.2004. [DOI] [PubMed] [Google Scholar]
- 32.Koenig W. Fibrin(ogen) in cardiovascular disease: an update. Thrombosis and haemostasis. 2003;89:601–609. [PubMed] [Google Scholar]
- 33.Koscielny J, Jung EM, Mrowietz C, Kiesewetter H, Latza R. Blood fluidity, fibrinogen, and cardiovascular risk factors of occlusive arterial disease: results of the Aachen study. Clinical hemorheology and microcirculation. 2004;31:185–195. [PubMed] [Google Scholar]
- 34.Kwaan HC. Role of plasma proteins in whole blood viscosity: a brief clinical review. Clinical hemorheology and microcirculation. 2010;44:167–176. doi: 10.3233/CH-2010-1271. [DOI] [PubMed] [Google Scholar]
- 35.Lee BK, Durairaj A, Mehra A, Wenby RB, Meiselman HJ, Alexy T. Hemorheological abnormalities in stable angina and acute coronary syndromes. Clinical hemorheology and microcirculation. 2008;39:43–51. [PubMed] [Google Scholar]
- 36.Levy DE, del Zoppo GJ, Demaerschalk BM, Demchuk AM, Diener HC, Howard G, Kaste M, Pancioli AM, Ringelstein EB, Spatareanu C, Wasiewski WW. Ancrod in acute ischemic stroke: results of 500 subjects beginning treatment within 6 hours of stroke onset in the ancrod stroke program. Stroke. 2009;40:3796–3803. doi: 10.1161/STROKEAHA.109.565119. [DOI] [PubMed] [Google Scholar]
- 37.Matsuda H, Shoemaker WC. Survivor's and nonsurvivors' responses to dextran 40. Hemodynamic and oxygen transport changes in critically ill patients. Arch Surg. 1975;110:301–305. doi: 10.1001/archsurg.1975.01360090071015. [DOI] [PubMed] [Google Scholar]
- 38.McHedlishvili G, Gobejishvili L, Beritashvili N. Effect of intensified red blood cell aggregability on arterial pressure and mesenteric microcirculation. Microvascular research. 1993;45:233–242. doi: 10.1006/mvre.1993.1021. [DOI] [PubMed] [Google Scholar]
- 39.Morariu AM, Gu YJ, Huet RC, Siemons WA, Rakhorst G, Oeveren WV. Red blood cell aggregation during cardiopulmonary bypass: a pathogenic cofactor in endothelial cell activation? European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2004;26:939–946. doi: 10.1016/j.ejcts.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 40.Mutter TC, Ruth CA, Dart AB. Hydroxyethyl starch (HES) versus other fluid therapies: effects on kidney function. Cochrane Database Syst Rev. 2013;7:CD007594. doi: 10.1002/14651858.CD007594.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nash G, Wenby R, SOWEMIMOCOKER S, Meiselman H. INFLUENCE OF CELLULAR PROPERTIES ON RED-CELL AGGREGATION. Clinical Hemorheology. 1987;7:93–108. [Google Scholar]
- 42.Ong PK, Jain S, Namgung B, Woo YI, Kim S. Cell-free layer formation in small arterioles at pathological levels of erythrocyte aggregation. Microcirculation. 2011;18:541–551. doi: 10.1111/j.1549-8719.2011.00114.x. [DOI] [PubMed] [Google Scholar]
- 43.Perner A, Haase N, Winkel P, Guttormsen AB, Tenhunen J, Klemenzson G, Muller RG, Aneman A, Wetterslev J. Long-term outcomes in patients with severe sepsis randomised to resuscitation with hydroxyethyl starch 130/0.42 or Ringer's acetate. Intensive Care Med. 2014;40:927–934. doi: 10.1007/s00134-014-3311-y. [DOI] [PubMed] [Google Scholar]
- 44.Piety NZ, Reinhart WH, Pourreau PH, Abidi R, Shevkoplyas SS. Shape matters: the effect of red blood cell shape on perfusion of an artificial microvascular network. Transfusion. 2016;56:844–851. doi: 10.1111/trf.13449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pries AR, Neuhaus D, Gaehtgens P. Blood viscosity in tube flow: dependence on diameter and hematocrit. Am J Physiol. 1992;263:H1770–1778. doi: 10.1152/ajpheart.1992.263.6.H1770. [DOI] [PubMed] [Google Scholar]
- 46.Qureshi SH, Rizvi SI, Patel NN, Murphy GJ. Meta-analysis of colloids versus crystalloids in critically ill, trauma and surgical patients. Br J Surg. 2015 doi: 10.1002/bjs.9943. [DOI] [PubMed] [Google Scholar]
- 47.Rampling MW, Whittingstall P, Martin G, Bignall S, Rivers RP, Lissauer TJ, Bailey PC. A comparison of the rheologic properties of neonatal and adult blood. Pediatric research. 1989;25:457–460. doi: 10.1203/00006450-198905000-00006. [DOI] [PubMed] [Google Scholar]
- 48.Razavian SM, Guillemin MT, Guillet R, Beuzard Y, Boynard M. Assessment of red blood cell aggregation with dextran by ultrasonic interferometry. Biorheology. 1991;28:89–97. doi: 10.3233/bir-1991-281-209. [DOI] [PubMed] [Google Scholar]
- 49.Reinhart WH, Lutolf O, Nydegger UR, Mahler F, Straub PW. Plasmapheresis for hyperviscosity syndrome in macroglobulinemia Waldenstrom and multiple myeloma: influence on blood rheology and the microcirculation. J Lab Clin Med. 1992;119:69–76. [PubMed] [Google Scholar]
- 50.Reinhart WH, Piety NZ, Deuel JW, Makhro A, Schulzki T, Bogdanov N, Goede JS, Bogdanova A, Abidi R, Shevkoplyas SS. Washing stored red blood cells in an albumin solution improves their morphologic and hemorheologic properties. Transfusion. 2015 doi: 10.1111/trf.13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reinhart WH, Piety NZ, Goede JS, Shevkoplyas SS. Effect of osmolality on erythrocyte rheology and perfusion of an artificial microvascular network. Microvascular research. 2015;98:102–107. doi: 10.1016/j.mvr.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rochwerg B, Alhazzani W, Sindi A, Heels-Ansdell D, Thabane L, Fox-Robichaud A, Mbuagbaw L, Szczeklik W, Alshamsi F, Altayyar S, Ip WC, Li G, Wang M, Wludarczyk A, Zhou Q, Guyatt GH, Cook DJ, Jaeschke R, Annane D. Fluid resuscitation in sepsis: a systematic review and network meta-analysis. Ann Intern Med. 2014;161:347–355. doi: 10.7326/M14-0178. Fluids in S, Septic Shock G. [DOI] [PubMed] [Google Scholar]
- 53.Secomb TW, Pries AR, Gaehtgens P. Hematocrit fluctuations within capillary tubes and estimation of Fahraeus effect. Int J Microcirc Clin Exp. 1987;5:335–345. [PubMed] [Google Scholar]
- 54.Shevkoplyas SS, Gifford SC, Yoshida T, Bitensky MW. Prototype of an in vitro model of the microcirculation. Microvascular research. 2003;65:132–136. doi: 10.1016/s0026-2862(02)00034-1. [DOI] [PubMed] [Google Scholar]
- 55.Shevkoplyas SS, Yoshida T, Gifford SC, Bitensky MW. Direct measurement of the impact of impaired erythrocyte deformability on microvascular network perfusion in a microfluidic device. Lab Chip. 2006;6:914–920. doi: 10.1039/b601554a. [DOI] [PubMed] [Google Scholar]
- 56.Sowemimo-Coker SO, Whittingstall P, Pietsch L, Bauersachs RM, Wenby RB, Meiselman HJ. Effects of cellular factors on the aggregation behavior of human, rat and bovine erythrocytes. Clinical Hemorheology. 1989;9:723–737. [Google Scholar]
- 57.Spengler MI, Svetaz MJ, Leroux MB, Bertoluzzo SM, Carrara P, Van Isseldyk F, Petrelli D, Parente FM, Bosch P. Erythrocyte aggregation in patients with systemic lupus erythematosus. Clinical hemorheology and microcirculation. 2011;47:279–285. doi: 10.3233/CH-2011-1409. [DOI] [PubMed] [Google Scholar]
- 58.Surgenor DMN. The Red Blood Cell. Elsevier Science; 2013. [Google Scholar]
- 59.Suzuki Y, Tateishi N, Soutani M, Maeda N. Flow behavior of erythrocytes in microvessels and glass capillaries: effects of erythrocyte deformation and erythrocyte aggregation. Int J Microcirc Clin Exp. 1996;16:187–194. doi: 10.1159/000179172. [DOI] [PubMed] [Google Scholar]
- 60.Tikhomirova IA, Oslyakova AO, Mikhailova SG. Microcirculation and blood rheology in patients with cerebrovascular disorders. Clinical hemorheology and microcirculation. 2011;49:295–305. doi: 10.3233/CH-2011-1480. [DOI] [PubMed] [Google Scholar]
- 61.Toker S, Rogowski O, Melamed S, Shirom A, Shapira I, Berliner S, Zeltser D. Association of components of the metabolic syndrome with the appearance of aggregated red blood cells in the peripheral blood. An unfavorable hemorheological finding. Diabetes/metabolism research and reviews. 2005;21:197–202. doi: 10.1002/dmrr.502. [DOI] [PubMed] [Google Scholar]
- 62.Tripette J, Alexy T, Hardy-Dessources MD, Mougenel D, Beltan E, Chalabi T, Chout R, Etienne-Julan M, Hue O, Meiselman HJ, Connes P. Red blood cell aggregation, aggregate strength and oxygen transport potential of blood are abnormal in both homozygous sickle cell anemia and sickle-hemoglobin C disease. Haematologica. 2009;94:1060–1065. doi: 10.3324/haematol.2008.005371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tripette J, Nguyen LC, Allard L, Robillard P, Soulez G, Cloutier G. In vivo venous assessment of red blood cell aggregate sizes in diabetic patients with a quantitative cellular ultrasound imaging method: proof of concept. PLoS One. 2015;10:e0124712. doi: 10.1371/journal.pone.0124712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tseng MY, Hutchinson PJ, Kirkpatrick PJ. Effects of fluid therapy following aneurysmal subarachnoid haemorrhage: a prospective clinical study. Br J Neurosurg. 2008;22:257–268. doi: 10.1080/02688690701832100. [DOI] [PubMed] [Google Scholar]
- 65.Vaya A, Calvo J, Alcala C, Mico L, Todoli J, Ricart JM. Rheological alterations and thrombotic events in patients with systemic lupus erythematosus. Clinical hemorheology and microcirculation. 2012;51:51–58. doi: 10.3233/CH-2011-1508. [DOI] [PubMed] [Google Scholar]
- 66.Verkeste CM, Boekkooi PF, Saxena PR, Peeters LL. Increased red cell aggregation does not reduce uteroplacental blood flow in the awake, hemoconcentrated, late-pregnant guinea pig. Pediatric research. 1992;31:91–93. doi: 10.1203/00006450-199201000-00017. [DOI] [PubMed] [Google Scholar]
- 67.Vicaut E. Opposite effects of red blood cell aggregation on resistance to blood flow. J Cardiovasc Surg (Torino) 1995;36:361–368. [PubMed] [Google Scholar]
- 68.Vicaut E, Hou X, Decuypere L, Taccoen A, Duvelleroy M. Red blood cell aggregation and microcirculation in rat cremaster muscle. Int J Microcirc Clin Exp. 1994;14:14–21. doi: 10.1159/000178201. [DOI] [PubMed] [Google Scholar]
- 69.Yalcin O, Uyuklu M, Armstrong JK, Meiselman HJ, Baskurt OK. Graded alterations of RBC aggregation influence in vivo blood flow resistance. American journal of physiology Heart and circulatory physiology. 2004;287:H2644–2650. doi: 10.1152/ajpheart.00534.2004. [DOI] [PubMed] [Google Scholar]
- 70.Yaylali YT, Susam I, Demir E, Bor-Kucukatay M, Uludag B, Kilic-Toprak E, Erken G, Dursunoglu D. Increased red blood cell deformability and decreased aggregation as potential adaptive mechanisms in the slow coronary flow phenomenon. Coron Artery Dis. 2013;24:11–15. doi: 10.1097/MCA.0b013e32835b0bdf. [DOI] [PubMed] [Google Scholar]
- 71.Zhong JZ, Wei D, Pan HF, Chen YJ, Liang XA, Yang ZY, Tang HB. Colloid solutions for fluid resuscitation in patients with sepsis: systematic review of randomized controlled trials. J Emerg Med. 2013;45:485–495. doi: 10.1016/j.jemermed.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 72.Zilberman-Kravits D, Harman-Boehm I, Shuster T, Meyerstein N. Increased red cell aggregation is correlated with HbA1C and lipid levels in type 1 but not type 2 diabetes. Clinical hemorheology and microcirculation. 2006;35:463–471. [PubMed] [Google Scholar]
- 73.Zorio E, Murado J, Arizo D, Rueda J, Corella D, Simo M, Vaya A. Haemorheological parameters in young patients with acute myocardial infarction. Clinical hemorheology and microcirculation. 2008;39:33–41. [PubMed] [Google Scholar]