Abstract
Beta cell death may occur both after islet isolation and during infusion back into recipients undergoing total pancreatectomy with islet autotransplantation (TPIAT) for chronic pancreatitis. We measured the novel beta cell death marker unmethylated insulin (INS) DNA in TPIAT recipients before and immediately after islet infusion (n=21), and again 90 days post-TPIAT concurrent with metabolic functional assessments (n=25). As expected, INS DNA decreased after pancreatectomy (p=0.0002). Following islet infusion, all TPIAT recipients had an elevated unmethylated INS DNA ratio in the first hours. In 4 samples (3 patients), INS DNA was also assessed immediately after islet isolation and again before islet infusion to assess the impact of the isolation process: unmethylated and methylated INS DNA fractions both increased over this interval, suggesting death of beta cells and exocrine tissue before islet infusion. Higher mixed meal testing glucose excursion was associated with persistently elevated INS DNA at day 90. In conclusion, we observed universal early elevations in the beta cell death marker INS DNA after TPAIT, with pronounced elevations in the islet supernatant pre-infusion, likely reflecting beta cell death induced by islet isolation. Persistent post-transplant elevation of INS DNA predicted greater hyperglycemia at 90 days.
Introduction
Total pancreatectomy with islet autotransplantation (TPIAT) is performed to relieve pain in patients with severe chronic pancreatitis (1). The IAT component is intended to minimize post-operative diabetes by preserving endogenous beta cell mass, but success is complicated by post-isolation and post-infusion loss of beta cells. The islet isolation procedure itself induces high rates of beta cell apoptosis (2, 3). Subsequently, infusion of islets directly into the portal vasculature stimulates an instant blood-mediated inflammatory reaction (IBMIR) (4), which along with hypoxia and hyperglycemia (5–9) may further contribute to beta cell loss. These same limitations apply to allogenic islet transplantation for type 1 diabetes (T1D).
Islet mass is measured in the islet isolation lab before intraportal islet infusion (1). However, until recently, the islet transplant field has lacked biomarkers to detect beta cell loss (10, 11). Such markers may be critically important in addressing barriers to success in islet transplantation. Recently, our group has developed a method to measure beta cell-derived unmethylated insulin (INS) DNA in serum and used it to identify beta cell loss in patients with T1D (12, 13). This assay differentiates unmethylated and methylated CpG sites within an exon in the insulin gene found in bisulfite-treated circulating DNA. Unmethylated CpG sites are characteristic of actively transcribed genes. Because cellular DNA is released into the serum with cell death, the relative level of INS DNA with unmethylated CpG sites versus methylated CpG sites reflects beta cell loss because only within the beta cell is INS unmethylated for transcription (14–17). We reported previously that the ratio unmethylated:methylated INS DNA is elevated in early onset T1D, when beta cell destruction is occurring; is low in late-stage T1D, when few beta cells remain; and decreases following successful treatment of patients with new-onset T1D with anti-CD3 mAb (14). We also previously found that the relative level of unmethylated INS DNA increased following TPIAT (18), but the basis for the increase and levels of beta cell death in the days following transplantation had not been addressed. This prospective study assessed the utility of unmethylated INS DNA as a beta cell biomarker in TPIAT recipients.
Methods
Subject and study protocol
Patients age ≥11 years undergoing TPIAT at the University of Minnesota for intractable chronic or recurrent acute pancreatitis were prospectively enrolled. Repeated serum samples were drawn from 21 TPIAT recipients before surgery (day -2), post-pancreatectomy but pre-islet infusion, and then at +15, 30, and 60 minutes, 3, 6, 12, and 24 hours, and 3, 5, 7, 14, 21, 30, and 90 days after end of islet infusion to measure unmethylated insulin DNA levels; based on observations in the first 4 participants, the post-pancreatectomy, +15 min, and +30 min times were added and collected only for the last 17 participants. The same 21 participants and an additional 4 TPIAT recipients returned at 90 days post-TPIAT for unmethylated insulin DNA sampling and tests of islet function and glycemic control.
To investigate whether beta cell loss resulted from islet isolation versus islet infusion, in 3 further TPIAT recipients we collected supernatant from the islet graft immediately after islet isolation in the islet lab and then again immediately before islet infusion.
The study was approved by the University of Minnesota and Yale University Institutional Review Boards. Informed consent or parental informed consent with patient assent were obtained.
Surgical procedure and islet isolation techniques
Total or completion pancreatectomy, with partial duodenectomy, roux-en-Y duodenojejunostomy, splenectomy, and cholecystectomy were performed as previously described (1, 19). Islet isolation was performed with collagenase distention followed by mechanical digestion using the semi-automated method of Ricordi (20, 21). COBE purification was performed only when tissue volume exceeded 0.25 mL/kg body weight (22). Islet counts, expressed as total equivalents (IEQ), were obtained by manual count with dithizone staining. Following islet isolation and islet counting, the final islet product was washed twice with fresh transplant media and then placed in a single bag or divided between two bags depending on tissue volume.
For the three patients in whom samples from the islet product were obtained, supernatant samples were collected twice: (1) first from the islet product suspended in 100 mL of CMRL media immediately after islet washing; and (2) second from the final islet product suspended in 200 mL CMRL, collected in the operating room (OR) during infusion Paired samples were obtained from 4 bags of islet product in 3 TPIAT recipients in the lab and OR supernatant for analysis.
Islets were infused primarily into the portal vein with monitoring of portal pressures and/or flow. If portal hypertension prohibited infusion of all islets intraportally (n=8 cases) then a portion of the islets were placed in the peritoneal cavity or similar site. The end time of the portal infusion was designated as time 0 for lab draws.
Assays for unmethylated insulin DNA
The unmethylated insulin (INS) DNA assay used droplet digital PCR (ddPCR) as previously described (12). DNA was purified from 200ml of serum using QIAamp DNA Blood Kits as suggested by the manufacturer (Qiagen, Valencia, CA), with a modified incubation period of 20 min at 45°C in the final step. DNA was bisulfite treated using EZ DNA Methylation Kit (Zymo Research, Irvine, CA). We measured the levels of unmethylated INS DNA by ddPCR targeting two methylation-sensitive sites of the human insulin gene in positions +396 and +399 from the transcription start site (hg19_knownGene_uc021qcd.1 range=chr11:2181009-2182439) (13, 18). Each 25μl volume consisted of Droplet PCR supermix (BioRad), 900nM of primer, 250nM of probe, and 5μl of sample. The mixture and droplet generation oil were loaded onto a droplet generator (Biorad) and the generated droplets were transferred to a 96 well PCR plate. The PCR reaction was run on a thermal cycler with 10 min activation at 95ΰC, 40× a two-step amplification protocol (30s at 94ΰC denaturation and 60s at 58ΰC), and a 10 min inactivation step at 98ΰC. The DNA content of the droplets was analyzed with a QX100 droplet reader (Biorad), and QuantaSoft (Bio-Rad) Analysis software. Discrimination between droplets that did and did not contain the target (positives and negatives respectively) was achieved by applying a fluorescence amplitude threshold based on the amplitude read from the negative template control (NTC). For each sample the “ratio” of unmethylated INS DNA was calculated as the unmethylated counts/the sum of the unmethylated and methylated counts (U/[M+U]). The normal ratio was defined from healthy non-diabetic control subjects (n=49, mean±SD of 0.129±0.045) as ≤0.219, i.e. mean +2SD. Higher values were considered elevated.
Assays for islet engraftment and beta cell function at day 90 post-transplant
Patients returned at day 90±21 days post-TPIAT for metabolic assessments. Day 90 was selected because islet revascularization is complete (9). On two separate mornings, following an overnight fast, patients underwent three tests: on the first morning, mixed meal tolerance testing (MMTT) and on the second, intravenous glucose tolerance testing (IVGTT) followed immediately by a glucose-potentiated arginine-induced insulin secretion (GPAIS) study.
MMTT was performed by measuring glucose and C-peptide before and at 30 minute intervals for 2 hours after drinking Boost HP (6 mL/kg, max 360 mL) (23). The area under the curve (AUC) glucose and AUC C-peptide were calculated by the trapezoidal rule. IVGTT and GPAIS protocols have been previously described in islet transplant recipients (23, 24). IVGTT was performed by administering 0.3 grams/kg dextrose. Samples were collected before and +2, 3, 4, 5, 7, and 10 minutes after administering dextrose. Acute insulin response to glucose (AIRg) and acute C-peptide response to glucose (ACRg) were calculated from the AUC for the 10 minute interval subtracting out the baseline. At +10 minutes, a variable rate infusion of 20% dextrose was started and continued for 45 minutes, adjusted to maintain a target blood glucose of 230 mg/dL. At the end of the 45 minute clamp period, two baseline serum samples were obtained, 5 grams arginine was administered over 30 seconds, and insulin and C-peptide were obtained at times +2, 3, 4, and 5 minutes. Glucose-potentiated acute insulin response to arginine (AIRpot) and acute C-peptide response to arginine (ACRpot) were calculated as the mean of the 2–5 minute values minus the pre-arginine baseline value (25).
Statistics
Summary data are reported as median (interquartile range). Changes in U/(U+M) after baseline were tested using a mixed linear model (which generalizes a repeated-measures analysis using person and time after baseline as the fixed and random effect respectively. Levels of unmethylated and methylated INS DNA and the ratio were compared immediately post-isolation versus the time of infusion using paired t-tests. Finally, testing the association of functional measures with elevated ratio at follow up used multiple linear regression with primary predictor elevated INS DNA ratio (yes vs. no) and adjuster IEQ/kg body weight. Separate analyses were done for elevated ratio during 3 to 30 days post-TPIAT and at 90 days post-TPIAT.
The half-life of unmethylated INS DNA was calculated by fitting an exponential function to the repeated measures of unmethylated insulin DNA levels after islet infusion, where each patient had his/her own baseline values. The parameter λ characterizing the exponential decay was obtained by maximum likelihood estimation. The half-life was then calculated as t0.5 = log(0.5)/log(λ).
Results
Subject characteristics
TPIAT recipients had a median age of 28.3 (IQR 18.0, 42.1) years and 314,600 IEQ (169649, 425500) transplanted (Table 1).
Table 1.
Demographic characteristics of the 25 TPIAT recipients with serum unmethylated INS DNA measures in the first 30 days post-TPIAT and/or at day 90 for comparison with metabolic parameters.
| Median | IQR or % | |
|---|---|---|
| Age (y) | 28.3 | 17.6, 44.6 |
| Pediatric, n (%) | 6 | 24% |
| Sex F, n (%) | 15 | 60% |
| BMI (kg/m2) | 25.0 | 21.5, 28.1 |
| HbA1c (%) pre-transplant | 5.3 | 5.1, 5.6 |
| Etiology, n (%) | ||
| Hereditary | 9 | 36% |
| Idiopathic | 8 | 32% |
| Pancreas Divsium | 4 | 16% |
| Other | 4 | 16% |
| IEQ total | 314,600 | 157,324, 453,300 |
| IEQ/kg | 3,912 | 2,677, 6,571 |
| IEQ total intraportal | 296,100 | 144,950, 388,200 |
| IEQ/kg intraportal | 3,912 | 2,181, 5,975 |
| Day 90 insulin use* (u/kg/d) | 0.27 | 0.12, 0.38 |
| Day 90 HbA1c | 6.0 | 5.6, 6.4 |
| Day 90 metabolic measures | ||
| AUC glucose (mg/dL*min) | 15870 | 13,725, 20,070 |
| AUC C-peptide (ng/mL*min) | 150 | 93, 306 |
| AIRg (mIU/mL*min) | 135 | 22, 233 |
| ACRg (ng/mL*min) | 7.55 | 1.40, 12.95 |
| AIRpot (mIU/mL) | 31.9 | 19.1, 65.7 |
| ACRpot (ng/mL) | 1.45 | 0.68, 3.08 |
Legend: IQR = interquartile range; IEQ = islet equivalents; AUC = area under the curve; AIR= acute insulin response to glucose; AIRpot = glucose potentiated acute insulin response to arginine; ACR = acute C-peptide response to glucose; ACRpot = glucose potentiated acute C-peptide response to arginine; Patients are maintained on insulin per protocol through day 90 to maintain euglycemia.
Unmethylated insulin DNA before and after islet infusion
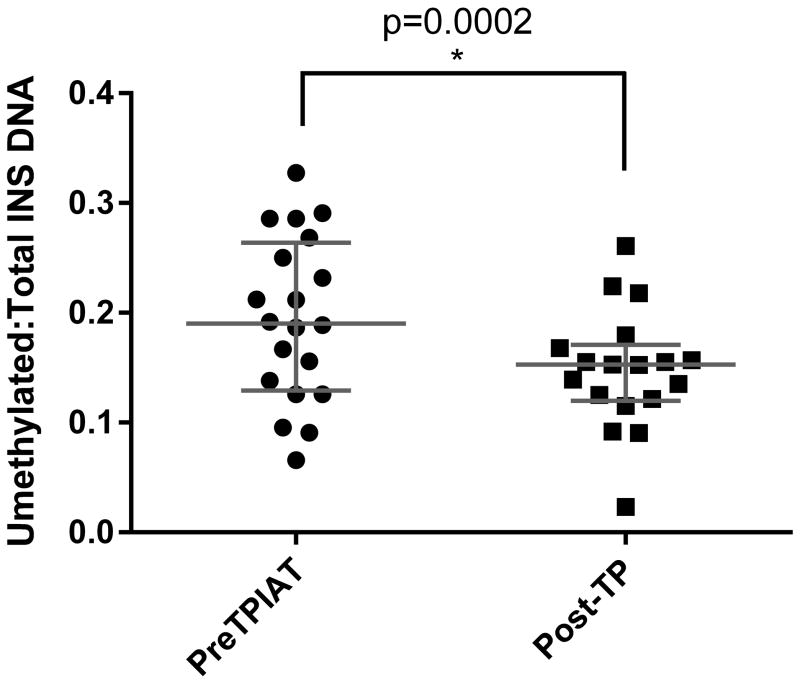
Serum samples were obtained before and after complete pancreatectomy. As expected, the ratio of INS DNA was significantly lower after the procedure (p=0.0002; Figure 1): Before pancreatectomy, samples from 7/21 subjects were above the normal range ratio for unmethylated to total INS DNA (>0.219), suggesting increased beta cell death related to chronic pancreatitis. After pancreatectomy and before islet infusion, 2/18 levels were above the normal range and the median ratio was 0.152 (IQR 0.121, 0.179) indicating that the targeted exon is unmethylated in a small but detectable proportion of DNA molecules from non-pancreatic tissue.
Figure 1.
Unmethylated to methylated INS DNA ratios measured before TPIAT and post-pancreatectomy but before islet infusion (measured at 1- 4 hours post-pancreatectomy). Horizontal lines for each time indicate median and IQR. A greater ratio suggests greater beta cell death.
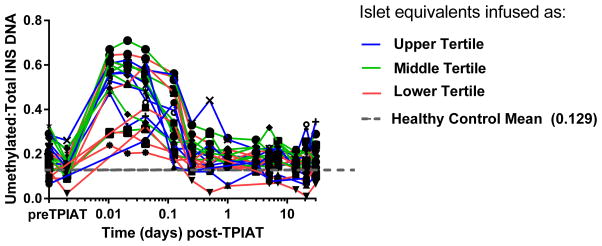
The ratio was elevated immediately after islet infusion in all recipients in the first 3 hours, with variable later peaks over the 30 day sampling period (Figure 2). Ratios of unmethylated to total INS DNA peaked within the first several hours post-TPIAT at median 2.5-fold (IQR 2.0, 3.3) above pre-TPIAT baseline (p<0.001). The ratios did not differ in those with intraportal vs intraportal+peritoneal islet grafts; adolescents had lower elevations in the ratio than adults in the 15 minute to 1 hour post-infusion samples only (p=0.005) but similar levels beyond 1 hour. The half-life of unmethylated INS DNA in serum was 2.2 hours (95% CI: 1.7–2.9 hours).
Figure 2.
Trajectory of unmethylated INS DNA ratio post-TPIAT, with all patients showing elevation of this ratio in the first day post-TPIAT infusion and variable elevations late after transplant and at the day 90 follow up assessment. In this graphic, the first two measures indicate pre-pancreatectory baseline and values in the pancreatectomized patient just prior to islet transplant; subsequent measures were obtained after infusing the islet product. The grey hash line indicated the mean for healthy non-diabetic controls without pancreatitis for reference; while the color lines indicate islet mass transplanted divided into tertiles with blue line indicating the highest tertile (452,500- 624,800 IEQ), green line middle tertile (301,000- 409,600 IEQ) and the red line the lowest tertile (111,700- 292,300 IEQ).
Source of unmethylated INS DNA
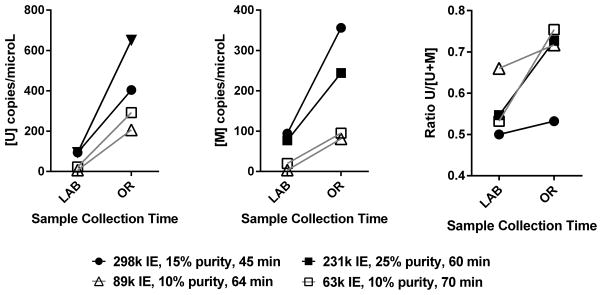
To investigate whether these early ratio elevations were due to beta cell death that occurred before infusion, from the trauma of islet isolation, we measured the levels of INS DNA in 4 preparations of islet product: (1) in the the lab immediately post-isolation and (2) in the OR at infusion. Between end of isolation and subsequent infusion (40–75 min), there was a significant increase in the total counts of unmethylated INS DNA (p=0.02), methylated INS DNA (p=0.04), and to a lesser extent the ratio of unmethylated to total INS DNA in the cell free supernatant of the islet product (p=0.07) (Figure 3). The islet products had low purity, ~ 10–25%, so elevation in unmethylated INS DNA reflects death of the beta cell component, while elevation in methylated INS DNA in the supernatant reflects death of non-beta islet cell components and exocrine tissue composing the transplant graft.
Figure 3.
A. Change in unmethylated INS DNA ([U] copies/ microL), B. methylated INS DNA ([M] copies per microL) components, and C. ratio of unmethylated INS DNA to total INS DNA (Ratio U/[U+M]) from supernatant of the islet product immediately post-isolation (LAB) to islet product infusion (OR). Values from OR are adjusted to account for the 2-fold dilution of the islet product with CMRL Media following the LAB sample to allow direct comparison. Legend indicates IEQ, purity, and time between isolation and infusion in OR (time the islet product is in the infusion bag) for each product. The closed and open squares represent two different bags (suspensions) of islet product from the same patient.
Unmethylated insulin DNA and functional beta cell testing after TPIAT
Because all patients had elevated unmethylated INS DNA ratios immediately after islet infusion, we considered whether elevation of this ratio late after TPIAT was a predictor of 90 day outcomes. In the measures between days 3 and 30, 23/119 ratios in 11/21 (52%) patients were elevated (i.e. >0.219), which we termed ‘late elevations’. We considered day 90 separately (post-islet engraftment), elevated ratios were still present in 7/24 (29%) patients, termed “day 90 elevations”.
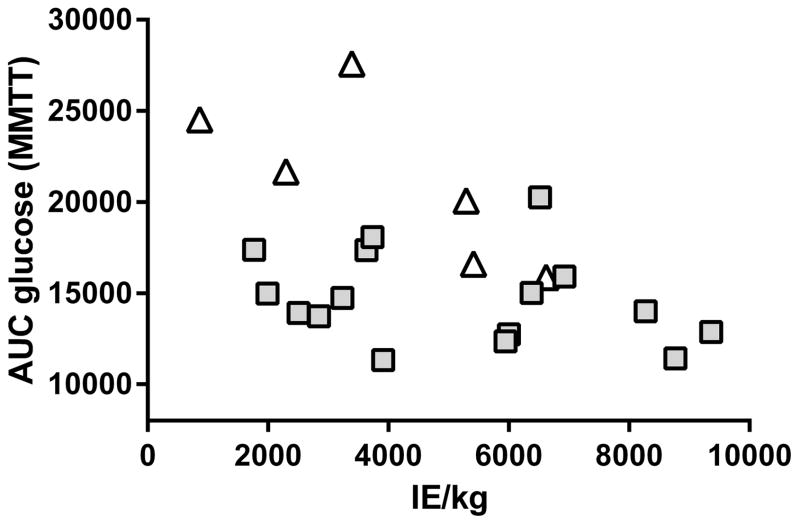
After adjusting for IE/kg transplanted, patients with late or day 90 elevations did not differ significantly from those without elevations in insulin or C-peptide secretory measures including AUC C-peptide, AIRg, ACRg, AIRpot, or ACRpot. However, high MMTT AUC glucose was significantly associated with an elevated unmethylated INS DNA ratio at day 90, after adjusting for IE/kg transplanted (P = 0.0009; Figure 4), suggesting ongoing beta cell loss at day 90 was associated with meal-induced hyperglycemia.
Figure 4.
AUC glucose from MMTT by IE/kg, with patients indicated as having persistent elevated ratios of unmethylated INS DNA at day 90 (≥0.219, open triangles), or normal levels (<0.219, grey squares). Elevated ratio and higher AUC glucose at day 90 were significantly associated (P = 0.0009), adjusting for islet mass transplanted (p<0.0004).
Measures of islet function at day 90 did not differ between those with intraportal only vs intraportal+peritoneal grafts, or in adults versus adolescents (p>0.15 for all).
Discussion
Diabetes outcomes after TPIAT vary for any transplanted islet mass, likely due to differences in beta cell survival following both isolation and transplantation (1). Beta cell apoptosis can be induced by islet isolation itself (2, 26, 27) and by the innate inflammatory responses that follow intraportal islet infusion (4, 10, 28), but until recently beta cell biomarkers to assess post-transplant beta cell death have been largely lacking. We observed that the ratio of unmethylated INS DNA declines after removal of the pancreas. It then is elevated several-fold over pre-pancreatectomy baseline in all recipients immediately after isolation and infusion of the islet product, confirming our group’s previous observations that this is a marker of beta cell death. A dramatic rise in unmethylated INS DNA and methylated INS DNA within the islet supernatant in the short (~1 hour) period before islet infusion suggests that much of the immediate post-islet infusion peak results from islet isolation itself. Late elevations beyond the first post-operative day varied between recipients, suggesting inconsistent later beta cell loss. These results highlight an opportunity to better protect beta cells during islet isolation and the first several months post-islet transplant to optimize diabetes results after TPIAT.
Indeed, a striking rise in INS DNA was observed in the islet product supernatant before infusion. This suggests a significant die-off of beta cells, other islet cell components, and exocrine tissue induced by the isolation procedure; we hypothesize that this accounts for a substantial proportion of the peak in the unmethylated INS DNA ratio in the first hour after islet infusion. In contrast, later elevations may be better explained by detrimental post-infusion events including IBMIR and beta cell hypoxia (9, 28).
Elevations in the unmethylated INS DNA ratio did not predict insulin secretory measures at 3 months, the period after which islet revascularization is largely complete and beta cell functionality restored (8, 9). Because the half-life of the unmethylated INS DNA is short, much more frequent monitoring may be needed to accurately account for all beta cell death occurring during islet engraftment. However, compared to a previously established normal healthy control cohort, more than half of our TPIAT recipients clearly had periods of beta cell loss even beyond the first 24 hours post-transplant. The nearly 30% of patients with persistent elevated ratios of unmethylated INS DNA at day 90 exhibited a greater glycemic excursion to a mixed meal test, adjusting for IE/kg transplanted. This suggests that either these recipients’ islet grafts have poorer viability, resulting in hyperglycemia, or, that recurrent hyperglycemia itself triggers ongoing loss of beta cells (6, 29).
Further study is warranted to determine the clinical utility of the unmethylated INS DNA marker. This measure may useful for studying strategies to improve early beta cell survival, including refinements in isolation, and anti-inflammatory strategies that have been proposed but remain untested in randomized trials. While we hypothesize that much of the early elevation in unmethylated INS DNA is attributable to beta-cell loss induced by isolation, the relative impact of isolation, IBMIR, and co-transplanted acinar tissue on beta cell death merits further mechanistic study. Post hoc analyses suggest lesser elevations in the INS DNA ratio in the adolescents versus adults, although functional outcomes were no different between these groups. This raises speculation about better resilience of ‘young’ beta cells to isolation and transplant injury. Very young children- previously shown in our population to have better diabetes outcomes than adolescents and adults - were excluded (19).
Our data as well as previous studies support that the unmethylated INS DNA is specific for beta cell loss-- CpG sites within the INS gene of β cells are unmethylated to transcribe insulin (13–16, 18). However, it is possible that these same sites may be unmethylated rarely in non-β cells leading to a non-specific background ‘signal’ present even after pancreatectomy. Our studies from high throughput sequencing suggest this occurs in about 5% of reads (unpublished observations). Furthermore, we cannot exclude that surgical pancreas manipulation might affect INS DNA during and immediately after surgery, but the reduction in INS DNA ratios after pancreatectomy suggests this is not a significant confounder.
We conclude that beta cell death induced by islet isolation is nearly universal in TPIAT recipients. Beta cell death may persist through islet engraftment even up to 3 months post-TPIAT. Unmethylated INS DNA may represent a useful beta cell biomarker for monitoring beta cell death in the islet product before transplant as islet isolation strategies evolve, and as a marker for ongoing post-islet infusion loss in TPIAT recipients.
Acknowledgments
Research reported in this publication was supported in part by awards from the National Center for Advancing Translational Sciences (UL1TR000114), the National Institutes of Diabetes and Digestive and Kidney Diseases (R03-DK102469, PI Bellin; R01DK057846, R42DK095639, DP3DK101122, and UC4DK104205, PI Herold) and the Juvenile Diabetes Research Foundation (2014-142-S-B, PI Herold).
Abbreviations
- ACRg
Acute C-peptide response to glucose
- ACRpo t
Glucose potentiated acute C-peptide response to arginine
- AIRg
Acute insulin response to glucose
- AIRpot
Glucose potentiated acute insulin response to arginine
- AUC
Area under the curve
- GPAIS
Glucose-potentiated arginine-induced insulin secretion
- IVGTT
Intravenous glucose tolerance tests
- HbA1c
Hemoglobin A1c level
- IEQ
Islet equivalents
- IEQ/kg
Islet equivalents/kilogram body weight
- MMTT
Mixed meal tolerance test
- T1D
Type 1 diabetes
- TPIAT
Total pancreatectomy with islet autotransplantation
Footnotes
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Juvenile Diabetes Research Foundation.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Sutherland DE, Radosevich DM, Bellin MD, Hering BJ, Beilman GJ, Dunn TB, et al. Total pancreatectomy and islet autotransplantation for chronic pancreatitis. J Am Coll Surg. 2012;214(4):409–24. doi: 10.1016/j.jamcollsurg.2011.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paraskevas S, Maysinger D, Wang R, Duguid TP, Rosenberg L. Cell loss in isolated human islets occurs by apoptosis. Pancreas. 2000;20(3):270–6. doi: 10.1097/00006676-200004000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Hanley S, Liu S, Lipsett M, Castellarin M, Rosenberg L, Tchervenkov J, et al. Tumor necrosis factor-alpha production by human islets leads to postisolation cell death. Transplantation. 2006;82(6):813–8. doi: 10.1097/01.tp.0000234787.05789.23. [DOI] [PubMed] [Google Scholar]
- 4.Naziruddin B, Iwahashi S, Kanak MA, Takita M, Itoh T, Levy MF. Evidence for instant blood-mediated inflammatory reaction in clinical autologous islet transplantation. Am J Transplant. 2014;14(2):428–37. doi: 10.1111/ajt.12558. [DOI] [PubMed] [Google Scholar]
- 5.Giuliani M, Moritz W, Bodmer E, Dindo D, Kugelmeier P, Lehmann R, et al. Central necrosis in isolated hypoxic human pancreatic islets: evidence for postisolation ischemia. Cell Transplant. 2005;14(1):67–76. doi: 10.3727/000000005783983287. [DOI] [PubMed] [Google Scholar]
- 6.Negi S, Park SH, Jetha A, Aikin R, Tremblay M, Paraskevas S. Evidence of endoplasmic reticulum stress mediating cell death in transplanted human islets. Cell Transplant. 2012;21(5):889–900. doi: 10.3727/096368911X603639. [DOI] [PubMed] [Google Scholar]
- 7.Biarnes M, Montolio M, Nacher V, Raurell M, Soler J, Montanya E. Beta-cell death and mass in syngeneically transplanted islets exposed to short- and long-term hyperglycemia. Diabetes. 2002;51(1):66–72. doi: 10.2337/diabetes.51.1.66. [DOI] [PubMed] [Google Scholar]
- 8.Pepper AR, Gala-Lopez B, Ziff O, Shapiro AM. Revascularization of transplanted pancreatic islets and role of the transplantation site. Clinical & developmental immunology. 2013;2013:352315. doi: 10.1155/2013/352315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olsson R, Olerud J, Pettersson U, Carlsson PO. Increased numbers of low-oxygenated pancreatic islets after intraportal islet transplantation. Diabetes. 2011;60(9):2350–3. doi: 10.2337/db09-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanak MA, Takita M, Shahbazov R, Lawrence MC, Chung WY, Dennison AR, et al. Evaluation of MicroRNA375 as a Novel Biomarker for Graft Damage in Clinical Islet Transplantation. Transplantation. 2015 doi: 10.1097/TP.0000000000000625. [DOI] [PubMed] [Google Scholar]
- 11.Bellin MD, Gelrud A, Arreaza-Rubin G, Dunn TB, Humar A, Morgan KA, et al. Total Pancreatectomy With Islet Autotransplantation: Summary of an NIDDK Workshop. Ann Surg. 2015;261(1):21–9. doi: 10.1097/SLA.0000000000001059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herold KC, Usmani-Brown S, Ghazi T, Lebastchi J, Beam CA, Bellin MD, et al. beta cell death and dysfunction during type 1 diabetes development in at-risk individuals. J Clin Invest. 2015;125(3):1163–73. doi: 10.1172/JCI78142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Usmani-Brown S, Lebastchi J, Steck AK, Beam C, Herold KC, Ledizet M. Analysis of beta-cell death in type 1 diabetes by droplet digital PCR. Endocrinology. 2014;155(9):3694–8. doi: 10.1210/en.2014-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lebastchi J, Deng S, Lebastchi AH, Beshar I, Gitelman S, Willi S, et al. Immune Therapy and beta-Cell Death in Type 1 Diabetes. Diabetes. 2013;62(5):1676–80. doi: 10.2337/db12-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lehmann-Werman R, Neiman D, Zemmour H, Moss J, Magenheim J, Vaknin-Dembinsky A, et al. Identification of tissue-specific cell death using methylation patterns of circulating DNA. Proc Natl Acad Sci U S A. 2016;113(13):E1826–34. doi: 10.1073/pnas.1519286113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Husseiny MI, Kaye A, Zebadua E, Kandeel F, Ferreri K. Tissue-specific methylation of human insulin gene and PCR assay for monitoring beta cell death. PloS one. 2014;9(4):e94591. doi: 10.1371/journal.pone.0094591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher MM, Watkins RA, Blum J, Evans-Molina C, Chalasani N, DiMeglio LA, et al. Elevations in Circulating Methylated and Unmethylated Preproinsulin DNA in New-Onset Type 1 Diabetes. Diabetes. 2015;64(11):3867–72. doi: 10.2337/db15-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herold KC, Usmani-Brown S, Ghazi T, Lebastchi J, Beam CA, Bellin MD, et al. beta Cell death and dysfunction during type 1 diabetes development in at-risk individuals. J Clin Invest. 2015;125(3):1163–73. doi: 10.1172/JCI78142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chinnakotla S, Bellin MD, Schwarzenberg SJ, Radosevich DM, Cook M, Dunn TB, et al. Total pancreatectomy and islet autotransplantation in children for chronic pancreatitis: indication, surgical techniques, postoperative management, and long-term outcomes. Ann Surg. 2014;260(1):56–64. doi: 10.1097/SLA.0000000000000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balamurugan AN, Loganathan G, Bellin MD, Wilhelm JJ, Harmon J, Anazawa T, et al. A new enzyme mixture to increase the yield and transplant rate of autologous and allogeneic human islet products. Transplantation. 2012;93(7):693–702. doi: 10.1097/TP.0b013e318247281b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37(4):413–20. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- 22.Wilhelm JJ, Bellin MD, Dunn TB, Balamurugan AN, Pruett TL, Radosevich DM, et al. Proposed thresholds for pancreatic tissue volume for safe intraportal islet autotransplantation after total pancreatectomy. Am J Transplant. 2013;13(12):3183–91. doi: 10.1111/ajt.12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lundberg R, Beilman GJ, Dunn TB, Pruett TL, Chinnakotla SC, Radosevich DM, et al. Metabolic Assessment Prior to Total Pancreatectomy and Islet Autotransplant: Utility, Limitations and Potential. Am J Transplant. 2013;13(10):2664–71. doi: 10.1111/ajt.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rickels MR, Liu C, Shlansky-Goldberg RD, Soleimanpour SA, Vivek K, Kamoun M, et al. Improvement in beta-cell secretory capacity after human islet transplantation according to the CIT07 protocol. Diabetes. 2013;62(8):2890–7. doi: 10.2337/db12-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rickels MR, Naji A, Teff KL. Acute insulin responses to glucose and arginine as predictors of beta-cell secretory capacity in human islet transplantation. Transplantation. 2007;84(10):1357–60. doi: 10.1097/01.tp.0000287595.16442.a7. [DOI] [PubMed] [Google Scholar]
- 26.Abdelli S, Ansite J, Roduit R, Borsello T, Matsumoto I, Sawada T, et al. Intracellular stress signaling pathways activated during human islet preparation and following acute cytokine exposure. Diabetes. 2004;53(11):2815–23. doi: 10.2337/diabetes.53.11.2815. [DOI] [PubMed] [Google Scholar]
- 27.Bottino R, Balamurugan AN, Tse H, Thirunavukkarasu C, Ge X, Profozich J, et al. Response of human islets to isolation stress and the effect of antioxidant treatment. Diabetes. 2004;53(10):2559–68. doi: 10.2337/diabetes.53.10.2559. [DOI] [PubMed] [Google Scholar]
- 28.Kanak MA, Takita M, Kunnathodi F, Lawrence MC, Levy MF, Naziruddin B. Inflammatory Response in Islet Transplantation. International journal of endocrinology. 2014;2014:451035. doi: 10.1155/2014/451035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walters SN, Luzuriaga J, Chan JY, Grey ST, Laybutt DR. Influence of chronic hyperglycemia on the loss of the unfolded protein response in transplanted islets. Journal of molecular endocrinology. 2013;51(2):225–32. doi: 10.1530/JME-13-0016. [DOI] [PubMed] [Google Scholar]