Abstract
While most people take identification with their body for granted, conditions like phantom limb pain, alien hand syndrome, and xenomelia suggest that the feeling of bodily congruence is constructed and susceptible to alteration. Individuals with xenomelia typically experience one of their limbs as over-present and aversive, leading to a desire to amputate the limb. Similarly, many transgender individuals describe their untreated sexed body parts as incongruent and aversive, and many experience phantom body parts of the sex they identify with (Ramachandran, 2008). This experience may relate to differences in brain representation of the sexed body part, as suggested in xenomelia (McGeoch et al., 2011). We utilized magnetoencephalography imaging to record brain activity during somatosensory stimulation of the breast – a body part that feels incongruent to most pre-surgical female-to-male (FtM) identified transgender individuals – and the hand, a body part that feels congruent. We measured the sensory evoked response in right hemisphere somatosensory and body-related brain areas and found significantly reduced activation in the supramarginal gyrus and secondary somatosensory cortex but increased activation at the temporal pole for chest sensation in the FtM group (N = 8) relative to non-transgender females (N = 8). In addition, we found increased white matter coherence in the supramarginal gyrus and temporal pole and decreased white matter diffusivity in the anterior insula and temporal pole in the FtM group. These findings suggest that dysphoria related to gender-incongruent body parts in FtM individuals may be tied to differences in neural representation of the body and altered white matter connectivity.
Keywords: FtM, body image, body representation, gender identity, parietal lobes, transgender
INTRODUCTION
Many transgender individuals experience the sex of their body as incongruent with their gender identity (sense of gendered self). A feeling of being the “wrong” sex often extends beyond psychological identity to the physical body, causing body image dissatisfaction and distortion (Lindgren & Pauly, 1975). Indeed, as early as ages 2–4, some children with Gender Dysphoria1 express desires to be the opposite sex and have the anatomy of the opposite sex (Zucker & Cohen-Kettenis, 2008). Transgender individuals who desire physical transition to another sex overwhelmingly feel more like “themselves” and experience a more positive body image after sex reassignment surgeries (or “affirmation surgeries”) (Kraemer, Delsignore, Schnyder, & Hepp, 2008). In “Second Skins: The Body Narratives of Transsexuality,” Prosser (1998) described sex reassignment surgery as “a nostalgic return to the sexed contours that should have been” (p. 84). He draws a comparison between the occurrence of phantom limbs after amputation and “phantomization” of desired body parts in many transgender individuals, suggesting that the strongly sensed “parts felt as more real” indicate an inherent “phantom sex” (p. 84). Could differences in representation of sexed body parts in the brain underlie the sensation of bodily incongruity experienced by many transgender individuals?
One report suggests that many presurgical female-to-male (FtM) transgender individuals experience a “phantom” penis – the sensation of having a penis in its physical absence – suggesting differences in representation of the sexed body in transgender individuals(Ramachandran, 2008).2 If congruent-feeling body parts generate phantom sensations, then incongruent body parts ought to produce fewer phantom sensations after their removal, due to their reduced cortical representation. Indeed, FtM and male-to-female (MtF) individuals who undergo sex reassignment surgery were reported in one study to have a lower incidence of phantom breasts and penises, respectively, than people who undergo this surgery for unrelated reasons, suggesting reduced neural representation of these body parts prior to their removal (Ramachandran, 2008; Ramachandran & McGeoch, 2007).
Transgender individuals who desire sex reassignment surgery bear some similarity to xenomelia, a form of Body Integrity Identity Disorder in which the patient desires to amputate a healthy limb because it feels incongruent with their internal body representation (but does not appear to result from negative experience with the limb or from any real or perceived defect of the limb) (First 2005). Patients with xenomelia respond to touch on their affected limb with heightened physiological arousal (Brang, McGeoch, & Ramachandran, 2008; McGeoch, Brang, Song, & Lee, 2009), and sensation from the affected limb activates the right superior parietal lobule less than touch from the unaffected limb (McGeoch et al., 2011). Patients also show reduced cortical thickness in the right superior parietal lobule (Hilti et al., 2013). This difference may reflect an inability of the brain to “map” or integrate sensation from the limb into body maps (McGeoch et al., 2011). A transgender identity often involves a similar discrepancy between body image and body morphology in the absence of known brain injury or pathology, and thus might also involve neural differences in body representation.
Little is known more generally about the biological correlates of gender identity. Some potential biological differences have been reported between transgender and cisgender (non-transgender) individuals including higher rates of certain chromosomal variations (in MtF individuals; Erickson-Schroth, 2013) and a higher rate of left-handedness in transgender (specifically transsexual) individuals, indicating possible differences in developmental brain lateralization (Erickson-Schroth, 2013). Some differences in patterns of white matter microstructure (Rametti et al., 2011a, 2011b), cortical thickness (Luders et al., 2012; Zubiaurre-Elorza et al., 2013), gray matter volume (Savic & Arver, 2011), structural hemispheric connectivity (Hahn et al., 2014), and hypothamalic anatomy (Bao & Swaab, 2011; Cohen-Kettenis & Gooren, 1999; Kruijver et al., 2000; Zhou, Hofman, Gooren, & Swaab, 1995) have been reported. Guillamon, Junque, & Gómez-Gil (2016) review studies of brain structure of FtM and MtF individuals and report that the cortex is generally more feminine (thicker cortical gray matter indicative of reduced cortical thinning) but in different cortical regions than seen in cisgender females. In FtM individuals some white matter tracts are masculinized. The researchers argue that these changes- seen primarily in the right hemisphere- create a unique FtM phenotype.
A number of functional imaging studies of transgender individuals have also been conducted. These studies have investigated brain response to visual erotic stimuli (e.g., Gizewski et al., 2009), differences in brain response to odorous steroids (Berglund, Lindström, Dhejne-Helmy, & Savic, 2008; Burke, Cohen-Kettenis, Veltman, Klink, & Bakker, 2014), and differences in engagement of various brain areas in cognitive (Schöning et al., 2010; Staphorsius et al., 2014) or affective (Soleman et al., 2014) tasks. Yet, only two brain imaging studies to our knowledge have investigated body representation in transgender individuals. Lin et al. (2014) found a number of differences in resting state connectivity in transgender individuals (identified as transsexual), including higher centrality of the primary somatosensory cortex and superior parietal lobule, as well as greater involvement of visual and auditory regions in the body network. These results suggest a heightened focus on sensory input in body representation. Manzouri, Kosidou, and Savic (2015) showed differences in the “own-body image network” and suggested that FtM individuals may have weaker connections between body perception networks and body self-ownership networks. They also reported reduced functional connectivity between the amygdala and the right precuneus and temporo-parietal junction, right extrastriate body area, and the fusiform cortex that might reflect greater separation of body perception and body-related emotion.
Scientific reductionism is unlikely to yield a simple explanation for a phenomenon as complex as gender identity. Recognizing this inherent limitation, we focus on only one aspect of gender identity in the current study – the neural correlates of sexed body image. On average, breasts cause the greatest physical gender identity conflict for FtM individuals (more than genitals; see Dutton, Koenig, & Fennie, 2008), and are the most important predictor of body dissatisfaction in FtM individuals (Davis & Meier, 2013). Indeed, bilateral mastectomy is currently considered medically necessary for FtM individuals to live safely and effectively as men (Richards & Barrett, 2013). The current study aimed to investigate body perception directly by examining neural response to stimulation of body parts that are dysphoric.
FtM individuals experience sensation arising from their breasts and are cognizant that their breasts are part of their physical body. Yet, their sense of ownership of and identification with their breasts is reduced. Tsakiris (2010) proposed a neurocognitive model of bodily ownership that may partly explain this reduced sense of ownership. According to Tsakiris, a visual form, such as a hand that you see, is first compared against an internal model of a human body in the right temporo-parietal junction. Second, the posture and anatomical features of the visual form are compared to the posture and anatomy of one’s own body in the primary and secondary somatosensory cortices and, if they align, multisensory input from the body part recalibrates the brain’s visual and tactile coordinate systems in the posterior parietal cortex. Third, sensory input gives rise to a sense of body “ownership” in the posterior insula to the extent that the sensory input is integrated into one’s egocentric body representation. In line with Tsakiris’ model, activation of the posterior insula correlates with the phenomenological experience of bodily ownership during body ownership illusions like the Rubber Hand Illusion (Baier & Karnath, 2008; Tsakiris, Hesse, Boy, Haggard, & Fink, 2007).
In the current study, we aimed to determine whether brain areas involved in sensory and affective body representation are activated differently in FtM individuals than in control females by tactile input from the breasts, a body part rated highly incongruent by all FtM participants in the current study. We conducted magnetoencephalography (MEG) recordings during tactile stimulation (tapping) of the breasts and hands of presurgical female-to-male (FtM) transgender individuals and cisgender female controls. If we apply Tsakiris’ model to FtM individuals, in the first step, FtM individuals may compare the body part receiving sensation (the breasts) to their internal structural body representation (no breasts), leading to a lack of integration of the sensory information in the parietal lobe and reduced activation of the posterior insula relating to reduced body ownership. We therefore examined early sensory response in eight regions of interest (ROIs) covering the parietal lobe and insula “body-matrix” (Moseley, Gallace, & Spence, 2012), as well as the amygdala, due to the affective aversion to the breasts. Analysis was restricted to the right hemisphere where sensation from the left side of the body (where we applied sensory stimulation) is primarily processed.
We predicted that sensation from incongruent-feeling body parts would cause reduced activation of the secondary somatosensory cortex, the posterior insula, and the supramarginal gyrus given their role in conscious perception of sensation, body ownership, and attribution of sensation to the self (Blanke et al., 2005; Dijkerman & de Haan, 2007; Tsakiris et al., 2007). Similarly, the numerous parallels between individuals with xenomelia and individuals who are transgender led us to predict reduced right superior parietal lobule activation to incongruent-feeling body parts in FtM individuals. We also predicted reduced activation of the intraparietal sulcus, given its role in sensory integration and multimodal body representation (e.g., Mauguière et al., 1997). In contrast, we predicted heightened affective response to breast sensation, including a heightened response in the medial temporal lobe, related to the amygdala (also predicted by heightened skin conductance response observed for the chest relative to the leg in FtM individuals relative to control females) (Case, 2013), and in the anterior insula, due to disgust towards an incongruent-feeling body part (e.g., Craig, 2009). We did not predict differences in primary somatosensory cortex activation, as there is no evidence of abnormal sensory acuity in transgender individuals (however, binding of the chest, as practiced by some FtM individuals, might affect sensory acuity). Finally, we conducted diffusion tensor imaging (DTI) to test for differences in white matter connectivity in the same right hemisphere regions as we conducted our MEG analyses in.
METHOD
Participants
This study was conducted with approval from the University of California, San Diego Human Research Protections Program. Eight female-to-male (FtM) transgender individuals and eight female cisgender (non-transgender) control individuals completed the study. The current study was designed to study transsexual individuals. Due to changing preferences in gender identities categories we now refer to these participants as transgender, but they represent a subset of transgender individuals who desire to physically alter their bodies. FtM participants were recruited through fliers and email announcements distributed to local LGBT centers and groups; cisgender participants were recruited through email and word-of-mouth advertisement. Our recruitment materials called for volunteers who were anatomically female but identified as male (FtM), as well as for volunteers who were anatomically female and identified strongly as female. Study inclusion was based on two screening questions that were embedded among other identity-related questions: “I feel my biological sex (e.g., anatomy) feels inconsistent with who I feel I am,” and “I feel like my body should have breasts on it.” FtM transgender participants who agreed and disagreed, respectively, with these questions were invited to participate in the study. The opposite responses were required for cisgender female participants. Only one prospective participant who self-identified as “in between male and female” was determined to be ineligible on the basis of these screening questions. The mean age of participants was 29 (range, 20–50 years) and did not differ by group. Participants were asked in an open-ended manner whether they identified as male, female, genderqueer, or other, and whether they identified as transgender. FtM participants varied in self-identification from “male” to “transsexual female-to-male” to “genderqueer and primarily male.” All agreed they were transgender and all desired a male or mostly-male anatomy and strongly desired to have their breasts removed. All individuals in the control group strongly identified as female and rated their breasts as congruent with their internal body image. In response to the question “Should your body have breasts? (1 = strongly disagree; 5 = strongly agree),” all control participants marked “5” and all FtM participants marked either 1 or 2 (M = 1.3). An additional question on the screening form asking participants to rate how much they felt their breasts belonged to them was used as a covariate in analyses of the brain imaging data. All FtM participants wished to have their breasts removed (one expressed uncertainty about surgery but desired to have a male-appearing chest). Four of the eight participants in the FtM group were taking testosterone, but none had undergone any type of surgery related to their gender (see Table 1 for participant characteristics). Sexual orientation was obtained after the study from four of the FtM participants; three reported being predominantly gynephilic, and one was bisexual/other. The control females were predominantly androphilic (seven androphilic; one bisexual/other). All participants were right-handed as assessed by self-report.
Table 1.
Eight FtM individuals participated in the current study.
| Participant | Hormones | Self-identification | My breasts belong to me (1–5) |
My body should have breasts (1–5) |
Desired anatomy |
|---|---|---|---|---|---|
| 1 | genderqueer, leaning towards male |
1 | 1 | No breasts | |
| 2 | male; transgender FtM | 1 | 1 | Anatomic male | |
| 3 | Yes | transmale | 5 | 1 | Anatomic male |
| 4 | mostly male | 2.5 | 1 | Anatomic male | |
| 5 | Yes | male | 3 | 1 | Anatomic male |
| 6 | genderqueer, mostly male |
1 | 1 | More masculine, no breasts |
|
| 7 | Yes | male | 3 | 2 | Anatomic male |
| 8 | Yes | transmale, FtM, or transgender |
2 | 2 | Anatomic male |
Demographic and descriptive data were collected before the MEG and MRI sessions. 1 = strongly disagree; 5 = strongly agree.
Measures
MEG recordings were conducted at the Radiological Imaging Laboratory at the University of California, San Diego using a whole-head Elekta Neuromag Vectorview 306-channel system in an enhanced multi-layer magnetically shielded room. Head position was digitally recorded using four non-magnetic head position indicator coils. The magnetic field surrounding each participant’s scalp was measured continuously at a sampling rate of 1000 Hz.
Imaging was conducted on a General Electric 1.5 T Excite HDx MRI scanner. A T1-weighted anatomical scan was acquired in the sagittal plane with a 3D MPRAGE sequence (TR = 10.73 ms, TE = 2.8 ms, TI = 1000 ms, FOV = 25 cm, flip angle = 8°, whole brain, slice thickness = 1 mm, 176 slices). DTI was then collected to measure white matter coherence in the brain (51 directions, b = 1000 s/mm2, TR = 13.2 s, TE = 80.4 ms, FOV = 24 cm, 47 oblique slices aligned to the AC/PC encompassing the whole brain, 2.5 mm slice thickness).
Procedure
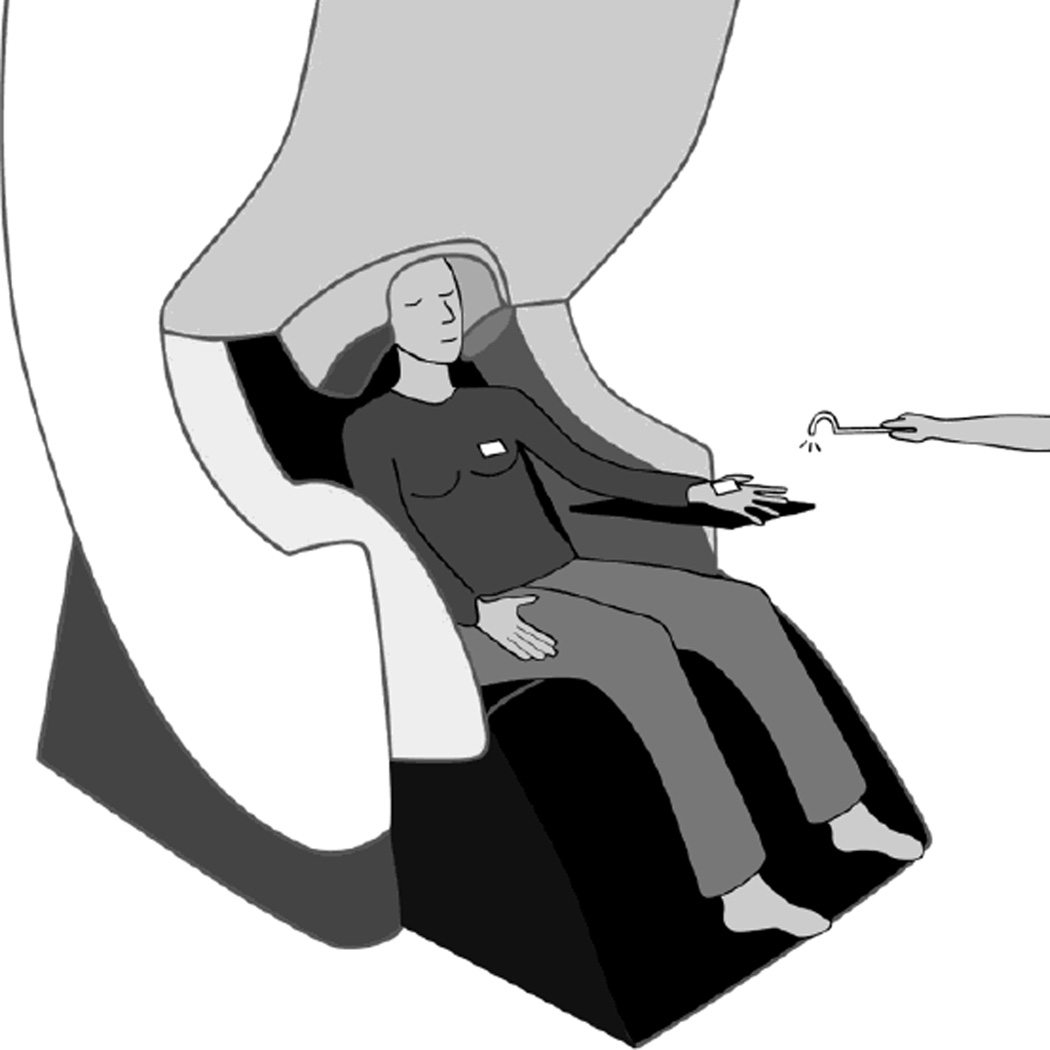
Somatosensory evoked fields (SEFs) were recorded in the MEG scanner for each participant during tactile stimulation (mechanical tapping) of the palm of the left hand (near the base of the thumb) and of the left breast (approximately 1–2 inches above the nipple). During each block, a female research assistant masked to the hypothesis of the study tapped the participant’s body part at approximately 1 Hz intervals using a plastic tip. This 1 Hz rate of tapping is lower than the temporal window for temporal summation seen in MEG with interstimulus intervals below 50–75 ms (Yamashiro, Inui, Otsuru, Urakawa, & Kakigi, 2011; Zhu, Disbrow, Zumer, McGonigle, & Nagarajan, 2007). Tapping occurred on the chest over a thin, tight-fitting shirt (with no undergarments). Two separate blocks of approximately 200 taps were collected for both the Hand and Chest conditions, except only one hand block was obtained for two of the FtM participants and only one chest block was obtained for two other FtM participants due to technical difficulties with the sensors. Head position was measured digitally at the start of each block. To time lock the taps, the tapper contained two bundled fiber-optic filaments. A low-energy laser beam emitted from one of the filaments scattered upon contact with the body and the scatter was detected by the second filament, triggering an optical switch to time lock the tap to the MEG recording. A piece of white tape was placed on each location of tapping to assist with laser scatter (see Fig. 1). A block of median nerve stimulation of the left hand was also collected after the tapping blocks to confirm localization of primary somatosensory cortex. Median nerve stimulation began at 5 milliamperes and increased in 1 milliampere increments until perceptible movement of the thumb was observed. During all blocks of tapping or median nerve stimulation, the participant was instructed to relax but remain aware of the tapping, and to not meditate or fall asleep. Participants kept their eyes closed throughout the blocks of tapping. An MRI scan (structural scan and diffusion weighted imaging) was collected for each participant after the MEG session. Including setup, the MEG session took about two hours and the MRI scan took about 30 minutes.
Figure 1. Illustration of MEG somatosensory stimulation paradigm.
White rectangles show placement of reflective tape where the participant was tapped.
Data Analysis
MEG
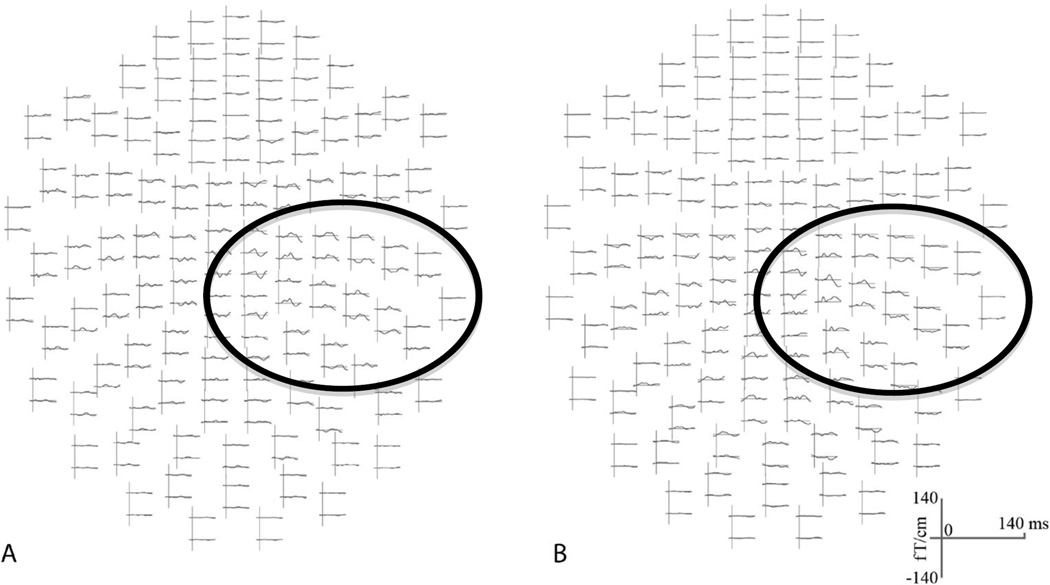
To align the MEG data to the MRI scan, each participant’s cortical surface was reconstructed from his or her structural MRI in FreeSurfer. Anatomical head digitization from the MEG session was manually aligned to the MRI in mrilab (Neuromag software). An inner skull surface boundary element model was calculated from the MRI in seglab (Neuromag software) for use in the forward solution (used to model the origin of signal from each sensor). MEG data from each condition were spatially filtered in maxfilter (Neuromag software) to eliminate non-neural sources of variation. A band pass frequency filter was applied between 0.1–50 Hz. The data were then downsampled to 250 Hz and epoched from −100 ms to 400 ms. Individual trials were reviewed and outlier trials (extreme sensory readings) were deleted from each block based on overall magnitude of sensor activity. For blocks with more than 200 trials remaining, automatic rejection thresholds for the gradiometers, magnetometers, and EOG channel were raised iteratively until 200 (+/−3) trials remained (several blocks had only 160–180 events after initial outlier rejection: one hand block for one FtM participant, one hand block for one control participant, and two hand blocks and two chest blocks for one control participant). Epoched data were then averaged in matlab (see Fig. 2 for sample filtered average waveforms in sensor space).
Figure 2. Example averaged Sensory Evoked Field in sensor space after spatial filtering and trial rejection.
Average waveforms (0–140 ms) from a chest block from (A) a representative FtM participant and (B) a representative cisgender female (control) participant. The circles highlight the general region of sensors in the right hemisphere showing an evoked sensory response.
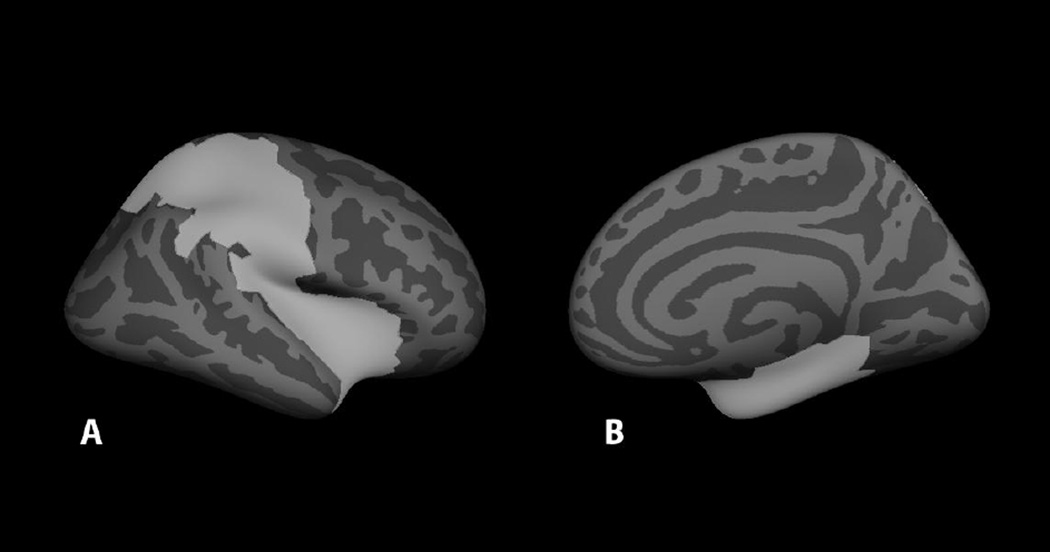
Individual distributed statistical parametric maps (dSPMs) (Dale et al., 2000) were constructed for each block for each participant. Analysis was constrained to the right hemisphere (contralateral to sensory stimulation). Label boundaries were demarcated by hand in FreeSurfer on an average cortical surface (FreeSurfer fsaverage template) based on the average functional activation from all 16 participants and guided by the FreeSurfer atlas. Eight right hemisphere brain areas were labeled: primary somatosensory cortex, secondary somatosensory cortex, the superior parietal lobule, the intraparietal sulcus, the supramarginal gyrus, the posterior insula, the anterior insula, and the medial temporal lobe (see Fig. 3) and morphed onto each individual participant’s brain using spherical morphing (Fischl, Sereno, Tootell, & Dale, 1999). For each of these brain areas for each participant, waveforms were extracted from the dSPM and averaged within each condition (Hand, Chest). For two control participants, the first chest block was excluded from analysis because of excessively noisy raw waveforms (confirmed by a rater masked to group identity). A whole-brain permutation analysis (freesurfer program mri_glmfit) masked with the selected ROIs was conducted to compare the dSPM in the 0–156 ms range in time bins of 0–36 ms, 40–76 ms, 80–116 ms, and 120–156 ms for the Hand, Chest, and Hand x Chest interaction. These analyses were corrected for multiple comparisons using freesurfer automatic clustering to identify clusters of voxels unlikely to occur by chance alone (p < .05). This time window was selected to span the approximate time window studied by McGeoch et al. (2011), who studied the SEF at 0–140 ms. Time bins were chosen to capture the time preceding sensory response, the early response in somatosensory cortex (generally around 40–50 ms), the late response in secondary somatosensory cortex and other secondary sensory processing areas (generally after 70 ms), and the beginning of conscious processing (generally observed after 100 ms). By averaging across these time bins we were able to roughly capture the peaks we saw in sensory processing in the raw waveforms as well as increase the signal-to-noise of the data.
Figure 3. FreeSurfer average brain displaying the mask for whole-brain analysis.
A: lateral view of right hemisphere; B: medial view of right hemisphere. The mask was the conjunction of pre-selected ROIs based on whole-group functional activation and the FreeSurfer atlas.
We also computed follow-up Monte Carlo p-values for each timepoint in secondary somatosensory cortex, the supramarginal gyrus, and the medial temporal lobe in an effort to identify at which specific timepoints significant group differences occurred. The Monte Carlo t-test is a permutation t-test that tests the group difference against permutation distributions based on all possible assignments of participants to each group, avoiding assumptions about the distribution. Monte Carlo p-values were computed from 1000 random assignments of group at each timepoint. Finally, Monte Carlo t-tests were computed to determine whether FtM participants taking testosterone differed from those not taking testosterone.
DTI
DTI measures of white matter roughly index the strength and direction of white matter paths. Differences in DTI measures can be caused by a variety of underlying differences in white matter, including differences in the number of fibers, myelination, and axonal degeneration. Fractional anisotropy (FA) is a measure derived from DTI that roughly indexes white matter coherence and microstructural integrity, including numbers of axons, axonal diameter, reduced fiber crossing, and increased myelination (Johansen-Berg & Rushworth, 2009). Higher FA in a white matter tract suggests greater connectivity while reduced FA can indicate neuropathology. In contrast, mean diffusivity (MD) is the overall magnitude of diffusion, derived by averaging across the three axes of the tensor ellipsoid that models the direction of diffusion. Higher overall diffusion can be caused by demyelination or axonal degeneration. Axial diffusivity (AD) is diffusion along the principal axis of the tensor (along the main direction of the white matter) and radial diffusivity (RD) is the average diffusion along the two minor axes of the tensor (perpendicular to the main direction of the white matter).
Diffusion weighted images were pre-processed in the FMRIB Diffusion Toolbox (FDT), part of the FMRIB Software Library (FSL) (Smith et al., 2004). DWI images were eddy current corrected and masked by the individual brain volume using the Brain Extraction Tool. FDT was used to compute FA, MD, AD, and RD by fitting a tensor model to the raw diffusion data. TBSS (Tract-Based Spatial Statistics, part of FSL) (Smith et al., 2007) was used to perform standardized registration and tract alignment. Each volume was nonlinearly registered into standard space (MNI152) using FNIRT (Andersson, Jenkinson, & Smith, 2007). The mean FA image was thresholded at 0.2 to form a group white matter skeleton and individual data were projected onto this FA skeleton. Voxel-wise permutation-based testing and inference was performed using Randomise in FSL (5000 permutations). The statistical threshold was set at p < .05 FWE-corrected and clusters were defined using the Threshold-Free Cluster Enhancement (TFCE) method to adjust voxel-wise t-statistics based on local spatial clusters (Smith et al., 2006). A whole brain two-sample t-test was then performed to compare FA, MD, AD, and RD between FtM and control female participants. Finally, FA, MD, AD, and RD were extracted from the eight ROIs utilized in the MEG analysis (transformed from Freesurfer surface-based labels into FSL, and expanded into the white matter). Two-tailed t-tests were conducted to compare mean values within each ROI between FtM and control participants and to compare FtM participants taking testosterone with those not taking testosterone. Pairwise Pearson correlations were computed between DTI and MEG ROI values and FtM participants’ ratings of breast ownership.
RESULTS
MEG
Hand and chest stimulation activated the expected somatotopic areas of primary somatosensory cortex; we observed hand activation in the hand area of primary somatosensory cortex and chest activation in the superior dorsal part of primary somatosensory cortex.
Hand
No significant differences were found between the FtM and control group for the Hand condition.
Chest
In the Chest condition, significantly less activation was observed in the FtM group than in the control group in the supramarginal gyrus and secondary somatosensory cortex in the 40–76 ms time window, but significantly more activation was observed in the FtM group in the medial temporal lobe in the 120–156 ms time window (see Table 2).
Table 2.
Size and location of significant clusters of voxels in the right hemisphere for control > FtM and FtM > control
| Contrast | Size (mm2) |
Clusterwise probability |
MNI coordinates (X, Y, Z) |
Location | ||
|---|---|---|---|---|---|---|
|
A) Chest 40–76 ms control > FtM |
1424.95 | .022 | 60.6 | −36.3 | 34.9 | Supramarginal gyrus/ secondary somatosensory cortex |
|
B) Chest 120–156 ms FtM > control |
1708.58 | .006 | 37.8 | 15.6 | −36.2 | Temporal pole |
|
C) Chest-Hand 0–36 ms control > FtM |
2654.29 | .002 | 45.6 | −8.3 | 36.7 | Primary somatosensory cortex, primary motor cortex, intraparietal sulcus |
|
D) Chest-Hand 120- 156 ms FtM > control |
1363.15 | .018 | 39.9 | −5.8 | −35.6 | Temporal pole |
Chest vs. Hand
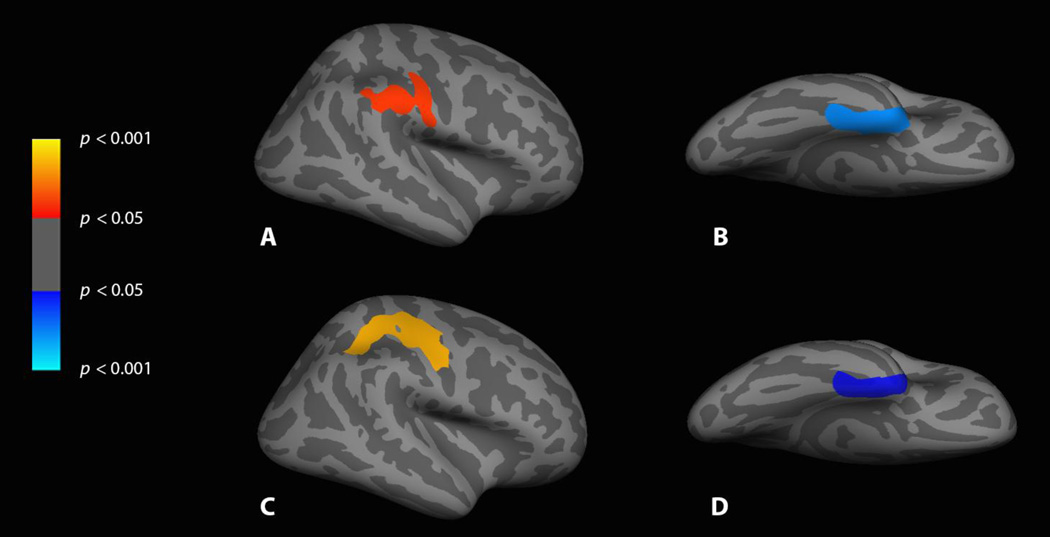
The group x body site interaction yielded two significant clusters of voxels – one for lower activation in the FtM group for chest versus hand sensation in primary sensorimotor cortex and the intraparietal sulcus in the 0–36 ms time window, and the other for greater activation for chest versus hand sensation in the FtM group in the medial temporal lobe in the 120–156 ms time window (see Fig. 4).
Figure 4. FreeSurfer average brain displaying the significant differences for cisgender versus FtM participants.
A: Chest, cisgender > FtM, lateral view of right hemisphere, 40–76 ms; B: Chest, FtM > cisgender, ventral view of right hemisphere, 120–156 ms; C: Chest-Hand subtraction, cisgender > FtM, lateral view of right hemisphere, 0–36 ms; D: Chest-Hand subtraction, FtM > cisgender, ventral view of right hemisphere, 120–156 ms.
Timepoints with Monte Carlo group-difference p-values below .05 were found in secondary somatosensory cortex at 20–24 ms and 68–72 ms. Marginally significant timepoints (p < .08) were identified in the medial temporal lobe at 120–124 ms and in the supramarginal gyrus at 52 ms. Comparison of these regions at these timepoints between FtM participants taking versus not taking testosterone did not yield statistically significant differences (p > .10).
We also confirmed that the tapping intervals were randomly distributed across trials and participants by examining the average interstimulus interval. On average, participants were tapped every 1.01s (SD 0.13) on the hand and every 1.05s (SD 0.14) on the chest; average interstimulus interval across blocks for each participant did not differ significantly between groups (p > .20 for both Hand and Chest).
DTI
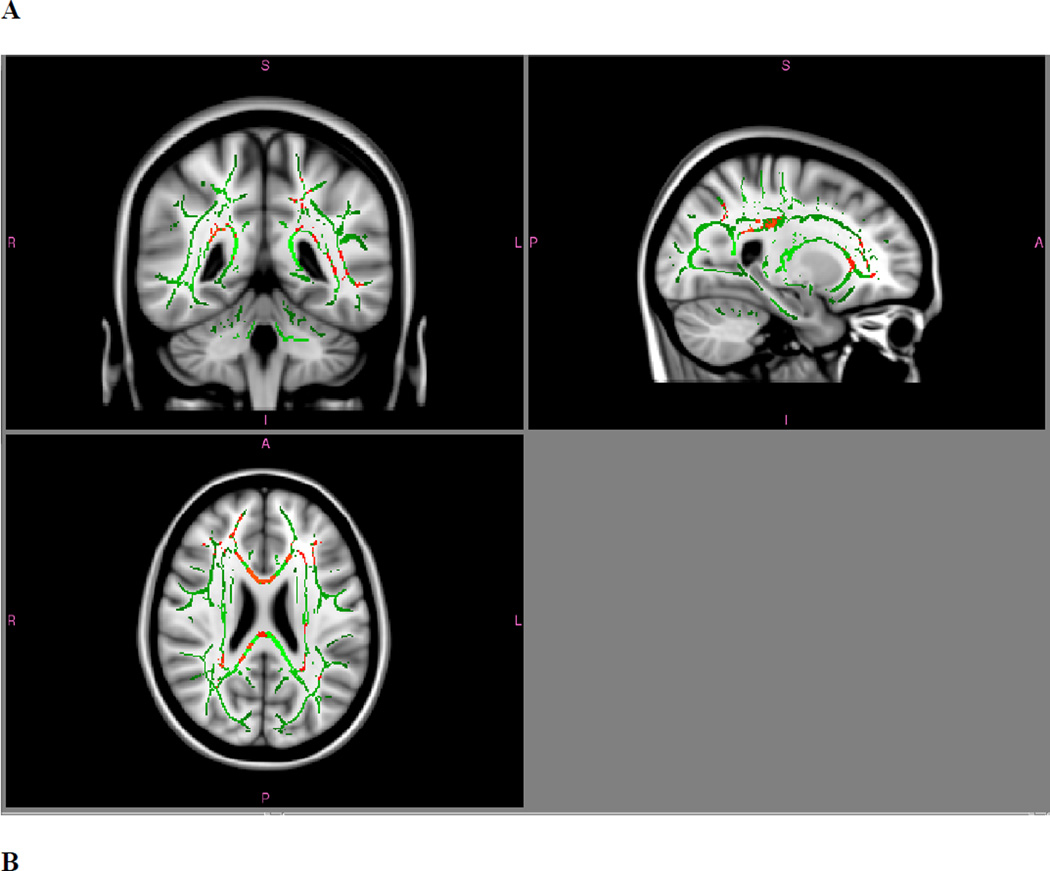
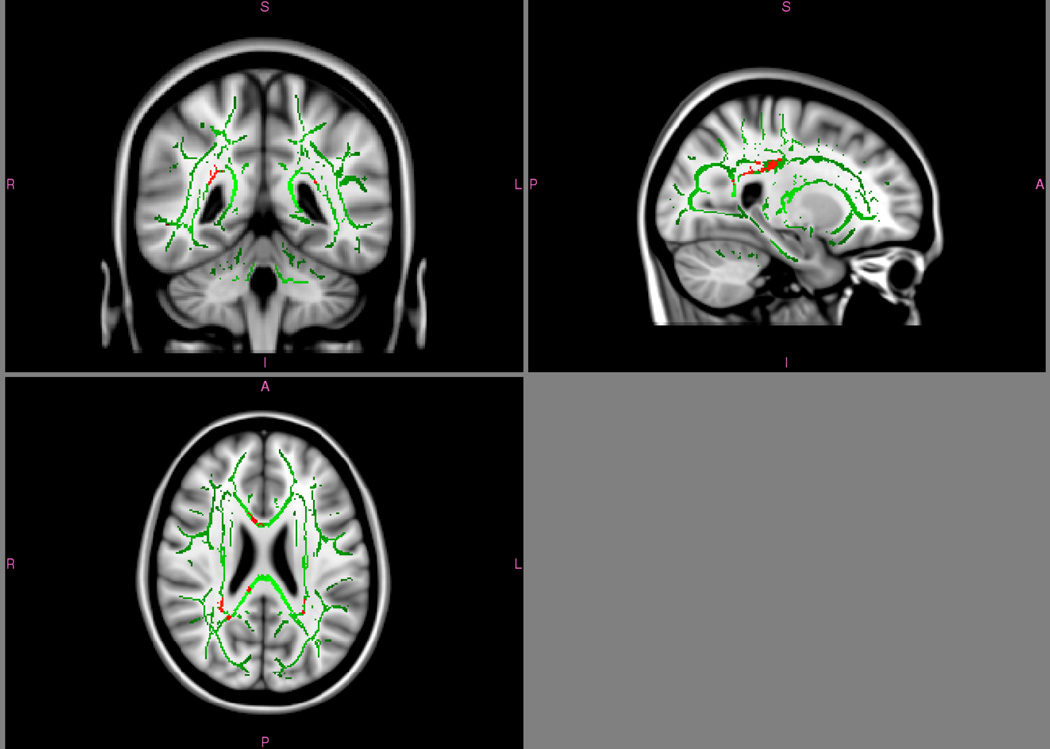
In the whole-brain TBSS analysis, FA did not differ between FtM and control female individuals. However, in analysis of eight pre-selected ROIs, higher FA was found in FtM participants than in control participants in the supramarginal gyrus (p = .02) and in the medial temporal lobe (p = .008; see Table 3.). Whole-brain TBSS analysis of MD did differ between groups; significant clusters were found with lower MD in FtM participants in numerous tracts, including the left inferior longitudinal fasciculus, left corticospinal tract, right posterior corona radiata, the forceps minor, and the body and splenium of the corpus callosum. These differences appeared to be driven primarily by radial diffusivity (see Fig. 5); axial diffusivity did not show any group differences in the whole-brain analysis. In the eight pre-selected ROIs, FtM participants had significantly lower mean diffusivity in the medial temporal lobe and anterior insula that appeared to be driven by differences in both axial and radial diffusivity. Testosterone status had no effect on DTI measures except a trend for FA in the supramarginal gyrus, which was marginally higher in FtM individuals taking testosterone than in those not taking testosterone (p = .08).
Table 3.
Comparisons of white matter between FtM and control female participants.
| FA (all FtM > control) |
MD (all control > FtM) |
AD (all control > FtM) |
RD (all control > FtM) |
|
|---|---|---|---|---|
|
Primary somatosensory cortex |
t(14) = .03, p = .98 |
t(14) = .59, p = .57 |
t(14) = .92, p = .37 |
t(14) = .50, p = .63 |
|
Secondary somatosensory cortex |
t(14) = 1.17, p = .26 |
t(14) = .31 p = .76 |
t(14) = .42, p = .68 |
t(14) = .49, p = .63 |
| Superior parietal lobule |
t(14) = 1.66, p = .12 |
t(14) = 1.40, p = .18 |
t(14) = 1.21, p = .25 |
t(14) = 1.68, p = .11 |
| Intraparietal sulcus |
t(14) = .04, p =.97 |
t(14) = .85, p = .41 |
t(14) = 1.02, p = .33 |
t(14) = .64, p = .53 |
| Supramarginal gyrus |
t(14) = 2.59, p = .02* |
t(14) = 1.43, p = .17 |
t(14) = 1.57, p = .14 |
t(14) = 1.53, p = .15 |
| Anterior insula |
t(14) = .37, p = .71 |
t(14) = 2.09, p = .055 |
t(14) = 2.24, p = .04* |
t(14) = 2.09, p =. 055 |
| Posterior insula |
t(14) = .18, p = .86 |
t(14) = 1.34, p = .20 |
t(14) = 1.68, p = .11 |
t(14) = 1.29, p = .22 |
| Medial temporal lobe |
t(14) = 3.09, p = .008* |
t(14) = 2.56, p = .02* |
t(14) = 2.19, p = .05* |
t(14) = 2.97, p = .01* |
Fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) were computed from diffusion weighted images in the FMRIB Diffusion Toolbox (FDT; FSL; Smith et al., 2004). FA, MD, AD, and RD values for each participants were extracted from the eight ROIs utilized in the MEG analysis and compared between groups.
Figure 5. TBSS whole-brain differences in white matter diffusivity for cisgender versus FtM transgender participants.
A: Mean diffusivity, cisgender > FtM; p < .05, FWE corrected. B: Radial diffusivity, cisgender > FtM; p < .05, FWE corrected. No significant clusters were found for FtM > cisgender mean diffusivity or for axial diffusivity (either contrast).
Hormones and Body Ratings
FtM participants taking testosterone rated their breasts as belonging to them more than FtM participants not taking testosterone, t(6) = 2.56, p = .04. Testosterone status had no significant effect on MEG sensory response to the breast or hand in any of the ROIs where group differences were found.
Body ownership ratings did not correlate with MEG sensory response but did correlate with FA in the supramarginal gyrus in the FtM group (r = 0.82, two-tailed p = .01).
MEG sensory response (at the timepoints with group differences) did not correlate significantly with DTI measures in the supramarginal gyrus or secondary somatosensory cortex, but did negatively predict MD in the medial temporal lobe across both groups for the breast (r = −0.65, two-tailed p = .006) but not for the hand (r = −0.35, two-tailed p = .18).
DISCUSSION
In the current study, we investigated the discrepancy between body image (or “phantom body”) and external morphology experienced by many transgender individuals by investigating early sensory integration of somatosensation from body parts that felt congruent (hand) and incongruent (breast). As predicted, FtM transgender and control female participants did not differ in neural response to hand sensation. For breast sensation, however, FtM participants showed a reduced early sensory response in the supramarginal gyrus and secondary somatosensory cortex in the 40–76 ms time window, but heightened sensory-evoked activity in the medial temporal lobe. When chest response was directly compared with hand response, the medial temporal lobe continued to show a heightened response in the FtM group for the chest relative to hand sensation, confirming heightened response to the chest in FtM individuals in the peri-amygdaloid area. A region falling primarily in the motor cortex showed reduced sensory response for the chest compared to hand condition in FtM individuals (relative to controls); the difference between groups appeared more related to the Hand condition. These results confirm several of our predicted differences in neural processing of incongruent-feeling body parts including reduced parietal integration but heightened amygdala response for incongruent-feeling sensation coming from the chest.
We also examined white matter using DTI analysis to see whether differences in sensory response related to differences in white matter coherence in FtM individuals. Indeed, we observed increased white matter coherence in the FtM participants in the supramarginal gyrus and medial temporal lobe, the same areas identified to have altered sensory response to the incongruent-feeling breast sensation. Mean diffusivity, on the other hand, showed widespread reduction in FtM participants, consistent with the findings of Kranz et al. (2014). ROI analyses identified reduced diffusivity in the medial temporal lobe and the anterior insula; lower mean diffusivity in the medial temporal lobe was correlated with heightened MEG breast response. DTI is sensitive to properties of tissue microstructure and higher MD or lower FA values often reflect damaged or impaired integrity of the white matter fibers (Soares, Marques, Alves, & Sousa, 2013). In contrast, our findings indicate greater coherence in these brain areas in FtM individuals, perhaps owing to greater focus on the body and thus greater use of these networks.
Reduced sensory evoked response to chest sensation in FtM individuals in the supramarginal gyrus may relate to the role of this brain area in feelings of bodily ownership. The broader region of the temporo-parietal junction is implicated in self-other distinctions and inference of self-agency (Decety & Lamm, 2007), body image, and attributions of agency (Blanke et al., 2005). Electrical stimulation of the angular gyrus, just posterior to the supramarginal gyrus, has been found to elicit out-of-body experience (Blanke, Ortigue, Landis, & Seeck, 2002). Indeed, several researchers suggest that visual body form representation is encoded in the supramarginal gyrus and the inferior parietal lobe in general (e.g., Carruthers, 2008; Tsakiris, Costantini, & Haggard, 2008). Speculatively, reduced processing in the supramarginal gyrus suggests that breast sensation may not be treated as strongly as “self” in FtM individuals. The supramarginal gyrus is critically involved in self-other distinctions and FtM participants in the current study provided low ratings for their feeling of ownership of their breasts (see Table 1).
Similarly, diminished secondary somatosensory cortex activation in FtM participants to chest sensation may reflect diminished integration of breast sensation and diminished conscious awareness of the sensation. Neurons in secondary somatosensory cortex show gross somatotopy (Zhu et al., 2007) and activation of secondary somatosensory cortex has been shown to relate to conscious perception of touch and to the body schema (Dijkerman & de Haan, 2007; Lin & Forss, 2002). Indeed, secondary somatosensory cortex is one of the parietal regions found to have reduced cortical thickness in patients with xenomelia, who also feel reduced ownership and aversion to a body part (Hilti et al., 2013). While we did not observe any significant differences in the right posterior insula, overlapping function has been seen in posterior insula and secondary somatosensory cortex, so it is possible that the difference in secondary somatosensory cortex may also reflect differences in the posterior insula mislocalized to secondary somatosensory cortex. Monte Carlo analysis of the sensory evoked responses suggested that group differences were greatest in secondary somatosensory cortex around 70 ms, consistent with the simultaneous activation of the parietal operculum and posterier parietal cortex in the 70–140 ms range in similar somatosensory studies (e.g., Mauguière et al., 1997). The right posterior insula has also been related to egocentric representation (Fink et al., 2003), self-recognition (Devue et al., 2007), and body ownership (Baier & Karnath, 2008; Tsakiris et al., 2007).
We did not observe the secondary somatosensory cortex and supramarginal gyrus group differences in the Chest-Hand comparison that were observed in the Chest condition. While this necessitates caution about the group differences observed in the Chest condition, we believe that the lack of interaction may be due to noise added by comparing SEFs from two very different body parts. The hands, for instance, have a strong motor representation that breasts do not have, making the Hand condition a poor comparison for sensory integration and body representation. In both the Chest and Chest-Hand conditions, however, we observed greater sensory response to chest sensation in the medial temporal lobe in the FtM group than in the control female group, suggesting that heightened periamygdaloid activation in FtM participants was related specifically to sensation from the chest. This may reflect an alarm response in the amygdala/periamygdaloid cortex to sensation from an incongruous or disliked body part. While the amygdala is further from the cortical surface and thus difficult to observe directly using MEG, periamygdaloid activity has been reported in MEG in relation to negative emotion (Cornwell et al., 2008; Garolera et al., 2007). This finding may relate to the observations of Manzouri et al. (2015), who reported reduced connectivity between body perception areas and the amygdala in resting state scans of FtM individuals.
Finally, in the group by body site interaction, we observed less activation in the Chest condition relative to Hand condition in the FtM than in the control group in primary motor cortex, with some activation extending into primary somatosensory cortex and the intraparietal sulcus. As we did not observe main effects in this area in the Chest or Hand conditions alone, the interpretation of this result is less clear. However, there was a trend towards greater activation in primary somatosensory cortex in the Hand condition in FtM individuals (p < .08), suggesting that the interaction was driven by differences between groups in the Hand condition.
No significant differences were observed in either the Hand or Chest conditions in SEFs in primary somatosensory cortex, the superior parietal lobule, the intraparietal sulcus, or the insula. The lack of difference in primary somatosensory cortex suggests equal registration of the somatosensory stimulus in this area. The location of chest activation in primary somatosensory cortex in the current study is also of interest as little research has been conducted on the “hermunculus,” the female version of the homunculus (Di Noto, Newman, Wall, & Einstein, 2013). In the current study, we observed chest activation on the superior dorsal part of primary somatosensory cortex. The few studies that have examined the somatosensory representation of the female breast and nipple report the same location for the female breast in primary somatosensory cortex as for the male chest (Aurbach, Heller, & Eagleman, 2009; Komisaruk et al., 2011; Rothemund, Schaefer, Grüsser, & Flor, 2005). Our data are in agreement with these studies. The lack of difference in the superior parietal lobule, however, is surprising given its role in xenomelia (McGeoch et al., 2011). We speculate that lesser representation of the breasts in the superior parietal lobule due to decreased motor relevance may contribute to this difference in findings. The lack of difference in the insula is also surprising and is discussed below in relation to theories of body ownership.
DTI
Higher FA was found in FtM participants than in cisgender participants in the supramarginal gyrus and in the medial temporal lobe, indicating greater white matter coherence, but not in the other right hemisphere body-related ROIs utilized in the MEG and DTI analyses; indeed, mean FA in primary somatosensory cortex was virtually identical between the two groups. This is significant given that the medial temporal lobe and supramarginal gyrus were the two regions in which FtM and control female participants differed in their response to chest sensation, but not in their response to hand sensation. Altered FA in these regions could result from body dysphoria and altered attention to incongruent-feeling body parts, or it could reflect inherent neural differences in transgender and cisgender individuals. Indeed, higher FA has been observed in untreated FtM individuals in the posterior part of the right superior longitudinal fasciculus, the forceps minor, and the corticospinal tract, similar to control males (Rametti et al., 2011a). We also found reduced mean diffusivity and axial diffusivity in the FtM participants in many regions, similar to previous findings in FtM individuals (Kranz et al., 2014) and for cisgender males versus females (Inano, Takao, Hayashi, Abe, & Ohtomo, 2011). ROI analyses identified reduced white matter diffusivity in FtM participants in the medial temporal lobe, corroborating the MEG and FA findings, and in the anterior insula.
Body Image and Body Ownership
We were surprised to find that FtM participants taking testosterone rated greater belonging of their breasts than FtM participants not taking testosterone. However, hormones had no significant effect on MEG sensory response. Hormones did show a trend in FtM individuals towards heightened FA in the supramarginal gyrus, but the effect was marginal. A clearer relationship was found between FA in the supramarginal gyrus and breast belonging ratings, suggesting that FtM participants with higher FA in the supramarginal gyrus (as seen overall in the FtM group) felt more ownership of their breasts. We speculate that hormones had a positive effect on body image and allowed participants to feel more ownership of their bodies, although this did not change the reduced MEG response to breast sensation.
Group differences in MEG sensory response in the supramarginal gyrus and secondary somatosensory cortex were not clearly related to any group differences in white matter. However, a heightened medial temporal lobe response to breast sensation was correlated with reduced MD in the medial temporal lobe; this was specific for breast sensation and not hand sensation. This finding suggests that individuals with reduced MD (as found overall in transgender individuals and in males relative to females) (Inano et al., 2011; Kranz et al., 2014) had more alarm response to breast sensation, though the causal relationship between these observations is not known.
The current study cannot discriminate between innate differences in body representation and alterations of body representation over time. Experiments with infants and individuals with congenital absence of a limb suggest some innate multimodal body representation in humans (De Preester & Tsakiris, 2009; Meltzoff & Moore, 1983; Morgan & Rochat, 1997), and gender identity may be affected by organizational effects of testosterone both in utero and at puberty (Bao & Swaab, 2011). FtM individuals experience sensation arising from their breasts, yet their sense of ownership of their breasts is reduced. One possible explanation for this phenomenon is that FtM individuals may have internal body representations that are anatomically male. Following the neurocognitive model of bodily ownership proposed by Tsakiris (2010), FtM individuals compare their sensed body form (breasts) to their internal structural body representation (no breasts). A poor structural match leads to lesser integration of the sensation, leading to a reduced sense of bodily ownership. Our findings support this theory to some extent, as we observed reduced sensory response in the secondary somatosensory cortex and supramarginal gyrus that may reflect reduced sensory integration. We were surprised to not observe differences in the insula, but it is possible that our secondary somatosensory cortex finding partly reflects differences in the posterior insula owing to the limitations of MEG (discussed under Limitations).
However, differences in body representation are likely to be affected by culture and personal experience in complex ways (Giummarra, Bradshaw, Nicholls, Hilti, & Brugger, 2011). Higher-level differences in gender-identification might lead to an acquired aversion to sex-specific body parts, and over time might modify representation of that body part. Indeed, Brugger, Lenggenhager, and Giummarra (2013) have suggested that the cortical thickness differences in xenomelia could result from bottom-up differences in neural body representation-or, equally plausibly, from “years, if not decades, of a hostile attitude directed to a part of the body.” The heightened peri-amygdala response in FtM participants may reflect such dislike of the female-identified body part, adding an affective element to body integration not addressed by Tsakiris (2010).
Limitations
MEG suffers a general limitation on source localization, as it records primarily from sulcal neurons. This affects localization of sources like secondary somatosensory cortex in the parietal operculum, making our secondary somatosensory cortex finding difficult to confirm. However, the temporal pattern of the secondary somatosensory cortex response in the current study was consistent with converging evidence suggesting that sensory response in this brain area begins around 60 or 70 ms (e.g., Cheyne et al., 2000 in Aine et al.; Frot & Mauguière, 1999; Zhu et al. 2007). In addition, our small sample size limited our power to observe potential differences between groups. MEG sample sizes are often smaller than other neuroimaging modalities. This is especially true for studies of motor and sensory evoked responses, due to the robust nature of the signal. Our sample size is on the lower end of MEG studies, but it is noteworthy that McGeoch et al. (2009) demonstrated differences in sensory evoked response outside of primary somatosensory cortex in a within-subject study of only four individuals with xenomelia.
In addition, it is difficult to know whether differences in sensory response in transgender and cisgender individuals reflect long-standing differences in body representation in transgender individuals or transient alterations in sensory processing related to mood and attention. Since the FtM participants were uncomfortable with their breasts, they might have employed strategies to diminish conscious perception of them. However, bottom-up attentional biasing in the sensory domain appears to be driven by the temporo-parietal junction, while top-down processing appears to be driven by the superior parietal lobule and precuneus (Behrmann, Geng, & Shomstein, 2004). We did not find any significant differences in primary somatosensory cortex or in the superior parietal lobule, suggesting that any attention-related effects in the current study are related to bottom-up processes rather than top-down modulation of attention to the sensation. In addition, recent work on xenomelia reported heightened parietal response to sensory stimulation in general, despite strong feelings of disownership for one of the limbs stimulated (van Dijk et al., 2013). This suggests that disownership and negative affect are unlikely to decrease attention, and that other differences may better explain the reduced inferior parietal response we observed in the FtM group. Mental distraction could also underlie reduced sensory processing for the chest in FtM individuals, especially in secondary somatosensory cortex (e.g., Fujiwara et al., 2002; Hamada, Okita, & Suzuki, 2003; Hoechstetter et al., 2000). In post-study debriefing, however, each participant confirmed remaining aware of the sensation throughout the task. Furthermore, the FtM participants exhibited heightened response in the medial temporal lobe (peri-amygdaloid cortex) during both early and late parts of the SEF, suggesting a heightened emotional response that is somewhat inconsistent with a lack of attention or with active distraction. We believe the results of the current study are thus best explained by a model of reduced early and automatic integration of sensation, through conflict with neural representations of body and/or self. This reduced integration might drive bottom-up differences in salience of the sensation and contribute to reduced feelings of body ownership.
Conclusion
In sum, we find preliminary evidence of differences in the neural response to sensation from the chest in FtM individuals, possibly mediated by differences in anatomical connectivity, compared with cisgender (non-transgender) female individuals. FtM individuals show less activation of supramarginal gyrus and secondary somatosensory cortex, and more activation of the temporal pole in response to sensation on the chest, suggesting less integration and more anxiety and alarm for sensation from this body part. Further, increased white matter FA was found in these same regions in the same group of FtM individuals, suggesting that altered sensory processing could be related to underlying differences in white matter in these brain regions. While the current study cannot discern the origin of differences in sensory-related body representation in the brain in transgender individuals, our results suggest that aversion to gender-incongruous body parts is rapid and automatic at the sensory level. These differences may contribute to the experience of discomfort and incongruence caused by sensation in these body parts.
Acknowledgments
We thank Dr. Mingxiong Huang, Dr. Roland Lee, and Dr. Tao Song, for support in collecting the MEG data and Dr. Eric Halgren and Burke Rosen for assistance in processing the MEG data. We thank Zack Taich, Dave Deriso, and Radhika Gosavi for assistance carrying out the study and Rachel Case for assistance preparing the figures.
This work was supported by the University of California, San Diego through an Academic Senate Award (2011) and through general support of several of the authors while the study was conducted. This work was also supported by research funds from Herb Lurie. Finally, the first author thanks NCCIH for support during the preparation of the manuscript. The views presented in this paper are solely those of the authors and do not necessarily reflect views of the National Center for Complementary and Integrative Health.
Footnotes
Gender Dysphoria is diagnosed in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5; American Psychiatric Association, 2013) on the basis of an individual’s dysphoria about the discrepancy between the gender they identify with versus the gender assigned to them at birth. This dysphoria is sometimes treated through hormones and surgery to align the body’s appearance with the individual’s gender identity.
It is unlikely that transgender individuals willfully construct these representations. While some phantom sexed body parts are welcome, others can conflict with gender identity, as seen in the case of a recurrent, unwanted phantom penile erection after sex reassignment surgery removing the penis (Namba et al., 2008). Indeed, phantom limbs in amputees are often characterized by severe and chronic pain and thus unlikely to be willfully constructed.
REFERENCES
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Washington, DC: Author; 2013. [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S. Non-linear registration aka spatial normalisation. 2007 FMRIB Analysis Group Technical Report. Retrieved from http://www.fmrib.ox.ac.uk/analysis/techrep/tr07ja2/tr07ja2.pdf.
- Aurbach E, Heller L, Eagleman DM. Plasticity of somatosensory cortex after surgical body recontouring. Society for Neuroscience Annual Meeting Abstracts. 2009;39:363.25. [Google Scholar]
- Baier B, Karnath HO. Tight link between our sense of limb ownership and self awareness of actions. Stroke. 2008;39:486–488. doi: 10.1161/STROKEAHA.107.495606. [DOI] [PubMed] [Google Scholar]
- Bao A-M, Swaab DF. Sexual differentiation of the human brain: relation to gender identity, sexual orientation and neuropsychiatric disorders. Frontiers in Neuroendocrinology. 2011;32:214–226. doi: 10.1016/j.yfrne.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Geng JJ, Shomstein S. Parietal cortex and attention. Current Opinion in Neurobiology. 2004;14:212–217. doi: 10.1016/j.conb.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Berglund H, Lindström P, Dhejne-Helmy C, Savic I. Male-to-female transsexuals show sex-atypical hypothalamus activation when smelling odorous steroids. Cerebral Cortex. 2008;18:1900–1908. doi: 10.1093/cercor/bhm216. [DOI] [PubMed] [Google Scholar]
- Blanke O, Ortigue S, Landis T, Seeck M. Stimulating own-body perceptions. Nature. 2002;419:269–270. doi: 10.1038/419269a. [DOI] [PubMed] [Google Scholar]
- Blanke O, Mohr C, Michel C, Pascual-Leone A, Brugger P, Seeck M, et al. Linking out-of-body experience and self processing to mental own-body imagery at the temporoparietal junction. Journal of Neuroscience. 2005;25:550–557. doi: 10.1523/JNEUROSCI.2612-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brang D, McGeoch PD, Ramachandran VS. Apotemnophilia: A neurologic disorder. NeuroReport. 2008;19(13):1305–1306. doi: 10.1097/WNR.0b013e32830abc4d. [DOI] [PubMed] [Google Scholar]
- Brugger P, Lenggenhager B, Giummarra MJ. Xenomelia: a social neuroscience view of altered bodily self-consciousness. Frontiers in Psychology. 2013;4:204. doi: 10.3389/fpsyg.2013.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SM, Cohen-Kettenis PT, Veltman DJ, Klink DT, Bakker J. Hypothalamic response to the chemo-signal androstadienone in gender dysphoric children and adolescents. Frontiers in Endocrinology. 2014;5:1–10. doi: 10.3389/fendo.2014.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers G. Types of body representation and the sense of embodiment. Consciousness & Cognition. 2008;17:1302–1316. doi: 10.1016/j.concog.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Case LK. How the body can feel wrong: Sensory processing and neural body representation in transsexuality and anorexia nervosa. 2013 Retrieved from ProQuest Dissertations and Theses database (UMI No. 3601113) [Google Scholar]
- Cheyne D, Roberts LE, Gaetz W, Bosnyak D, Weinberg H, Johnson B, Nahmias C, et al. EEG and MEG source analysis of somatosensory evoked responses to mechanical stimulation of the fingers. In: Aine C, Okada Y, Stronik G, Swithenby SJ, Wood CC, editors. Advances in biomagnetism. New York: Springer-Verlag; 2000. pp. 1130–1133. [Google Scholar]
- Cohen-Kettenis PT, Gooren LJ. Transsexualism: a review of etiology, diagnosis, and treatment. Journal of Psychosomatic Research. 1999;46(4):315–333. doi: 10.1016/s0022-3999(98)00085-3. [DOI] [PubMed] [Google Scholar]
- Cornwell BR, Carver FW, Coppola R, Johnson L, Alvarez R, Grillon C. Evoked amygdala responses to negative faces revealed by adaptive MEG beamformers. Brain Research. 2008;1244:103–112. doi: 10.1016/j.brainres.2008.09.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel-now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Dale AM, Liu AK, Fischl BR, Buckner RL, Belliveau JW, Lewine JD, Halgren E. Dynamic statistical parametric mapping: Combining fMRI and MEG for high resolution imaging of cortical activity. Neuron. 2000;26:55–67. doi: 10.1016/s0896-6273(00)81138-1. [DOI] [PubMed] [Google Scholar]
- Davis SA, Meier SC. Effects of testosterone treatment and chest reconstruction surgery on mental health and sexuality in female-to-male transgender people. International Journal of Sexual Health. 2013;26:113–128. [Google Scholar]
- Decety J, Lamm C. The role of the right temporoparietal junction in social interaction: how low-level computational processes contribute to meta-cognition. The Neuroscientist. 2007;13:580–593. doi: 10.1177/1073858407304654. [DOI] [PubMed] [Google Scholar]
- De Preester H, Tsakiris M. Body-extension versus body-incorporation: is there a need for a body-model? Phenomenology & Cognitive Sciences. 2009;8(3):307–319. [Google Scholar]
- Devue C, Collette F, Balteau E, Degueldre C, Luxen A, Maquet P, Brédart S. Here I am: The cortical correlates of visual self-recognition. Brain Research. 2007;1143:169–182. doi: 10.1016/j.brainres.2007.01.055. [DOI] [PubMed] [Google Scholar]
- Dijkerman HC, de Haan EH. Somatosensory processes subserving perception and action. Behavioural Brain Science. 2007;30:189–201. doi: 10.1017/S0140525X07001392. [DOI] [PubMed] [Google Scholar]
- Di Noto PM, Newman L, Wall S, Einstein G. The hermunculus: What is known about the representation of the female body in the brain? Cerebral Cortex. 2013;23:1005–1013. doi: 10.1093/cercor/bhs005. [DOI] [PubMed] [Google Scholar]
- Dutton L, Koenig K, Fennie K. Gynecologic care of the female-to-male transgender man. Journal of Midwifery & Women’s Health. 2008;53:331–337. doi: 10.1016/j.jmwh.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson-Schroth L. Update on the biology of transgender identity. Journal of Gay & Lesbian Mental Health. 2013;17:150–174. [Google Scholar]
- Fink GR, Marshall JC, Weiss PH, Stephan T, Grefkes C, Shah NJ, et al. Performing allocentric visuospatial judgments with induced distortion of the egocentric reference frame: An fMRI study with clinical implications. Neuroimage. 2003;20:1505–1517. doi: 10.1016/j.neuroimage.2003.07.006. [DOI] [PubMed] [Google Scholar]
- First MB. Desire for amputation of a limb: Paraphilia, psychosis, or a new type of identity disorder. Psychological Medicine. 2005;35:919–928. doi: 10.1017/s0033291704003320. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Human Brain Mapping. 1999;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frot M, Mauguière F. Timing and spatial distribution of somatosensory responses recorded in the upper bank of the sylvian fissure (SII area) in humans. Cerebral Cortex. 1999;9:854–863. doi: 10.1093/cercor/9.8.854. [DOI] [PubMed] [Google Scholar]
- Fujiwara N, Imai M, Nagamine T, Mima T, Oga T, Takeshita K, et al. Second somatosensory area (SII) plays a significant role in selective somatosensory attention. Cognitive Brain Research. 2002;14:389–397. doi: 10.1016/s0926-6410(02)00141-6. [DOI] [PubMed] [Google Scholar]
- Garolera M, Coppola R, Muñoz KE, Elvevåg B, Carver FW, Weinberger DR, Goldberg TE. Amygdala activation in affective priming: A magnetoencephalogram study. NeuroReport. 2007;18:1449–1453. doi: 10.1097/WNR.0b013e3282efa253. [DOI] [PubMed] [Google Scholar]
- Giummarra MJ, Bradshaw JL, Nicholls ME, Hilti LM, Brugger P. Body integrity identity disorder: Deranged body processing, right fronto-parietal dysfunction, and phenomenological experience of body incongruity. Neuropsychology Review. 2011;21:320–333. doi: 10.1007/s11065-011-9184-8. [DOI] [PubMed] [Google Scholar]
- Gizewski ER, Krause E, Schlamann M, Happich F, Ladd ME, Forsting M, Senf W. Specific cerebral activation due to visual erotic stimuli in male-to-female transsexuals compared with male and female controls: An fMRI study. Journal of Sexual Medicine. 2009;6:440–448. doi: 10.1111/j.1743-6109.2008.00981.x. [DOI] [PubMed] [Google Scholar]
- Guillamon A, Junque C, Gómez-Gil E. Archives of Sexual Behavior. 2016 doi: 10.1007/s10508-016-0768-5. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Kranz GS, Küblböck M, Kaufmann U, Ganger S, Hummer A, et al. Structural connectivity networks of transgender people. Cerebral Cortex. 2014;25:3527–3534. doi: 10.1093/cercor/bhu194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada Y, Okita H, Suzuki R. Effect of interstimulus interval on attentional modulation of cortical activities in human somatosensory areas. Clinical Neurophysiology. 2003;114:548–555. doi: 10.1016/s1388-2457(02)00384-x. [DOI] [PubMed] [Google Scholar]
- Hilti LM, Hänggi J, Vitacco DA, Kraemer B, Palla A, Luechinger R, et al. The desire of healthy limb amputation: Structural brain correlates and features of xenomelia. Brain. 2013;136:318–329. doi: 10.1093/brain/aws316. [DOI] [PubMed] [Google Scholar]
- Hoechstetter K, Rapp A, Meinck HM, Weckesser D, Bornfleth H, Stippich C, et al. Magnetic source imaging of tactile input shows task-independent attention effects in SII. NeuroReport. 2000;11:2461–2465. doi: 10.1097/00001756-200008030-00024. [DOI] [PubMed] [Google Scholar]
- Inano S, Takao H, Hayashi N, Abe O, Ohtomo K. Effects of age and gender on white matter integrity. American Journal of Neuroradiology. 2011;32:2103–2109. doi: 10.3174/ajnr.A2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, Rushworth MFS. Using diffusion imaging to study human connectional anatomy. Annual Review of Neuroscience. 2009;32:75–94. doi: 10.1146/annurev.neuro.051508.135735. [DOI] [PubMed] [Google Scholar]
- Komisaruk B, Wise N, Frangos E, Liu WC, Allen K, Brody S. Women’s clitoris, vagina, and cervix mapped on the sensory cortex: fMRI evidence. Journal of Sexual Medicine. 2011;8:2822–2830. doi: 10.1111/j.1743-6109.2011.02388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer B, Delsignore A, Schnyder U, Hepp U. Body image and transsexualism. Psychopathology. 2008;41:96–100. doi: 10.1159/000111554. [DOI] [PubMed] [Google Scholar]
- Kranz GS, Hahn A, Kaufmann U, Küblböck M, Hummer A, Ganger S, et al. White matter microstructure in transsexuals and controls investigated by diffusion tensor imaging. Journal of Neuroscience. 2014;34:15466–15475. doi: 10.1523/JNEUROSCI.2488-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruijver FP, Zhou JN, Pool CW, Hofman MA, Gooren LJ, Swaab DF. Male-to-female transsexuals have female neuron numbers in a limbic nucleus. Journal of Clinical Endocrinology and Metabolism. 2000;85:2034–2041. doi: 10.1210/jcem.85.5.6564. [DOI] [PubMed] [Google Scholar]
- Lindgren TW, Pauly IB. A body image scale for evaluating transsexuals. Archives of Sexual Behavior. 1975;4:639–656. doi: 10.1007/BF01544272. [DOI] [PubMed] [Google Scholar]
- Lin YY, Forss N. Functional characterization of human second somatosensory cortex by magnetoencephalography. Behavioural Brain Research. 2002;135:141–145. doi: 10.1016/s0166-4328(02)00143-2. [DOI] [PubMed] [Google Scholar]
- Lin CS, Ku HL, Chao HT, Tu PC, Li CT, Cheng CM, et al. Neural network of body representation differs between transsexuals and cissexuals. PLoS ONE. 2014;9(1) doi: 10.1371/journal.pone.0085914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Sánchez FJ, Tosun D, Shattuck DW, Gaser C, Vilain E, Toga AW. Increased cortical thickness in male-to-female transsexualism. Journal of Behavioral and Brain Science. 2012;2:357–362. doi: 10.4236/jbbs.2012.23040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzouri A, Kosidou K, Savic I. Anatomical and functional findings in female-to-male transsexuals: testing a new hypothesis. Cerebral Cortex. 2015 doi: 10.1093/cercor/bhv278. [DOI] [PubMed] [Google Scholar]
- Mauguière F, Merlet I, Forss N, Vanni S, Jousmäki V, Adeleine P, Hari R. Activation of a distributed somatosensory cortical network in the human brain. A dipole modelling study of magnetic fields evoked by median nerve stimulation. Part I: location and activation timing of SEF sources. Electroencephalography and Clinical Neurophysiology/Evoked Potentials Section. 1997;104:281–289. doi: 10.1016/s0013-4694(97)00006-0. [DOI] [PubMed] [Google Scholar]
- McGeoch PD, Brang D, Song T, Lee RR. Apotemnophilia: The neurological basis of a ‘psychological’ disorder. Nature Precedings. 2009 Retrieved from http://precedings.nature.com/documents/2954/version/1.
- McGeoch PD, Brang D, Song T, Lee RR, Huang M, Ramachandran VS. Xenomelia: A new right parietal syndrome. Journal of Neurology, Neurosurgery, & Psychiatry. 2011;82:1314–1319. doi: 10.1136/jnnp-2011-300224. [DOI] [PubMed] [Google Scholar]
- Meltzoff AN, Moore MK. Newborn infants imitate adult facial gestures. Child Development. 1983;54:702–709. [PubMed] [Google Scholar]
- Morgan R, Rochat P. Intermodal calibration of the body in early infancy. Ecological Psychology. 1997;9:1–23. [Google Scholar]
- Moseley GL, Gallace A, Spence C. Bodily illusions in health and disease: Physiological and clinical perspectives and the concept of a cortical ‘body matrix’. Neuroscience and Biobehavioral Reviews. 2012;36:34–46. doi: 10.1016/j.neubiorev.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Namba Y, Sugiyama N, Yamashita S, Tokuyama E, Hasegawa K, Kimata Y. Phantom erectile penis after sex reassignment surgery. Acta Medical Okayama. 2008;62:213–216. doi: 10.18926/AMO/30981. [DOI] [PubMed] [Google Scholar]
- Prosser J. Second skins: The body narratives of transsexuality. New York: Columbia University Press; 1998. [Google Scholar]
- Ramachandran VS. Phantom penises in transsexuals: Evidence of an innate gender specific body image in the brain. Journal of Consciousness Studies. 2008;15:5–16. [Google Scholar]
- Ramachandran VS, McGeoch PD. Occurrence of phantom genitalia after gender reassignment surgery. Medical Hypotheses. 2007;69:1001–1003. doi: 10.1016/j.mehy.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Rametti G, Carrillo B, Gómez-Gil E, Junque C, Segovia S, Gomez Á, Guillamon A. White matter microstructure in female to male transsexuals before cross-sex hormonal treatment. A diffusion tensor imaging study. Journal of Psychiatric Research. 2011a;45:199–204. doi: 10.1016/j.jpsychires.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Rametti G, Carillo B, Gómez-Gil E, Junque C, Zubiarre-Elorza L, Segovia S, et al. The microstructure of white matter in male to female transsexuals before cross-sex hormonal treatment. A diffusion tensor imaging study. Journal of Psychiatric Research. 2011;45(7):949–954. doi: 10.1016/j.jpsychires.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Richards C, Barrett J. The case for bilateral mastectomy and male chest contouring for the female-to-male transsexual. Annals of The Royal College of Surgeons of England. 2013;95:93–95. doi: 10.1308/003588413X13511609957290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothemund Y, Schaefer M, Grüsser SM, Flor H. Localization of the human female breast in primary somatosensory cortex. Experimental Brain Research. 2005;164:357–364. doi: 10.1007/s00221-005-2257-2. [DOI] [PubMed] [Google Scholar]
- Savic I, Arver S. Sex dimorphism of the brain in male-to-female transsexuals. Cerebral Cortex. 2011;21:2525–2533. doi: 10.1093/cercor/bhr032. [DOI] [PubMed] [Google Scholar]
- Schöning S, Engelien A, Bauer C, Kugel H, Kersting A, Roestel C, et al. Neuroimaging differences in spatial cognition between men and male-to female transsexuals before and during hormone therapy. Journal of Sexual Medicine. 2010;7:1858–1867. doi: 10.1111/j.1743-6109.2009.01484.x. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen -Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith SM, Johansen-Berg H, Jenkinson M, Rueckert D, Nichols TE, Miller KL, et al. Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nature Protocols. 2007;2:499–503. doi: 10.1038/nprot.2007.45. [DOI] [PubMed] [Google Scholar]
- Soares JM, Marques P, Alves V, Sousa N. A hitchhiker’s guide to diffusion tensor imaging. Frontiers in Neuroscience. 2013;7:31. doi: 10.3389/fnins.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleman RS, Staphorsius AS, Cohen-Kettenis PT, Lambalk CB, Veltman DJ, van Trotsenburg MA, et al. Oestrogens are not related to emotional processing: a study of regional brain activity in female-to-male transsexuals under gonadal suppression. Cerebral Cortex. 2014;26:510–516. doi: 10.1093/cercor/bhu201. [DOI] [PubMed] [Google Scholar]
- Staphorsius AS, Kreukels BP, Cohen-Kettenis PT, Veltman DJ, Burke SM, Schagen SE, et al. Puberty suppression and executive functioning: An fMRI study in adolescents with gender dysphoria. Psychoneuroendocrinology. 2015;56:190–199. doi: 10.1016/j.psyneuen.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Tsakiris M. My body in the brain: A neurocognitive model of body-ownership. Neuropsychologia. 2010;48:703–712. doi: 10.1016/j.neuropsychologia.2009.09.034. [DOI] [PubMed] [Google Scholar]
- Tsakiris M, Costantini M, Haggard P. The role of the right temporo-parietal junction in maintaining a coherent sense of one’s body. Neuropsychologia. 2008;46:3014–3018. doi: 10.1016/j.neuropsychologia.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Tsakiris M, Hesse MD, Boy C, Haggard P, Fink GR. Neural correlates of body-ownership: A sensory network for bodily self-consciousness. Cerebral Cortex. 2007;17:2235–2244. doi: 10.1093/cercor/bhl131. [DOI] [PubMed] [Google Scholar]
- van Dijk MT, van Wingen GA, van Lammeren A, Blom RM, de Kwaasteniet BP, Scholte HS, Denys D. Neural basis of limb ownership in individuals with body integrity identity disorder. PLoS ONE. 2013;8(8) doi: 10.1371/journal.pone.0072212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro K, Inui K, Otsuru N, Urakawa T, Kakigi R. Temporal window of integration in the somatosensory modality: an MEG study. Clinical Neurophysiology. 2011;122:2276–2281. doi: 10.1016/j.clinph.2011.03.028. [DOI] [PubMed] [Google Scholar]
- Zhou JN, Hofman MA, Gooren LJ, Swaab DF. A sex difference in the human brain and its relation to transsexuality. Nature. 1995;378:68–70. doi: 10.1038/378068a0. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Disbrow EA, Zumer JM, McGonigle DJ, Nagarajan SS. Spatiotemporal integration of tactile information in human somatosensory cortex. BMC Neuroscience. 2007;8(21) doi: 10.1186/1471-2202-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubiaurre-Elorza L, Junque C, Gomez-Gil E, Segovia S, Carrillo B, Rametti G, Guillamon A. Cortical thickness in untreated transsexuals. Cerebral Cortex. 2013;23:2855–2862. doi: 10.1093/cercor/bhs267. [DOI] [PubMed] [Google Scholar]
- Zucker KJ, Cohen-Kettenis PT. Gender identity disorder in children and adolescents. In: Rowland DL, Incrocci L, editors. Handbook of sexual and gender identity disorders. New York: Wiley, Sons; 2008. pp. 376–422. [Google Scholar]