Abstract
Background:
The causes of many cases of preterm birth (PTB) remain enigmatic. Increased understanding of how epigenetic factors are associated with health outcomes has resulted in studies examining DNA methylation (DNAm) as a contributing factor to PTB. However, few studies on PTB and DNAm have included African American women, the group with the highest rate of PTB.
Methods:
The objective of this review was to systematically analyze the existing studies on DNAm and PTB among African American women.
Results:
Studies (N = 10) were limited by small sample size, cross-sectional study designs, inconsistent methodologies for epigenomic analysis, and evaluation of different tissue types across studies. African Americans comprised less than half of the sample in 50% of the studies reviewed. Despite these limitations, there is evidence for an association between DNAm patterns and PTB.
Conclusions:
Future research on DNAm patterns and PTB should use longitudinal study designs, repeated DNAm testing, and a clinically relevant definition of PTB and should include large samples of high-risk African American women to better understand the mechanisms for PTB in this population.
Keywords: preterm birth, African Americans, DNA methylation, gestational age, race, pregnancy
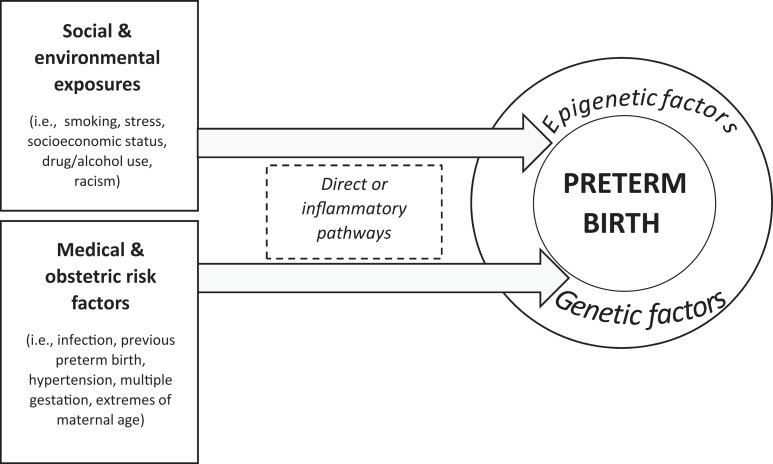
Preterm birth (PTB) is the leading contributor to infant mortality in the United States, and approximately 11% of infants in the United States are born preterm (<37 weeks’ gestation; Martin, Hamilton, Osterman, Curtin, & Matthews, 2015). Infants born preterm have significant cognitive, developmental, and physiological sequelae throughout life (Platt, 2014). Health disparities in birth outcomes by race persist, as African American women have approximately 1.5 times the risk of PTB (16%) as Caucasian women (11%) and are more than twice as likely as Caucasian women to experience infant mortality (Martin et al., 2015). These disparities persist even for African American women with higher levels of income and education (Colen, Geronimus, Bound, & James, 2006). Several types of PTB exist, and research has provided insight into direct and indirect pathways through which social, environmental, and medical/obstetric risk factors lead to PTB. Recently, investigators have begun paying increased attention to how these factors associate with epigenetic changes to result in PTB (Figure 1). However, few studies on PTB and epigenetic changes, specifically DNA methylation (DNAm), have included African American women. The purpose of this review is to provide a comprehensive discussion of DNAm and PTB with a focus on health disparities and studies done in minority populations in order to make recommendations for future research to advance the science.
Figure 1.
Contributors to preterm birth. Social and environmental exposures along with medical and obstetric risk factors interact with genetic and epigenetic factors to produce preterm birth.
Background
Risk Factors Associated With PTB and Racial Disparities in PTB
Clinicians often categorize PTB as either spontaneous or indicated, and mechanisms for prevention and treatment may differ between the two types. Spontaneous PTB can occur as a result of preterm labor (occurring in 45% of PTBs) or preterm premature rupture of membranes (PPROM; occurring in 25% of cases; Goldenberg, Culhane, Iams, & Romero, 2008). Indicated PTB refers to cases in which maternal or fetal medical risks necessitate delivery by prelabor cesarean section or vaginal induction. Pathways to PTB may differ by race, as the biggest contributor to spontaneous PTB in Caucasian women is preterm labor, while in African American women it is PPROM (Ananth & Vintzileos, 2006).
Research has identified a wide array of social and environmental risk factors that are associated with PTB (i.e., smoking, socioeconomic status, racism, and stress). Despite this knowledge, researchers have been unable to fully understand the causal mechanisms of PTB or determine how to reduce racial disparities in PTB (M. R. Kramer & Hogue, 2009). Smoking, for example, has been consistently linked with adverse birth outcomes (Goldenberg et al., 2008), yet the rate of smoking is lower among African American women (13.7%) than among Caucasian women (17.2%; Jamal et al., 2015). It may be, however, that African American women are less likely to quit smoking during pregnancy than Caucasian women (Harrison & Sidebottom, 2009). Further, investigators have reported that gene–smoking interactions are associated with hypertension in African Americans (Taylor, Schwander, et al., 2016). How this particular gene–environment interaction influences PTB risk is still unknown.
Social determinants of poor health outcomes, such as low socioeconomic status, often result in poor nutrition and inadequate maternal weight gain, which have been associated with spontaneous PTB (Goldenberg et al., 2008). Further, African American women are more likely than Caucasian women to exhibit signs of poor nutrition such as low serum folate levels and anemia in pregnancy (Dunlop, Kramer, Hogue, Menon, & Ramakrishan, 2011). Other social factors such as racism and neighborhood deprivation have also been associated with increased risk for PTB (M. R. Kramer & Hogue, 2009). A recent clinical trial among low-income, medically high-risk African American women attempted to ameliorate several individual-level risk factors leading to PTB (including inadequate housing, smoking, urogenital and periodontal infections, low literacy, and depression); however, researchers observed no change in outcome (Webb, Mathew, & Culhane, 2014).
Medical and obstetric factors (e.g., diabetes, hypertension, and prior PTB) also contribute to higher rates of PTB in African American women (M. R. Kramer & Hogue, 2009). For example, obesity affects 53% of African American women in the United States (compared to 32% of Caucasian women; May, Freedman, Sherry, Blanck, 2013; Centers for Disease Control and Prevention, 2013) and is implicated in the development of diseases associated with PTB such as hypertension and gestational diabetes (Yanit, Snowden, Cheng, & Caughey, 2012). Obese women are more likely than normal weight women to have hypertensive disorders during pregnancy, including both chronic hypertension and preeclampsia, placing them at higher risk of perinatal morbidity and mortality. Investigators from a large cohort study reported increased prevalence of mild (4.8%) and severe (3.5%) preeclampsia as well as gestational hypertension (3.6%) among African American women when compared to women of other races (Ghosh et al., 2014). Gestational diabetes, which is also related to obesity, affects 10.5% of African American women, compared to 6.8% of Caucasians (DeSisto, Kim, & Sharma, 2014). The rate of PTB is higher in women who have either pregestational diabetes (19.4%) or chronic hypertension (25.5%) than in women without these illnesses, and researchers have observed interactive effects for women with both disorders (35.5%; Yanit et al., 2012).
Short interpregnancy interval has also been linked to PTB, with the ideal interval between pregnancies for optimal birth outcomes being 18–24 months. African American women are more likely to have less than 6 months between pregnancies than Caucasian women (Hogue, Menon, Dunlop, & Kramer, 2011), placing them at higher risk for PTB. Further, women with a history of previous spontaneous PTB have a 2.5 times increased risk of a repeat spontaneous PTB and are 10 times as likely to have a PTB before 28 weeks’ gestation than women with no such history (Mercer et al., 1999). African American women have a rate of PTB before 27 weeks’ gestation that is more than 3 times greater than that of Caucasian women (Martin et al., 2015).
Although direct pathways to PTB exist, such as those we described above, research has suggested that inflammatory pathways may also be triggered that lead to this adverse birth outcome. For example, the prevalence of bacterial vaginosis is higher among African American women (51.6%) than Caucasian women (23.2%; Meis et al., 1995). Antimicrobial treatment results in limited improvement in outcomes (Sanu & Lamont, 2011), suggesting that infection may initiate an inflammatory process that continues despite treatment. Further, Gomez et al. (2010) found evidence of a gene–environment interaction between bacterial vaginosis infection and single nucleotide polymorphisms on inflammation-related genes that resulted in increased risk of PTB among a predominantly African American cohort of women. Nutritional deficiencies of iron, folic acid, zinc, calcium, magnesium, vitamin D, and polyunsaturated fats may also contribute to inflammation and may be associated with racial disparities in adverse birth outcomes (Dunlop et al., 2011).
Stress in pregnancy, which has also been associated with poor birth outcomes, may also act via inflammatory pathways (Olson et al., 2015). In a study of chronic stress in pregnancy, authors reported that African American and Latina women had impaired cytokine glucocorticoid feedback systems, limiting their ability to regulate inflammation in pregnancy when compared to Caucasian women (Corwin et al., 2013). Authors refer to chronic, accumulated stress as allostatic load, a concept that may be useful in quantifying stressors that contribute to inflammation and help identify women in early pregnancy at high risk for PTB (Olson et al., 2015).
Epigenetic and Genetic Contributions to PTB
This complex system of environmental, social, and genetic factors that surround PTB have begun to inspire more studies in the area of epigenomics and PTB, yet there remains a paucity of research in this area (Parets, Bedient, Menon, & Smith, 2014). Early epigenetic studies of the agouti mouse first identified how genetics and environmental factors interact to affect fetal programming (stressors experienced in utero) and influence phenotype (variation in coat color; Wolff, Kodell, Moore, & Cooney, 1998). Genetic factors alone may play a limited role in PTB, and studies in humans have begun to focus on how environmental exposures affect the epigenome of pregnant women and their fetuses. Some authors have suggested that environmental exposures have a stronger influence on PTBs than genetic factors, especially for African Americans (York, Eaves, Neale, & Strauss, 2014). However, despite being disproportionately affected by PTB, African Americans are underrepresented in genomic studies of PTB, and little is known about the epigenomic profiles that may contribute to this adverse outcome.
The shared physiology of the maternal/fetal environment presents an ideal landscape for examination of how maternal factors affect tissues and hormones to induce PTB. Epigenetic studies on PTB have commonly used DNAm as a biomarker for PTB. For example, in one study, researchers found that maternal smoking was associated with altered DNAm in offspring (Maccani, Koestler, Houseman, Marsit, & Kelsey, 2013). There is, thus, a need for studies that compare DNAm profiles between women who deliver preterm and those who deliver full term to determine if and how variation in methylation may contribute to timing of birth. The difference in direction of change identified in DNAm between term and PTB is also important to consider, as increasing or decreasing methylation at various genes may influence gene expression leading to PTB. Zhang et al. (2011) found that global DNAm differs by race/ethnicity. Indeed, some authors have suggested that observed racial differentials in DNAm may contribute to higher rates of adverse outcomes, including PTB, among African American women (Burris & Collins, 2010). These findings suggest that epigenomic paths of inquiry may be relevant to the study of the disproportionally high rate of PTB among African Americans. To date, however, African Americans have been underrepresented in genomic studies of PTB, and little is known about the epigenomic profiles that may contribute to this adverse outcome.
Methods
We conducted a systematic review of the literature to critically examine research on PTB that utilized DNAm. We searched the Medline (via PubMed) and CINAHL databases to find articles that met the inclusion criteria of describing data-based studies that included African American/Black populations and used DNAm technologies to examine PTB. Search terms included the MeSH terms DNAm AND PTB and DNAm AND gestational age. We identified 279 English-language articles published through January 29, 2016, in our initial database search and two additional studies through citations in the retrieved articles (Figure 2). After removing duplicates, we excluded studies that either did not specify the race of participants or did not include African Americans (n = 55), used animal models (n = 61), did not present results by PTB status, or adjusted for PTB in the analysis (n = 149). Two authors reviewed the abstracts to ensure inclusion of all eligible studies, and two authors also reviewed each of the full-text articles that met the study criteria.
Figure 2.
Preferred reporting items for systematic reviews and meta-analyses flow diagram. PTB = preterm birth.
Results
We included 10 studies in the final review (see Table 1). All of the studies included African Americans in their samples, and two had study populations comprising only African Americans (Parets et al., 2013; Parets, Conneely, Kilaru, Menon, & Smith, 2015). Only three of the studies had longitudinal or cohort designs (Burris et al., 2012; Liu et al., 2013; Vidal et al., 2013). Sample sizes in the studies ranged from 40 to 1,160 participants.
Table 1.
Selected Features of Studies on Preterm Birth (PTB) and DNA Methylation (DNAm) Whose Samples Included African Americans.
| Authors | Population | Sample Size | PTB Measurement | Tissue Source | Epityping Method (Candidate Gene/Targeted, EWAS, LINE-1) | Findings |
|---|---|---|---|---|---|---|
| Behnia et al. (2015) | Nashville, TN, Nashville Birth Cohort Biobank | N = 70 (13% AA) | 22–36 Weeks’ gestation with regular uterine contractions and cervical changes | Placental fetal membranes (amniochorion) | Candidate gene/targeted | Higher methylation status of CpG islands within the OXTR promoter in infants born preterm |
| Burris et al. (2012) | Boston, MA, Project Viva | N = 1,160 (10% AA) | <37 Weeks’ gestation; calculated from birth date and LMP gestational age | Maternal peripheral blood (1st and 2nd trimesters) and umbilical cord (at birth; LINE-1 DNAm) | LINE-1 | PTB is associated with lower LINE-1 in cord blood |
| Filiberto et al. (2011) | Providence, RI | N = 480 (10% AA) | Small for gestational age (<10th percentile for weight) and large for gestational age | Maternal placenta | Candidate gene/targeted | Significant relationship between differential (increased) methylation and large for gestational age |
| Lee et al. (2012) | Baltimore, MD, Tracking Health Related to Environmental Exposures | N = 141 (64% AA) | <37 weeks, abstraction for best obstetric estimate | Umbilical cord blood | EWAS | Three differentially methylated regions (NFIX, RAPGEF2, and MSRB3) with inverse correlation between DNAm and gene expression levels (gradual changes in DNAm associated with gestational age) |
| Liu et al. (2013) | Durham, NC, Newborn Epigenetics STudy | N = 73 (54% AA) | Preterm <37 weeks | Umbilical cord blood | Candidate gene/targeted | DNAm differed for infants with infections; no differences found between types of PTB |
| Michels, Harris, and Barault (2011) | Boston, MA, Epigenetic Birth Cohort | N = 319 dyads (12% AA) | Preterm <37 weeks, term ≥37 weeks | Umbilical cord blood and fetal placenta | LINE-1 | PTB associated with lower LINE-1 methylation compared to term |
| Parets et al. (2013) | Nashville, TN, Nashville Birth Cohort Biobank | PTB n = 22, term n = 28 (100% AA) | Preterm: 24–34 weeks’ gestation, term controls: >39 weeks’ gestation | Umbilical cord blood—fetal leukocytes | Candidate gene/targeted | 29 CpG sites associated with PTB |
| Parets, Conneely, Kilaru, Menon, & Smith (2015) | Nashville, TN, Nashville Birth Cohort Biobank | PTB n = 16, term n = 24 (100% AA) | Preterm: 24–34 weeks’ gestation, term controls: >39 weeks’ gestation | Maternal leukocytes (peripheral blood) | EWAS | No CpG sites associated with PTB, but DNAm between members of maternal–fetal pairs correlated |
| Schroeder et al. (2011) | Atlanta, GA, Women’s Mental Health Program Nashville, TN, Conditions Affecting Neurocognitive Development and Learning in Early Childhood | Cohort 1: n = 259 (10% AA); cohort 2: n = 194 (57% AA) | Preterm: <37 weeks’ gestation, determined via LMP and OB estimate | Umbilical cord blood | Candidate gene/targeted | CpG sites (41 first cohort, 26 replicated) on several genes (39 first cohort, 25 replicated) associated with gestational age, including AVP, OXT, CRHBP, and ESR1 |
| Vidal et al. (2013) | North Carolina, NEST Cohort | N = 397 (41% AA) | Low birth weight (<2,500 g), with preterm <37 weeks | Umbilical cord blood | Candidate gene/targeted | Methylation at five differentially methylated regions (DMRs) associated with maternal antibiotic use. Increased DNAm at PLAGL1 DMR was associated with higher birth weight |
Note. AA = African American; EWAS = epigenome-wide association study; LMP = last menstrual period; OB = obstetrician.
Outcome measures differed among these studies. While most studies used PTB as their primary outcome, definitions varied widely from 22–36 weeks’ gestation (Behnia et al., 2015) to 24–34 weeks’ gestation (Parets et al., 2013, 2015) and the clinical definition of <37 weeks’ gestation (Burris et al., 2012; Lee et al., 2012; Liu et al., 2013; Michels, Harris, & Barault, 2011; Schroeder et al., 2011; Vidal et al., 2013). Other researchers included outcomes of growth (small or large for gestational age; Filiberto et al., 2011) or birth weight (Vidal et al., 2013) along with or instead of PTB. Type of PTB (spontaneous, indicated, or PPROM) also differed by study. Some studies focused only on spontaneous PTB (Behnia et al., 2015; Parets et al., 2013, 2015), while others drew contrasts between types (Burris et al., 2012; Liu et al., 2013; Schroeder et al., 2011). Others did not specify PTB type (Lee et al., 2012; Michels et al., 2011; Vidal et al., 2013) or, as mentioned above, used fetal growth instead of PTB as the outcome (Filiberto et al., 2011). One study did not have a full-term comparison group (Liu et al., 2013), limiting analyses to preterm infants only, and another included very few cases (Vidal et al., 2013), limiting outcome assessment.
Most studies extracted DNA for DNAm analysis from umbilical cord blood or fetal placental tissue (Behnia et al., 2015; Burris et al., 2012; Lee et al., 2012; Liu et al., 2013; Michels et al., 2011; Parets et al., 2013; Schroeder et al., 2011; Vidal et al., 2013), while a few studies examined maternal tissues (placenta and leukocytes from whole blood; Burris et al., 2012; Filiberto et al., 2011; Parets et al., 2015). In only one study did investigators repeat tissue collection for DNAm, with samples taken during the first and second trimesters of pregnancy as well as at birth (Burris et al., 2012).
Methodology for methylation analysis also varied among studies. Some researchers used well-established techniques such as bisulfite polymerase chain reaction (PCR) and pyrosequencing (Burris et al., 2012; Filiberto et al., 2011; Liu et al., 2013; Vidal et al., 2013) and/or 27 K or 450 K BeadChip arrays (Parets et al., 2013, 2015; Schroeder et al., 2011). Many studies used targeted approaches based on specific candidate genes. Two studies (Burris et al., 2012; Michels et al., 2011) examined long interspersed nuclear element 1 (LINE-1), which has been studied as a surrogate measure for genome-wide methylation status (Yang et al., 2004). Some authors did not specify how many CpG sites (regions of DNA) they evaluated or how many they studied in each region (Liu et al., 2013).
Several studies reported a relationship between DNAm and PTB or lower birth weight; investigators observed decreased methylation in infants born preterm (Burris et al., 2012; Lee et al., 2012; Michels et al., 2011; Parets et al., 2013; Schroeder et al., 2011) or with lower birth weight (Vidal et al., 2013) or restricted growth (Filiberto et al., 2011). In one study, researchers found higher methylation in CpG islands within a specific gene (OXTR promoter) in preterm infants as compared to term infants (Behnia et al., 2015). In another study including only preterm infants with infections, investigators found differences in DNAm between types of infections (chorioamnionitis and funisitis), but the study was limited by not having a full-term comparison group (Liu et al., 2013). Finally, in one study of term (n = 16) and preterm (n = 24) African American infants, researchers found no CpG sites associated with PTB in an epigenome-wide investigation; however, this finding may have been due to the small sample size as well as the fact that the researchers corrected for multiple cell types in their analysis while others did not (Parets et al., 2015). The authors did find, however, that DNAm was significantly correlated between mothers and their infants.
Discussion
In this review of the existing literature on DNAm and PTB in African American women, we found that DNAm was associated with PTB in most studies. There is, however, a paucity of research in this area, and the reviewed studies were limited by small sample sizes, repeated use of samples from the same biobanks, largely cross-sectional study designs, inconsistent definitions of PTB, and limited inclusion of African American populations. In addition, methodology and technology used to measure DNAm differed among studies, a reflection of the speed at which the field of epigenomics is evolving.
The findings of the reviewed studies are consistent with some (Burris et al., 2014; Cruickshank et al., 2013; Kim et al., 2013; Maccani et al., 2013), but not all (Burris et al., 2013; Mitsuya, Singh, Sooranna, Johnson, & Myatt, 2014; Tobi et al., 2011), prior studies on DNAm and PTB among samples that did not include African Americans (or those that did not specify race). In one study, researchers evaluated DNAm across the genome in a cross-sectional sample (N = 24) of term versus preterm amnion from women in Iowa and Argentina (Kim et al., 2013). They reported significant differences in DNAm at 65 CpG sites among term-with-labor, term-without-labor, and preterm groups and validated these differences using both methylation-specific PCR and bisulfite sequencing. In another study examining genome-wide DNAm in a cohort of Caucasian and non-Caucasian women (with race/ethnicity not specified) in Rhode Island (N = 206), investigators found that hypermethylation of RUNX3 CpG sites was associated with decreased gestational age (Maccani et al., 2013). Cruickshank et al. (2013) used a genome-wide approach for a longitudinal study in Australia of preterm (<31 weeks’ gestation, n = 12) and term (n = 12) infants at birth and 18 years of age. They reported a discordance of DNAm between groups, indicating a long-term epigenetic legacy of PTB. Finally, Burris and colleagues (2014) employed a targeted approach in a Mexico City cohort (N = 80) and observed higher DNAm of long interspersed nuclear element-1 Homo sapiens-specific (LINE-1-HS) associated with shorter gestations. In contrast, a number of studies (Burris et al., 2013; Mitsuya et al., 2014; Tobi et al., 2011) of non-African American samples found no association between DNAm and PTB. These studies utilized a targeted candidate gene approach and varied in study design (cohort, case-control, and cross-sectional studies, respectively) and in the genes studied. In one, researchers examined infants categorized as small for gestational age rather than those born preterm specifically (Tobi et al., 2011). Interpretation of another was limited by the fact that the authors did not provide any information on race at all (Mitsuya et al., 2014).
Few of the studies reviewed here included epigenome-wide analyses, although this approach may be the most useful for complex etiologic outcomes such as PTB (Parets et al., 2015). The X chromosome, which plays an important role in many biological processes, should also be included in future epigenome-wide analyses of PTB (Klebaner et al., 2016). Researchers have commonly used markers of methylation such as LINE-1, which indicate the presence of inflammation. This global proxy of methylation has also been used in PTB studies and may be useful in investigating inflammatory pathways. However, genome-wide assessment of DNAm evaluating the direction of DNAm change may be most informative for identifying additional factors that contribute to risk of PTB. There is also a need to clearly identify genes associated with PTB, a process that can only be addressed via epigenome-wide studies.
Despite the fact that the studies reviewed here all included African Americans, they comprised less than half of the study sample in 50% of the studies. Although many current genetic studies include participants of varying ethnic and racial backgrounds in proportions that are representative of the whole U.S. population, this sampling technique often results in inadequate power to observe differences where they exist. Further, as the majority of African American women deliver full-term babies, there is an urgent need to study disparities within this high-risk group to distinguish what risk or protective factors exist. Distinct pathways to PTB may exist within the African American population, and whether they are socially induced (or mediated via resilience) or epigenome associated should be carefully examined in future studies.
Future genetic and epigenetic studies on DNAm and PTB should also address the concern that Yudell, Roberts, DeSalle, and Tishkoff (2016) raised regarding the inadequate classification of study participants by race (as defined by the U.S. government) due to the practice of relying on self-identification as African American or as having geographic ancestry. By utilizing ancestry-informative markers and epigenome-wide analysis, researchers could more accurately identify unique risk factors or epigenomic pathways for PTB among African Americans. Indeed, the concept of race in the United States may be a more useful proxy for negative social exposures that impact health than as a biologic precursor of disease. In addition, investigators should make an effort to improve representation of populations with diverse ancestry in genomic studies in order to address perinatal health disparities.
Another factor that may be impacting the number of studies looking specifically at race and associations between epigenomic changes and PTB is the inadequate number of nurse scientists with diverse ethnic and racial backgrounds who are ideally placed to conduct this research. According to the American Association of Colleges of Nursing (2015), only 13% of nursing faculty nationwide identify as African American, Latino, or another underrepresented minority group, while these groups made up 37% of the U.S. population in 2012 and are expected to grow to over 50% by 2043 (U.S. Census Bureau, 2014). The exclusion of minority communities from genomic studies may be due, in part, to researchers’ discomfort in dealing with issues of race and other barriers to recruiting minority populations (Knerr, Wayman, & Bonham, 2011). These barriers could result in genomic studies with insufficient samples among minority groups and, ultimately, the discarding of those groups from analyses. Advocacy efforts to improve recruitment and retention of diverse nursing student bodies and faculty is therefore an important part of meeting this challenge.
Limitations
One limitation of the present review is the potential bias resulting from the fact that three of the studies used tissues from the same biobank (Behnia et al., 2015; Parets et al., 2013, 2015). This reliance on a limited number of samples may be due to the cost and complexity of tissue collection and analysis as well as the relative lack of research labs focusing on this area. Researchers are currently engaged in larger studies with longitudinal study designs and diverse samples that hold promise for increasing our understanding of maternal–fetal epigenomic interactions that lead to PTB. Ongoing studies may also provide opportunities to improve our understanding of how DNAm is associated with PTB specifically in African American women and children (Crusto, Barcelona de Mendoza, Connell, Sun, & Taylor, 2016; Taylor, Wright, Crusto, & Sun, 2016). Another potential limitation is the inclusion of studies that used growth and gestational age as their primary outcome rather than PTB specifically. We included these studies because there is overlap in these adverse birth outcomes. All low birth weight babies are preterm, small for gestational age, or both (M. S. Kramer, 2013); therefore, biologic plausibility for shared pathways to these adverse outcomes exists. Finally, although there are other epigenetic factors/markers underlying epigenetic variation related to PTB, we focused on DNAm for the purposes of this review.
Recommendations for Future Research
Considering the limited number of existing epigenomic studies on DNAm and PTB, researchers should take advantage of data sharing and use of consortia to offset costs. Authors of future studies should also publish a detailed description of their methodology as well as a rationale for their chosen definition of PTB in order to facilitate replication of findings. It is imperative to identify and promote use of a clinically relevant definition of PTB in future studies, established via the gold standard for measurement of gestational age (i.e., ultrasound dating in the first half of pregnancy). Rationale for tissue type used for analysis should also be considered, as results may vary across tissues or cell composition ratios. DNAm is tissue-specific, and most of the studies reviewed used cord blood samples. Studies that examine overlap between maternal and fetal tissues could be crucial for determining whether maternal peripheral blood could be used as a marker for PTB, which would have great significance for clinical practice. Also, as the basic mechanism that triggers PTB is still unknown and the maternal and fetal environments are interdependent, studies that explore both maternal and fetal/infant DNAm are warranted. Finally, few epigenomic studies to date have distinguished between spontaneous and medically indicated PTB. Future research should consider PTB subtype.
Previous reviews on DNAm and PTB have presented considerable evidence for an association between altered DNAm and this adverse outcome, yet the samples covered by the reviews have been largely restricted to Northern European or U.S.-based Caucasian populations (Parets et al., 2014). Additionally, there has been wide variation in the approach to genomic analysis and research questions across studies, which limits generalizability of findings. Future research should be longitudinal in design, examining DNAm over time, including baseline (prepregnancy), during pregnancy (minimally at least once per trimester), and postpartum measurements. Such a design would allow researchers to investigate DNAm as a potential marker for PTB. As mentioned above, most studies in the present review used fetal-derived tissue. However, it remains unknown whether maternal factors, fetal factors, or a combination of the two drive spontaneous PTB. Examination of maternal samples longitudinally across pregnancy would help identify maternal factors that influence PTB, while long-term study of children born preterm may further inform our knowledge of life-course consequences (such as chronic disease). Finally and importantly, robust participation of African American mother–child dyads in future studies is also important so that studies will be sufficiently powered to examine the associations between epigenomic changes and PTB in this high-risk group.
Conclusion
Exploration of epigenomic changes association with PTB has the potential to provide important information that would allow clinicians to identify African American women who are at increased risk for PTB and to develop precise, individualized risk-specific interventions. Once research has revealed the mechanisms involved in PTB, including epigenomic pathways, future studies can focus on the reduction of health disparities in pregnancy outcomes. The ultimate goal of such research would be to provide the information necessary for nurses in the clinical setting to better care for vulnerable members of our communities and to prevent adverse pregnancy and birth outcomes.
Footnotes
Author Contributions: V. Barcelona de Mendoza contributed to conception and design, acquisition, analysis, and interpretation; drafted the manuscript; critically revised the manuscript; and gave final approval and agrees to be accountable for all aspects of work ensuring integrity and accuracy. M. Wright contributed to conception and design, acquisition, analysis, and interpretation; critically revised the manuscript; and gave final approval agrees to be accountable for all aspects of work ensuring integrity and accuracy. C. Agaba contributed to acquisition, analysis, and interpretation; drafted the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. L. Prescott contributed to acquisition, drafted the manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy. A. Desir contributed to acquisition, drafted the manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy. C. Crusto contributed to acquisition, critically revised the manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Y. Sun contributed to design and analysis, critically revised the manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy. J. Taylor contributed to conception, design, and analysis; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the National Institutes of Health and National Institute for Nursing Research (R01NR013520).
References
- American Association of Colleges of Nursing. (2015). Fact sheet: Enhancing diversity in the nursing workforce. Retrieved from www.aacn.nche.edu/media-relations/diversityFS.pdf
- Ananth C. V., Vintzileos A. M. (2006). Epidemiology of preterm birth and its clinical subtypes. Journal of Maternal-Fetal & Neonatal Medicine, 19, 773–782. doi:10.1080/14767050600965882 [DOI] [PubMed] [Google Scholar]
- Behnia F., Parets S. E., Kechichian T., Yin H., Dutta E. H., Saade G. R.…Menon R. (2015). Fetal DNA methylation of autism spectrum disorders candidate genes: Association with spontaneous preterm birth. American Journal of Obstetrics and Gynecology, 212, 533.e1–533.e9. doi:10.1016/j.ajog.2015.02.011 [DOI] [PubMed] [Google Scholar]
- Burris H. H., Baccarelli A. A., Motta V., Byun H. M., Just A. C., Mercado-Garcia A.…Wright R. O. (2014). Association between length of gestation and cervical DNA methylation of PTGER2 and LINE 1-HS. Epigenetics, 9, 1083–1091. doi:10.4161/epi.29170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris H. H., Braun J. M., Byun H. M., Tarantini L., Mercado A., Wright R. J.…Tellez-Rojo M. M. (2013). Association between birth weight and DNA methylation of IGF2, glucocorticoid receptor and repetitive elements LINE-1 and Alu. Epigenomics, 5, 271–281. doi:10.2217/epi.13.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris H. H., Collins J. W., Jr (2010). Race and preterm birth—The case for epigenetic inquiry. Ethnicity & Disease, 20, 296–299. [PubMed] [Google Scholar]
- Burris H. H., Rifas-Shiman S. L., Baccarelli A., Tarantini L., Boeke C. E., Kleinman K.…Gillman M. W. (2012). Associations of LINE-1 DNA methylation with preterm birth in a prospective cohort study. Journal of Developmental Origins of Health and Disease, 3, 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colen C. G., Geronimus A. T., Bound J., James S. A. (2006). Maternal upward socioeconomic mobility and black-white disparities in infant birthweight. American Journal of Public Health, 96, 2032–2039. doi:10.1080/14767050600965882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin E. J., Guo Y., Pajer K., Lowe N., McCarthy D., Schmiege S.…Stafford B. (2013). Immune dysregulation and glucocorticoid resistance in minority and low income pregnant women. Psychoneuroendocrinology, 38, 1786–1796. doi:10.1016/j.psyneuen.2013.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruickshank M. N., Oshlack A., Theda C., Davis P. G., Martino D., Sheehan P.…Craig J. M. (2013). Analysis of epigenetic changes in survivors of preterm birth reveals the effect of gestational age and evidence for a long term legacy. Genome Medicine, 5, 96 doi:10.1186/gm500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crusto C. A., Barcelona de Mendoza V., Connell C., Sun Y. V., Taylor J. Y. (2016). The Intergenerational Impact of Genetic and Psychological Factors on Blood Pressure Study (InterGEN): Design and methods for recruitment and psychological measures. Nursing Research, 65, 331–338. doi:10.1097/NNR.0000000000000163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSisto C. L., Kim S. Y., Sharma A. J. (2014). Prevalence estimates of gestational diabetes mellitus in the United States, pregnancy risk assessment monitoring system (PRAMS), 2007-2010. Preventing Chronic Disease, 11, E104 doi:10.5888/pcd11.130415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop A. L., Kramer M. R., Hogue C. J., Menon R., Ramakrishan U. (2011). Racial disparities in preterm birth: An overview of the potential role of nutrient deficiencies. Acta Obstetricia Et Gynecologica Scandinavica, 90, 1332–1341. doi:10.1111/j.1600-0412.2011.01274.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filiberto A. C., Maccani M. A., Koestler D., Wilhelm-Benartzi C., Avissar-Whiting M., Banister C. E.…Marsit C. J. (2011). Birthweight is associated with DNA promoter methylation of the glucocorticoid receptor in human placenta. Epigenetics, 6, 566–572. doi:10.4161/epi.6.5.15236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh G., Grewal J., Mannisto T., Mendola P., Chen Z., Xie Y., Laughon S. K. (2014). Racial/ethnic differences in pregnancy-related hypertensive disease in nulliparous women. Ethnicity & Disease, 24, 283–289. [PMC free article] [PubMed] [Google Scholar]
- Goldenberg R. L., Culhane J. F., Iams J. D., Romero R. (2008). Epidemiology and causes of preterm birth. Lancet, 371, 75–84. doi:10.1016/S0140-6736(08)60074-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez L. M., Sammel M. D., Appleby D. H., Elovitz M. A., Baldwin D. A., Jeffcoat M. K.…Parry S. (2010). Evidence of a gene–environment interaction that predisposes to spontaneous preterm birth: A role for asymptomatic bacterial vaginosis and DNA variants in genes that control the inflammatory response. American Journal of Obstetrics and Gynecology, 202, 386.e1–386.e6. doi:10.1016/j.ajog.2010.01.042 [DOI] [PubMed] [Google Scholar]
- Harrison P. A., Sidebottom A. C. (2009). Alcohol and drug use before and during pregnancy: An examination of use patterns and predictors of cessation. Maternal and Child Health Journal, 13, 386–394. doi:10.1007/s10995-008-0355-z [DOI] [PubMed] [Google Scholar]
- Hogue C. J., Menon R., Dunlop A. L., Kramer M. R. (2011). Racial disparities in preterm birth rates and short inter-pregnancy interval: An overview. Acta Obstetricia Et Gynecologica Scandinavica, 90, 1317–1324. doi:10.1111/j.1600-0412.2011.01081.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal A., Homa D. M., O’Connor E., Babb S. D., Caraballo R. S., Singh T.…King B. A. (2015). Current cigarette smoking among adults—United States, 2005–2014. Morbidity and Mortality Weekly Report, 64, 1233–1240. doi:10.15585/mmwr.mm6444a2 [DOI] [PubMed] [Google Scholar]
- Kim J., Pitlick M. M., Christine P. J., Schaefer A. R., Saleme C., Comas B.…Murray J. C. (2013). Genome-wide analysis of DNA methylation in human amnion. Scientific World Journal, 2013, 678156 doi:10.1155/2013/678156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebaner D., Huang Y., Hui Q., Taylor J. Y., Goldberg J., Vaccarino V., Sun Y. V. (2016). X chromosome-wide analysis identifies DNA methylation sites influenced by cigarette smoking. Clinical Epigenetics, 8, 20–016-0189–2 eCollection 2016 doi:10.1186/s13148-016-0189-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knerr S., Wayman D., Bonham V. L. (2011). Inclusion of racial and ethnic minorities in genetic research: Advance the spirit by changing the rules? Journal of Law, Medicine & Ethics, 39, 502–512. doi:10.1111/j.1748-720X.2011.00617.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer M. R., Hogue C. R. (2009). What causes racial disparities in very preterm birth? A biosocial perspective. Epidemiologic Reviews, 31, 84–98. doi:10.1093/ajerev/mxp003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer M. S. (2013). The epidemiology of low birthweight. Nestle Nutrition Institute Workshop Series, 74, 1–10. doi:10.1159/000348382 [DOI] [PubMed] [Google Scholar]
- Lee H., Jaffe A. E., Feinberg J. I., Tryggvadottir R., Brown S., Montano C.…Fallin M. D. (2012). DNA methylation shows genome-wide association of NFIX, RAPGEF2 and MSRB3 with gestational age at birth. International Journal of Epidemiology, 41, 188–199. doi:10.1093/ije/dyr237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Hoyo C., Murphy S., Huang Z., Overcash F., Thompson J.…Murtha A. P. (2013). DNA methylation at imprint regulatory regions in preterm birth and infection. American Journal of Obstetrics and Gynecology, 208, 395.e1–395.e7. doi:10.1016/j.ajog.2013.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccani J. Z., Koestler D. C., Houseman E. A., Marsit C. J., Kelsey K. T. (2013). Placental DNA methylation alterations associated with maternal tobacco smoking at the RUNX3 gene are also associated with gestational age. Epigenomics, 5, 619–630. doi:10.2217/epi.13.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. A., Hamilton B. E., Osterman M. J., Curtin S. C., Matthews T. J. (2015). Births: Final data for 2013. National Vital Statistics Reports: From the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System, 64, 1–65. [PubMed] [Google Scholar]
- May A. L., Freedman D., Sherry B., Blanck H. M. (2013). Obesity—United States, 1999–2010. Morbidity and Mortality Weekly Report, 62, 120–128. [PubMed] [Google Scholar]
- Meis P. J., Goldenberg R. L., Mercer B., Moawad A., Das A., McNellis D.…Andrews W. W. (1995). The preterm prediction study: Significance of vaginal infections. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. American Journal of Obstetrics and Gynecology, 173, 1231–1235. [DOI] [PubMed] [Google Scholar]
- Mercer B. M., Goldenberg R. L., Moawad A. H., Meis P. J., Iams J. D., Das A. F.…McNellis D. (1999). The preterm prediction study: Effect of gestational age and cause of preterm birth on subsequent obstetric outcome. National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. American Journal of Obstetrics and Gynecology, 181, 1216–1221. [DOI] [PubMed] [Google Scholar]
- Michels K. B., Harris H. R., Barault L. (2011). Birthweight, maternal weight trajectories and global DNA methylation of LINE-1 repetitive elements. PLoS ONE, 6, e25254 doi:10.1371/journal.pone.0025254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuya K., Singh N., Sooranna S. R., Johnson M. R., Myatt L. (2014). Epigenetics of human myometrium: DNA methylation of genes encoding contraction-associated proteins in term and preterm labor. Biology of Reproduction, 90, 98 doi:10.1095/biolreprod.113.113209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson D. M., Severson E. M., Verstraeten B. S., Ng J. W., McCreary J. K., Metz G. A. (2015). Allostatic load and preterm birth. International Journal of Molecular Sciences, 16, 29856–29874. doi:10.3390/ijms161226209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parets S. E., Bedient C. E., Menon R., Smith A. K. (2014). Preterm birth and its long-term effects: Methylation to mechanisms. Biology, 3(3), 498–513. doi:10.3390/biology3030498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parets S. E., Conneely K. N., Kilaru V., Fortunato S. J., Syed T. A., Saade G.…Menon R. (2013). Fetal DNA methylation associates with early spontaneous preterm birth and gestational age. PLoS ONE, 8, e67489 doi:10.1371/journal.pone.0067489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parets S. E., Conneely K. N., Kilaru V., Menon R., Smith A. K. (2015). DNA methylation provides insight into intergenerational risk for preterm birth in African Americans. Epigenetics, 10, 784–792. doi:10.1080/15592294.2015.1062964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt M. J. (2014). Outcomes in preterm infants. Public Health, 128, 399–403. doi:10.1016/j.puhe.2014.03.010 [DOI] [PubMed] [Google Scholar]
- Sanu O., Lamont R. F. (2011). Periodontal disease and bacterial vaginosis as genetic and environmental markers for the risk of spontaneous preterm labor and preterm birth. Journal of Maternal-Fetal & Neonatal Medicine, 24, 1476–1485. doi:10.3109/14767058.2010.545930 [DOI] [PubMed] [Google Scholar]
- Schroeder J. W., Conneely K. N., Cubells J. C., Kilaru V., Newport D. J., Knight B. T.…Smith A. K. (2011). Neonatal DNA methylation patterns associate with gestational age. Epigenetics, 6, 1498–1504. doi:10.4161/epi.6.12.18296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. Y., Schwander K., Kardia S. L., Arnett D., Liang J., Hunt S. C.…Sun Y. V. (2016). A genome-wide study of blood pressure in African Americans accounting for gene–smoking interaction. Scientific Reports, 6, 18812 doi:10.1038/srep18812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. Y., Wright M. L., Crusto C. A., Sun Y. V. (2016). The Intergenerational Impact of Genetic and Psychological Factors on Blood Pressure Study (InterGEN): Design and methods for complex DNA analysis. Biological Research for Nursing. Advance online publication. doi:10.1177/1099800416645399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobi E. W., Heijmans B. T., Kremer D., Putter H., Delemarre-van de Waal H. A., Finken M. J.…Slagboom P. E. (2011). DNA methylation of IGF2, GNASAS, INSIGF and LEP and being born small for gestational age. Epigenetics, 6, 171–176. doi:10.4161/epi.6.2.13516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Census Bureau. (2014). U.S. Census Bureau projections show a slower growing, older, more diverse nation a half century from now. Retrieved from https://www.census.gov/newsroom/releases/archives/population/cb12-243.html
- Vidal A. C., Murphy S. K., Murtha A. P., Schildkraut J. M., Soubry A., Huang Z.…Hoyo C. (2013). Associations between antibiotic exposure during pregnancy, birth weight and aberrant methylation at imprinted genes among offspring. International Journal of Obesity, 37, 907–913. doi:10.1038/ijo.2013.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb D. A., Mathew L., Culhane J. F. (2014). Lessons learned from the Philadelphia Collaborative Preterm Prevention Project: The prevalence of risk factors and program participation rates among women in the intervention group. BMC Pregnancy and Childbirth, 14, 368–014-0368–0 doi:10.1186/s12884-014-0368-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff G. L., Kodell R. L., Moore S. R., Cooney C. A. (1998). Maternal epigenetics and methyl supplements affect agouti gene expression in avy/a mice. FASEB Journal, 12, 949–957. [PubMed] [Google Scholar]
- Yang A. S., Estecio M. R., Doshi K., Kondo Y., Tajara E. H., Issa J. P. (2004). A simple method for estimating global DNA methylation using bisulfite PCR of repetitive DNA elements. Nucleic Acids Research, 32, e38 doi:10.1093/nar/gnh032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanit K. E., Snowden J. M., Cheng Y. W., Caughey A. B. (2012). The impact of chronic hypertension and pregestational diabetes on pregnancy outcomes. American Journal of Obstetrics and Gynecology, 207, 333.e1–333.e6. doi:10.1016/j.ajog.2012.06.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- York T. P., Eaves L. J., Neale M. C., Strauss J. F., 3rd (2014). The contribution of genetic and environmental factors to the duration of pregnancy. American Journal of Obstetrics and Gynecology, 210, 398–405. doi:10.1016/j.ajog.2013.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudell M., Roberts D., DeSalle R., Tishkoff S. (2016). Taking race out of human genetics. Science, 351, 564–565. doi:10.1126/science.aac4951 [DOI] [PubMed] [Google Scholar]
- Zhang F. F., Cardarelli R., Carroll J., Fulda K. G., Kaur M., Gonzalez K.…Morabia A. (2011). Significant differences in global genomic DNA methylation by gender and race/ethnicity in peripheral blood. Epigenetics, 6, 623–629. [DOI] [PMC free article] [PubMed] [Google Scholar]