Abstract
Oral fluid (OF) is an important matrix for monitoring drugs. Smoking cannabis is common, but vaporization and edible consumption also are popular. OF pharmacokinetics are available for controlled smoked cannabis, but few data exist for vaporized and oral routes. Frequent and occasional cannabis smokers were recruited as participants for 4 dosing sessions including one active (6.9% ∆9-tetrahydrocannabinol, THC) or placebo cannabis-containing brownie followed by one active or placebo cigarette or one active or placebo vaporized cannabis dose. Only one active dose was administered per session. OF was collected before and up to 54 (occasional) or 72 (frequent) h after dosing from cannabis smokers. THC, 11-hydroxy-THC (11-OH-THC), 11-nor-9-carboxy-THC (THCCOOH), tetrahydrocannabivarin (THCV), cannabidiol (CBD), and cannabigerol (CBG) were quantified by liquid chromatography-tandem mass spectrometry. OF cannabinoid Cmax occurred during or immediately after cannabis consumption due to oral mucosa contamination. Significantly greater THC Cmax and significantly later THCV, CBD, and CBG tlast were observed after smoked and vaporized cannabis compared to oral cannabis in frequent smokers only. No significant differences in THC, 11-OH-THC, THCV, CBD, or CBG tmax between routes were observed for either group. For occasional smokers, more 11-OH-THC and THCCOOH-positive specimens were observed after oral dosing than after inhaled routes, increasing % positive cannabinoid results and widening metabolite detection windows after oral cannabis consumption. Utilizing 0.3µg/L THCV and CBG cutoffs resulted in detection windows indicative of recent cannabis intake. OF pharmacokinetics after high potency CBD cannabis are not yet available precluding its use currently as a marker of recent use.
Keywords: Cannabinoids, Oral fluid, Smoking, Vaporizer, Edibles
INTRODUCTION
Cannabis remains the most commonly used illicit drug worldwide [1]. The main psychoactive compound in cannabis, ∆9-tetrahydrocannabinol (THC), was detected in 12.6% of U.S. weekend nighttime drivers’ blood or oral fluid (OF) samples[2]; increased crash risk is associated with cannabis intake[3–6]. OF is an important matrix for detecting drugs of abuse, particularly in driving under the influence of drugs (DUID) testing programs[7–13]. OF collection is advantageous over urine and blood, as it is collected under direct observation, deterring adulteration, without requiring specialized collection by medical personnel. Inhalation via smoking is the most common cannabis administration route, although inhalation via vaporization and oral consumption via edibles frequently occurs[14]. To date, data are available from a few OF cannabinoid disposition studies following controlled smoked cannabis[15–20]; however, fewer data exist after vaporized[13] and edible THC[17, 21]. As inhalation via vaporization and oral cannabis are becoming increasingly popular routes of intake[14], controlled administration studies examining these routes are crucial to understanding cannabinoid OF pharmacokinetics. Further, ideally these routes are studied in a within-subject, placebo-controlled design to best compare cannabinoid pharmacokinetics.[15–20][13][17, 21]
THC is metabolized to the active metabolite 11-hydroxy-THC (11-OH-THC), and to the inactive metabolite 11-nor-9-carboxy-THC (THCCOOH). Concentrations for THC alone[17] and with THCCOOH[21] were recently described in OF following an edible cannabis brownie administration. High THC OF concentrations primarily result from oral mucosa contamination during smoking or vaporization, with minor contribution from THC that partitions from blood into OF, especially during and shortly after intake. OF THCCOOH concentrations vary considerably between occasional and frequent cannabis smokers[13, 15–16]. Minor cannabinoids present in the cannabis plant include cannabinol (CBN), cannabidiol (CBD), cannabigerol (CBG), and ∆9-tetrahydrocannabivarin (THCV). These minor cannabinoids are possible markers of recent cannabis intake; however, limited CBN and CBD OF concentration profiles are available after controlled smoked[15] and vaporized[13] cannabis administration. There are no CBN or CBD OF data after oral cannabis administration. Additionally, to our knowledge, OF CBG and THCV disposition were not yet investigated.
With the increase in OF drug testing and increasing knowledge of OF drug disposition, cutoffs and testing criteria need to be established for clinical and forensic drug testing programs. For DUID, the European Driving under the Influence of Drugs, Alcohol, and Medicines (DRUID) project implemented a THC ≥1µg/L analytical cutoff in OF[22]. For workplace testing, the Substance Abuse and Mental Health Services Administration (SAMHSA) proposed a THC ≥2µg/L confirmatory cutoff in OF[23]. However, these cutoffs need to be fully evaluated following controlled cannabis administration via routes other than inhalation via smoking, (i.e. inhalation via vaporization or oral).
In order to fully characterize cannabinoid disposition in OF, we investigated THC, metabolites, and minor cannabinoids in OF (quantifying THC, 11-OH-THC, THCCOOH, THCV, CBD, and CBG) following controlled smoked, vaporized, and oral brownie cannabis administration in frequent and occasional cannabis smokers. Quantification of a wide spectrum of OF cannabinoids also permits assessment of detection windows for parent cannabinoids and metabolites improving interpretation of cannabinoid OF results.
MATERIALS AND METHODS
Participants
Healthy cannabis users (18–50 years) were recruited for this National Institute on Drug Abuse (NIDA) Intramural Research Program Institutional Review Board-, FDA-, and DEA-approved study. Individuals were recruited by radio and printed advertisements and participant referrals. All participants underwent a comprehensive medical and psychological evaluation. Inclusion criteria were self-reported cannabis intake ≥2x per month but <3x per week (occasional smokers) or ≥5x per week (frequent smokers) over the past three months, and frequent smokers had to produce a positive urine cannabinoid screen. Exclusion criteria included blood pressure >140/90mmHg or heart rate >100bpm at rest; clinically significant electrocardiogram abnormality; inability to discontinue contraindicated medication before study dosing; physical dependence on any drug other than cannabis, caffeine or nicotine; medicinal cannabis use; history of clinically significant medical or neurological illness or adverse event associated with cannabis intoxication; recent blood donation >450mL; pregnant or nursing women; recent interest or participation in a drug abuse treatment program; and any history of food allergy or sensitivity to gluten, dairy, egg, soy and/or chocolate. Pregnancy tests were administered at screening and on each session admission to women with reproductive potential. Individuals provided written, informed consent before admittance to the study.
Study Design
The study was randomized, double blind, and placebo-controlled with a crossover and double-dummy design. Participants entered the secure research unit ~19h before dosing to preclude acute intoxication. Cannabis cigarettes were obtained through the NIDA Drug Supply Program. Active cigarettes (0.734±0.05g) contained 6.9±0.95% (~50.6mg) THC and 0.20±.01% (~1.5mg) CBD. Placebo cigarettes (0.713±0.05g) contained 0.001±0.000% THC and no detectable CBD. Throughout 4 dosing sessions, participants were administered one active or placebo brownie followed by one active or placebo cigarette or one active or placebo vaporized ground cannabis dose (210°C, Volcano® Medic, Storz & Bickel, Tuttlingen, Germany). No more than one active dose was administered per session and the oral dose was followed by either smoking or vaporization in two sessions each. Participants had 10min to consume the oral dose ad libitum followed by 10min to consume the inhaled dose ad libitum. Frequent smokers remained on the unit 72h post-dose and were required to leave the unit for ≥72h before being admitted to their next session. Occasional smokers remained on the unit 54h post-dose but had the option of remaining on the unit for multiple sessions; they were not dosed more frequently than their self-reported intake frequency.
Brownies were prepared with Duncan Hines® Double Fudge brownie mix according to the manufacturer’s instructions and wet batter was portioned into a muffin tray. The contents of either an active or placebo cigarette were ground, placed into a greased foil packet, and baked at 121°C for 30min to ensure decarboxylation of the acid precursor to THC, then mixed into an individual portion of brownie batter. After cooling, brownies were stored at −20°C until the night before dosing, and thawed at 4°C.
OF specimens were collected with the Quantisal™ device (Immunalysis, Pomona, CA), which has a volume adequacy indicator for 1.0±0.1mL OF. OF was collected until the indicator turned blue or 5min elapsed, whichever occurred first due to the tight timeline. Oral intake was prohibited 10min prior to OF collection. OF was collected on admission (−19h), 1.5h before the initiation of smoking/vaporization (baseline, −1.5h) and at 0.17, 1.5, 3.5, 5, 8, 10, 12, 14, 20, 26, 32, 38, 44 and 50h after smoking/vaporization initiation, and at 54h for occasional smokers only, and at 56, 62, 68, and 72h for frequent smokers only.
Oral Fluid Analysis
Specimens were placed in 3mL elution/stabilizing buffer at 4°C for >12h prior to pad removal, followed by transfer of OF/buffer to polypropylene cryotubes and storage at 4°C until analysis. Specimens were analyzed within 1 month of collection based on our previous OF cannabinoid stability study[24–25] and quantified for THC, THCCOOH, 11-OH-THC, THCV, CBD, and CBG by a previously published method[26]. Briefly, samples (1mL elution buffer OF mixture containing 0.25mL OF) were mixed with 0.3mL 1M ammonium acetate buffer (pH 4) and hydrolyzed with 625 Units of β-glucuronidase (BG100®, Kura Biotec, Puerto-Varas, Chile), acidified and extracted with cation exchange solid-phase columns. Cannabinoids were quantified by liquid chromatography tandem mass spectrometry (LC-MS/MS) using atmospheric pressure chemical ionization with 0.2µg/L limits of quantification (except 15ng/L THCCOOH). Inter-assay accuracy and imprecision were 88.1–106% and 5.8–8.2%CV, respectively (n=92). Samples quantifying greater than the upper limits of quantification were re-analyzed after dilution with OF/buffer.
Data Analysis
Differences in demographic data between groups were evaluated with t-tests. Maximum concentration (Cmax), time to Cmax (tmax), and time of last detection (tlast) were calculated with concentrations observed post-dose and differences between administration routes were assessed with SPSS® Statistics 23 for Windows (IMB, Armonk, NY). For analytes detected after all three routes, differences were evaluated by repeated-measures ANOVA with separation of frequent and occasional smokers. If sphericity was violated, the Greenhous-Geisser correction was utilized. If a significant route effect was observed, planned Helmert contrasts were performed first by comparing mean oral dose to the combined mean of inhaled doses then comparing smoking to vaporization. Smoking group differences (frequent vs. occasional) were evaluated with separate repeated-measures ANOVA, with group included as a between-subject factor; if a significant route*group interaction was observed, then group differences after each administration route were evaluated with t-tests. Significance was attributed to a two-tailed p<0.05.
RESULTS
Participants
Demographics for 11 frequent and 9 occasional cannabis smokers are summarized in Table 1. Participants were 19–46 years old, 75% male and 75% African American. Participant K was originally admitted as an occasional smoker, but later reclassified as a frequent smoker based on baseline and post-dose blood cannabinoid pharmacokinetics. Participant H smoking frequency at admission to session 1 was inconsistent with self-reported frequency at screening so his demographic data were not included in summary statistics; smoking frequencies reported on admission to subsequent sessions were consistent with self-reported frequency at screening. Frequent smokers were all African American, began smoking at a significantly younger age, smoked significantly more frequently over the previous 14 days, and smoked significantly more per smoking occasion.
Table 1.
Demographic data and cannabis smoking histories for 11 frequent and 9 occasional smokers.
| Participant | Sex | Age (years) |
Racea | BMI (kg/m2) |
Age at first useb |
Lifetime Years Smokedb |
Cannabis Intake Frequencyb |
Time between last use and admissionc |
Number of days used in last 14c |
Average joint or joint equivalent per smoking occasionc |
|---|---|---|---|---|---|---|---|---|---|---|
| Frequent Smokers | ||||||||||
| A | M | 21 | AA | 26.5 | 16 | 5 | Daily | 17.2 h | 14 | 5 |
| B | M | 22 | AA, U | 31.0 | 15 | 7 | Daily | 19.3 h | 10 | 4 |
| C | M | 19 | AA | 19.8 | 13 | 6 | Daily | 18.7 h | 14 | 4 |
| D | F | 23 | AA | 31.9 | 13 | 10 | Daily | 7.9 h | 14 | 10 |
| E | M | 38 | AA | 32.2 | 12 | 26 | Daily | 2.4 h | 14 | 15 |
| F | F | 29 | AA | 31.0 | 11 | 18 | Daily | 1.9 h | 14 | 20 |
| G | M | 38 | AA | 22.0 | 16 | 22 | Daily | 2.1 h | 14 | 7 |
| H | M | 34 | AA | 23.0 | 14 | 20 | 5x/week | 239.7 hd | 2d | 2d |
| I | M | 21 | AA | 25.0 | 11 | 10 | Daily | 0.7 h | 14 | 5 |
| J | M | 25 | AA | 19.0 | 13 | 12 | 5x/week | 5.8 h | 14 | 2 |
| K | M | 31 | AA | 16.8 | 15 | 16 | 2–3x/weeke | 5.1 he | 4e | 2.5e |
| Mean | 27.4 | f | 25.3 | 13.5f | 13.9 | 8.4 h | 13.6f | 8.0f | ||
| SD | 6.9 | 5.6 | 1.8 | 6.9 | 7.8 h | 1.3 | 6.0 | |||
| Median | 25.3 | 25.0 | 13.0 | 12.3 | 5.8 h | 14.0 | 5.0 | |||
| Occasional Smokers | ||||||||||
| L | M | 24 | AA | 36.3 | 17 | 7 | 2x/month | 1.4 days | 3 | 2 |
| M | M | 21 | AA | 23.0 | 13 | 8 | 2x/week | 0.7 days | 4 | 2 |
| N | M | 25 | W | 24.2 | 21 | 4 | 2x/week | 13.0 days | 1 | 3 |
| O | M | 40 | W | 28.3 | 18 | 22 | 2x/week | 30.7 days | 0 | 2 |
| P | F | 46 | AA | 31.0 | 26 | 20 | 2x/week | 0.4 days | 4 | 4 |
| Q | M | 33 | AA | 30.7 | 16 | 17 | 2x/month | 22.8 days | 0 | 3 |
| R | F | 22 | W | 22.0 | 16 | 6 | 2x/week | 1.7 days | 4 | 1 |
| S | F | 22 | W | 23.0 | 14 | 8 | 2x/week | 1.1 days | 10 | 2 |
| T | M | 31 | W | 21.7 | 22 | 9 | 1–2x/week | 1.8 days | 2 | 2 |
| Mean | 29.4 | f | 26.7 | 18.1f | 11.3 | 8.2 days | 3.1f | 2.3f | ||
| SD | 8.6 | 5.1 | 4.2 | 6.3 | 11.4 days | 3.1 | 0.9 | |||
| Median | 24.9 | 24.2 | 17.0 | 8.5 | 1.7 days | 3.0 | 2.0 | |||
AA, African American; W, white; U, unknown
Data collected during screening
Data collected on admission to Session 1
Self-reported data on admission inconsistent with data received at screening. Data excluded from statistics.
Self-reported data inconsistent with biological sample concentrations. Data excluded from statistics.
Significant difference between groups (p<0.05)
In total, 1102 OF specimens (598 frequent, 504 occasional) were analyzed. OF specimens were not collected from 56–72h for participant K because he was originally recruited as an occasional smoker.
Pharmacokinetic Evaluation
Pharmacokinetic parameters and statistical evaluations for cannabinoids and metabolites are summarized in Table 2 for analytes detected after all three routes. Time course profiles for all six analytes in frequent and occasional smokers following three administration routes are shown in Figures 1 and Figure 2. THCCOOH concentrations represent free and hydrolyzed glucuronide concentrations. THC-glucuronide present in OF would also be hydrolyzed by this method (67% efficiency)[26] but biological concentrations are considered negligible based on previous research[27].
Table 2.
Summary of mean (range), maximum cannabinoid analyte concentrations (Cmax), time to Cmax (tmax), and time of last positive (tlast) after smoked, vaporized and oral cannabis doses (6.9% Δ9-tetrahydrocannabinol, THC; ~50.6mg THC) in 11 frequent and 9 occasional smokers. Repeated-measures analysis of variance F-statistic and p-value for overall route effect and planned Helmert contrasts are reported (contrast 1 evaluated the difference between the variance from oral dosing and the combined variance from smoked and vaporized dosings, contrast 2 evaluated the difference in variances from smoked and vaporized dosing). Route-specific significant difference between smoking groups were calculated and annotated in table. Only participants whose oral fluid was positive for analytes after all cannabis administrations were included in analyses.
| Analyte and Parameter | N | Oral | Smoking | Vaporization | F | p | Contrast 1 | Contrast 2 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | |||||||
| THC | ||||||||||
| Frequent Smokers | ||||||||||
| Cmax, µg/L | 11 | 297 (16.5–938) | 2789 (141–8503) | 1874 (68.6–7373) | 3.60 | 0.046 | 11.63 | 0.007 | 0.648 | 0.440 |
| tmax, h | 11 | 0.41 (0.17–1.5) | 0.17 | 0.17 | 2.22 | 0.167 | - | - | - | - |
| tlast, ha | 10 | 55.0 (20– >72) | 61.0 (32– >72) | 55.4 (26– >72) | 0.80 | 0.463 | - | - | - | - |
| Occasional Smokers | ||||||||||
| Cmax, µg/L | 9 | 202 (65–380) | 837 (81.4–5914) | 545 (7.6–3279) | 1.00 | 0.388 | - | - | - | - |
| tmax, h | 9 | 0.32 (0.17–1.5) | 0.17 | 0.32 (0.17–1.5) | 0.47 | 0.633 | - | - | - | - |
| tlast, h | 9 | 24.7 (10– >54) | 24.7 (8–50) | 18.6 (5– >54) | 2.37 | 0.126 | - | - | - | - |
| 11-OH-THC | ||||||||||
| Frequent Smokers | ||||||||||
| Cmax, µg/L | 1 | 0.23 | 2.2 | 0.27 | - | - | - | - | - | - |
| tmax, h | 1 | 0.17 | 0.17 | 0.17 | - | - | - | - | - | - |
| tlast, h | 1 | 0.17 | 0.17 | 0.17 | - | - | - | - | - | - |
| Occasional Smokers | ||||||||||
| Cmax, µg/L | 0 | - | - | - | - | - | - | - | - | - |
| tmax, h | 0 | - | - | - | - | - | - | - | - | - |
| tlast, h | 0 | - | - | - | - | - | - | - | - | - |
| THCCOOH§ | ||||||||||
| Frequent Smokers | ||||||||||
| Cmax, µg/L | 11 | 0.40 (0.13–1.2) | 0.41 (0.13–1.2) | 0.37 (0.05–0.97) | 0.09 | 0.911 | - | - | - | - |
| tmax, h | 11 | 25.7 (0.17–68) | 14.2 (0.17–68) | 8.4 (0.17–20) | 4.29 | 0.028 | 7.41 | 0.021 | 0.96 | 0.351 |
| tlast, ha | 10 | 71.6 (68– >72) | 66.6 (44– >72) | 66.6 (44– >72) | 1.74 | 0.204 | - | - | - | - |
| Occasional Smokers | ||||||||||
| Cmax, µg/L | 3 | 0.35 (0.02–0.80) | 0.18 (0.12–0.31) | 0.09 (0.02–0.17) | 0.96 | 0.457 | - | - | - | - |
| tmax, h | 3 | 8.5 (3.5–14) | 0.17 | 6.8 (0.17–20) | 0.84 | 0.498 | - | - | - | - |
| tlast, h | 3 | 38.7 (8– >54) | 15.7 (1.5–44) | 18.6 (0.17– >54) | 1.89 | 0.265 | - | - | - | - |
| THCV | ||||||||||
| Frequent Smokers | ||||||||||
| Cmax, µg/L | 11 | 4.5 (0.26–14.5) | 28.3 (1.6–82.4) | 40.2 (1.8–159) | 2.74 | 0.117 | - | - | - | - |
| tmax, h | 11 | 0.53 (0.17–1.5) | 0.29 (0.17–1.5) | 0.17 | 1.89 | 0.193 | - | - | - | - |
| tlast, h | 11 | 1.7 (0.17–3.5) | 4.7 (1.5–12)* | 3.9 (1.5–8) | 5.25 | 0.015 | 17.78 | 0.002 | 0.47 | 0.510 |
| Occasional Smokers | ||||||||||
| Cmax, µg/L | 7 | 3.5 (1.2–5.6) | 34.3 (1.0–146) | 17.5 (0.30–82.4) | 1.63 | 0.237 | - | - | - | - |
| tmax, h | 7 | 0.36 (0.17–1.5) | 0.17 | 0.17 | 1.00 | 0.356 | - | - | - | - |
| tlast, h | 7 | 2.7 (0.17–5) | 1.7 (0.17–8)* | 2.8 (0.17–8) | 0.647 | 0.541 | - | - | - | - |
| CBD | ||||||||||
| Frequent Smokers | ||||||||||
| Cmax, µg/L | 11 | 8.0 (0.48–26.3) | 93.3 (0.65–350) | 76.3 (2.3–339) | 2.92 | 0.077 | - | - | - | - |
| tmax, h | 11 | 0.53 (0.17–1.5) | 0.29 (0.17–1.5) | 0.29 (0.17–1.5) | 1.00 | 0.386 | - | - | - | - |
| tlast, h | 11 | 2.2 (1.5–3.5) | 8.1 (1.5–20) | 7.4 (3.5–20) | 5.40 | 0.013 | 21.55 | 0.001 | 0.09 | 0.769 |
| Occasional Smokers | ||||||||||
| Cmax, µg/L | 9 | 5.9 (2.1–11.4) | 55.9 (2.5–291) | 28.2 (0.23–167) | 1.731 | 0.209 | - | - | - | - |
| tmax, h | 9 | 0.47 (0.17–1.5) | 0.17 | 0.17 | 2.29 | 0.169 | - | - | - | - |
| tlast, h | 9 | 2.9 (1.5–5) | 4.0 (0.17–20) | 3.6 (0.17–20) | 0.19 | 0.728 | - | - | - | - |
| CBG | ||||||||||
| Frequent Smokers | ||||||||||
| Cmax, µg/L | 11 | 17.0 (0.77–60.6) | 165 (5.7–441) | 87.4 (2.7–394) | 4.63 | 0.022 | 10.56 | 0.009 | 1.87 | 0.201 |
| tmax, h | 11 | 0.41 (0.17–1.5) | 0.17 | 0.17 | 2.22 | 0.167 | - | - | - | - |
| tlast, h | 11 | 3.0 (1.5–8) | 10.6 (5–20) | 5.4 (1.5–8) | 8.64 | 0.002 | 15.52 | 0.003 | 5.64 | 0.039 |
| Occasional Smokers | ||||||||||
| Cmax, µg/L | 9 | 11.9 (3.2–26.3) | 118 (5.4–602) | 24.4 (0.30–142) | 2.19 | 0.176 | - | - | - | - |
| tmax, h | 9 | 0.47 (0.17–1.5) | 0.17 | 0.17 | 2.29 | 0.169 | - | - | - | - |
| tlast, h | 9 | 3.7 (1.5–10) | 4.9 (0.17–26) | 3.2 (0.17–20) | 0.63 | 0.465 | - | - | - | - |
THC, Δ9-tetrahydrocannabinol; 11-OH-THC, 11-hydroxy-THC; THCCOOH, 11-nor-9-carboxy-THC; THCV, Δ9-tetrahydrocannabivarin; CBD, cannabidiol; CBG, cannabigerol; bolded p-values designate significance
denotes route-specific significant difference between smoking groups (two-tailed t-test p<0.05)
N=10 because participant K (originally recruited as an occasional smoker, therefore oral fluid was only collected up to 54 h post-dose) was still positive at the final collection.
total THCCOOH (free + hydrolyzed glucuronide)
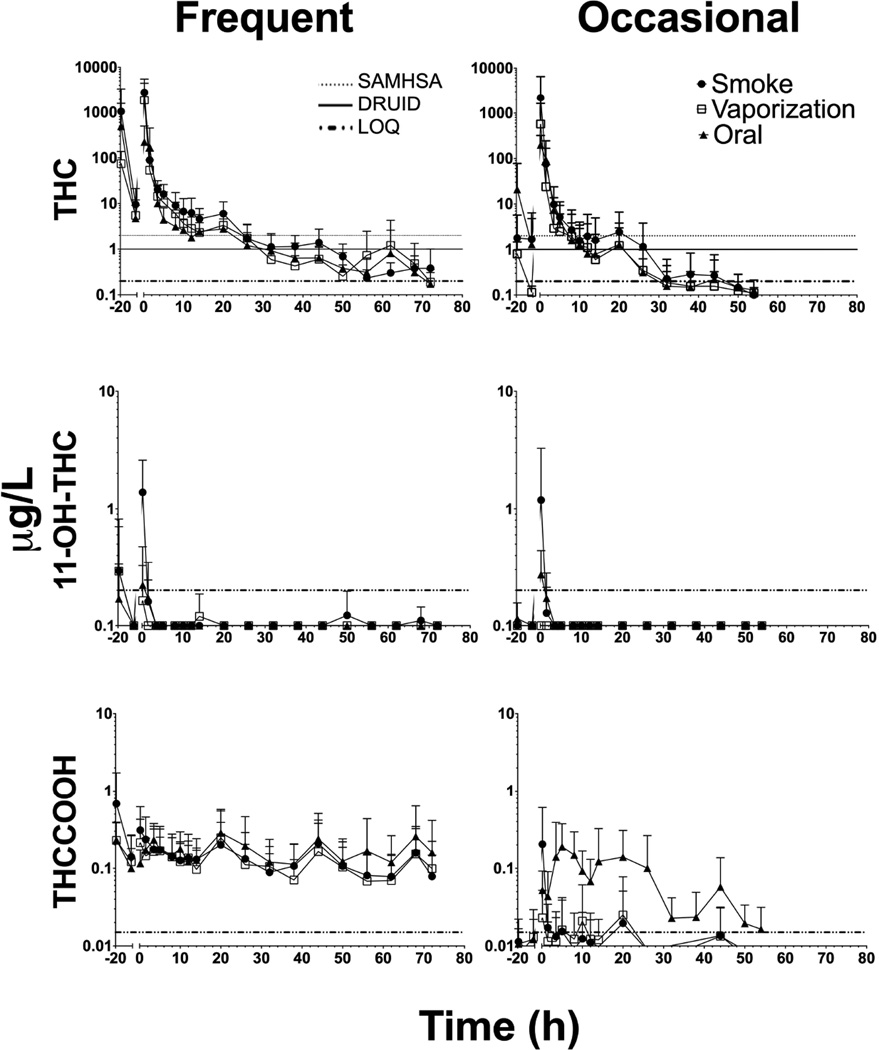
Figure 1.
Mean + standard deviation (SD) concentrations on a log-scale for ∆9-tetrahydrocannabinol (THC), 11-hydroxy-THC (11-OH-THC), and 11-nor-9-carboxy-THC (THCCOOH) in n=11 frequent (left) and n=9 occasional (right) smokers up to 72 and 54h, respectively, after smoked, vaporized, and oral cannabis (6.9% Δ9-tetrahydrocannabinol, THC; ~50.6mg THC) administration (0 h). Horizontal lines present at the limits of quantification (LOQ; 0.2 µg/L for all, except 15 ng/L for THCCOOH) and OF THC cutoffs for DRUID (1 µg/L) and SAMHSA (2 µg/L).
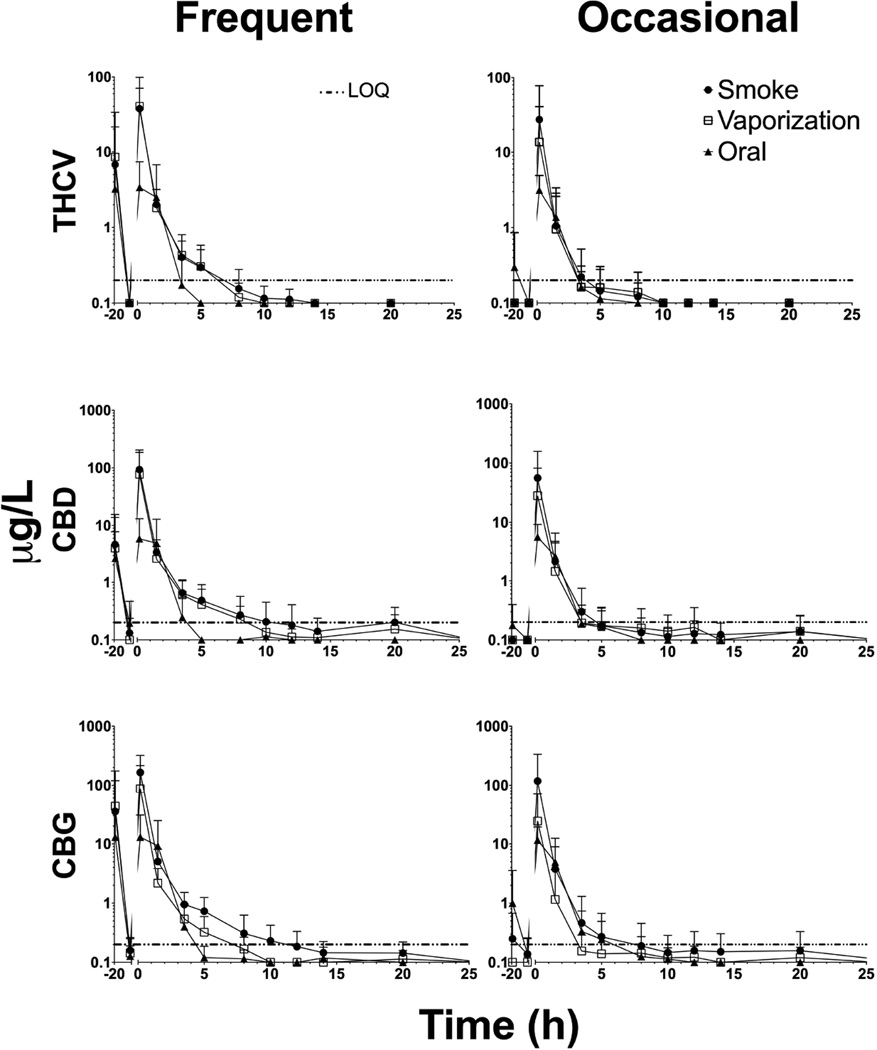
Figure 2.
Mean + standard deviation (SD) concentrations (up to 20h) for ∆9-tetrahydrocannabivarin (THCV), cannabidiol (CBD), and cannabigerol (CBG) in n=11 frequent (left) and n=9 occasional (right) smokers after smoked, vaporized, and oral cannabis (6.9% Δ9-tetrahydrocannabinol, THC; ~50.6mg THC) administration (0 h). Horizontal lines present at the limits of quantification (0.2 µg/L for all)
Observed mean THC, 11-OH-THC, THCV, CBD and CBG tmax occurred at or before the first OF collection (0.17h) immediately at the completion of cannabis intake, followed by rapid concentration decreases for frequent and occasional smokers after all routes of administration. There were no significant differences in THC, 11-OH-THC, THCV, CBD, and CBG tmax between routes. Frequent smokers’ THCCOOH mean (range) tmax was significantly later after oral dosing (25.7 [0.17–68]h) compared to smoking (14.2 [0.17–68]h) and vaporization (8.4 [0.17–20]h), as described in Table 2, Contrast 1. Only 3 occasional smokers were THCCOOH positive after all routes of administration; however, all occasional smokers were positive after the oral dose. 11-OH-THC was only detected in 1 frequent smoker after all doses; however, 11-OH-THC was present in 10/11, 2/11 and 4/11 frequent smokers after the smoked, vaporized, and oral doses, respectively. 11-OH-THC was detected in 3/9, 0/9, and 6/9 occasional smokers after smoked, vaporized, and oral cannabis administration. CBD and CBG were detected in all participants after all administrations, while THCV was detected in all frequent smokers and 9/9, 7/9, and 9/9 occasional smokers after smoked, vaporized, and oral cannabis.
Frequent smokers’ mean THC and CBG Cmax were significantly greater after inhaled routes than after oral cannabis; no difference was observed between smoked and vaporized cannabis. Mean (range) THC Cmax in frequent smokers after smoked (2789 [141–8503]µg/L) and vaporized (1874 [68.6–7373]µg/L) cannabis were significantly higher than in occasional smokers after smoked (837 [81.4–5914]µg/L) and vaporized (545 [7.6–3279]µg/L) cannabis. Mean (range) THC Cmax after oral administration for frequent and occasional smokers were 297 (16.5–938) and 202 (65.0–380)µg/L, respectively. Overall, frequent smokers’ observed THC Cmax were significantly greater than those in occasional smokers’, regardless of route; no statistically significant route*group interactions were observed.
Frequent smokers’ mean (range) THCV, CBD, and CBG tlast were significantly later after smoked (4.7 [1.5–12], 8.1 [1.5–20], and 10.6 [5–20]h, respectively) and vaporized (3.9 [1.5–8], 7.4 [3.5–20], and 5.4 [1.5–8]h respectively) cannabis compared to oral (1.7 [0.17–3.5], 2.2 [1.5–3.5], and 3 [1.5–8]h, respectively) administration. Minor cannabinoids THCV, CBD, and CBG were never detected beyond 26h in any participant after any administration route; CBD (up to 20h) and CBG (up to 26h) were detected longer than THCV (up to 12h). For frequent smokers, the only significant difference between inhaled routes was a later CBG tlast after smoked cannabis compared to the vaporized dose. Cannabinoid tlast in occasional smokers were not significantly different between smoked and vaporized administration. When comparing groups, a significantly later THCV tlast was observed for frequent smokers after smoking compared to occasional smokers.
At their final collection time (72h), 6/11, 3/11, and 2/11 frequent smokers were still THC positive at 0.2–2.2, 0.2–0.6, 0.3–0.5µg/L after smoked, vaporized, and oral cannabis, respectively. Only one occasional smoker was THC positive at the final collection time (54h) after the vaporized and oral sessions, with 0.2 and 0.4µg/L, respectively; this participant had the largest Cmax in these sessions among occasional smokers. Overall, frequent smokers’ THC tlast was significantly later than occasional smokers’, regardless of route; no statistically significant route*group interactions were observed. THCCOOH was present at discharge in 8/11, 7/11, and 10/11 frequent smokers and 0/9, 1/9, and 2/9 occasional smokers after smoked, vaporized, and oral cannabis, respectively. Occasional participants P and S with THCCOOH concentrations >LOQ at discharge were also THCCOOH positive at admission (−19h) and baseline (−1.5h) to the same session, with THCCOOH concentrations at admission comparable to those at discharge. THCCOOH tlast was significantly later in frequent smokers compared to occasional smokers, regardless of administration route. 11-OH-THC was detected infrequently and never beyond 1.5h in any participant after any administration route.
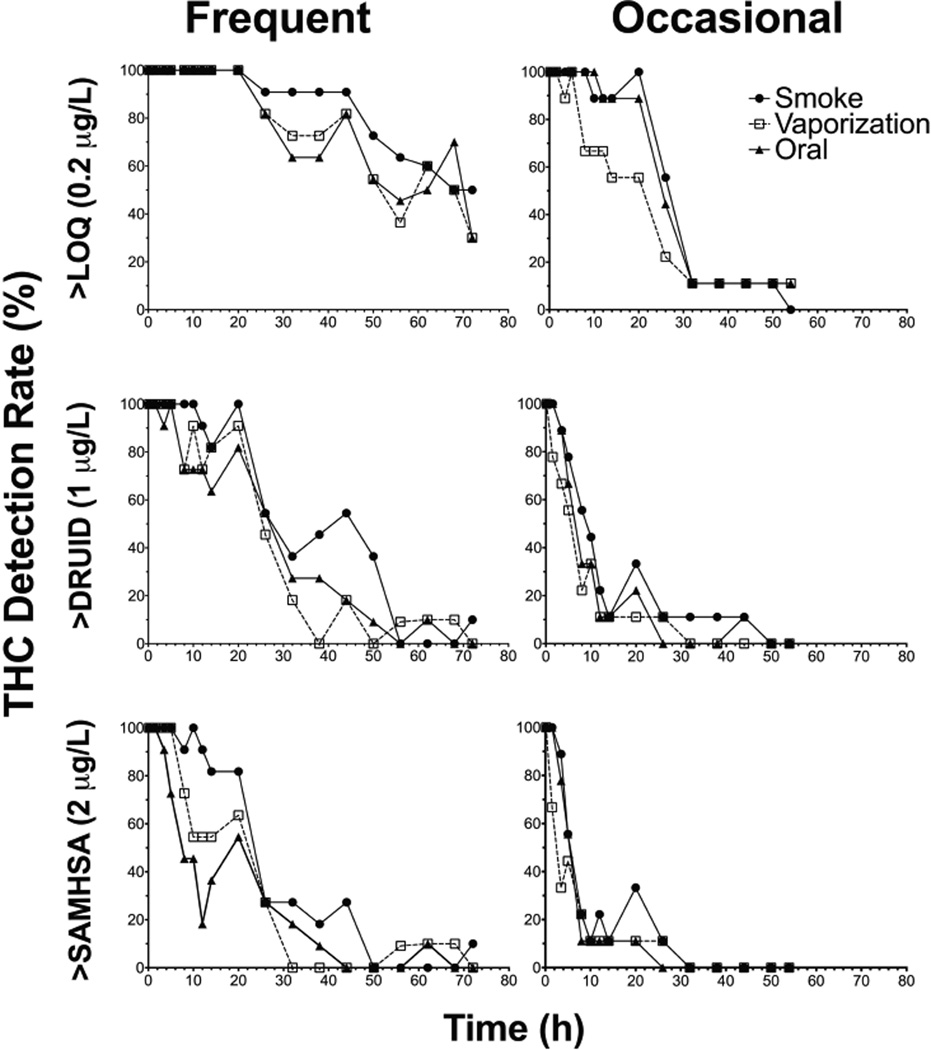
Cannabinoid Detection Rates
THC detection rates at three cutoffs (LOQ 0.2µg/L, DRUID 1µg/L, and SAMHSA 2µg/L) for frequent and occasional smokers are found in Figure 3. At the LOQ, DRUID, and SAMHSA cutoffs, THC was still observed in frequent smokers’ OF samples at discharge (72h), with detection rates never reaching 0%. At the LOQ, one occasional smoker’s OF samples were still THC positive at discharge (54h), while all samples were below DRUID and SAMHSA THC cutoffs by 50 and 32h, respectively. Detection rates dropped more quickly following oral administration. One frequent smoker was THC positive (2.2µg/L) above DRUID and SAMHSA cutoffs when discharged 72h after smoking. At baseline (−1.5h), following an overnight stay on the controlled research unit, 11/11, 9/11, and 10/11 frequent smokers remained positive for THC above DRUID cutoff (1µg/L) prior to smoked, vaporized, and oral cannabis sessions, respectively. Of the frequent smokers THC positive at baseline, all except 4 samples’ THC concentrations were also above the SAMHSA THC cutoff (2µg/L) prior to a dosing session. At baseline, only 1 occasional smoker was THC positive above the SAMHSA cutoff prior to smoking (14.3µg/L) and oral (10.6µg/L) sessions.
Figure 3.
∆9-tetrahydrocannabinol (THC) detection rates (%) at three cutoffs: limit of quantification (0.2µg/L, LOQ), DRUID (1µg/L), and SAMHSA (2µg/L) for n=11 frequent (left) and n=9 occasional (right) smokers up to 72 and 54h, respectively, after smoked, vaporized, and oral cannabis (6.9% Δ9-tetrahydrocannabinol, THC; ~50.6mg THC) administration (0h).
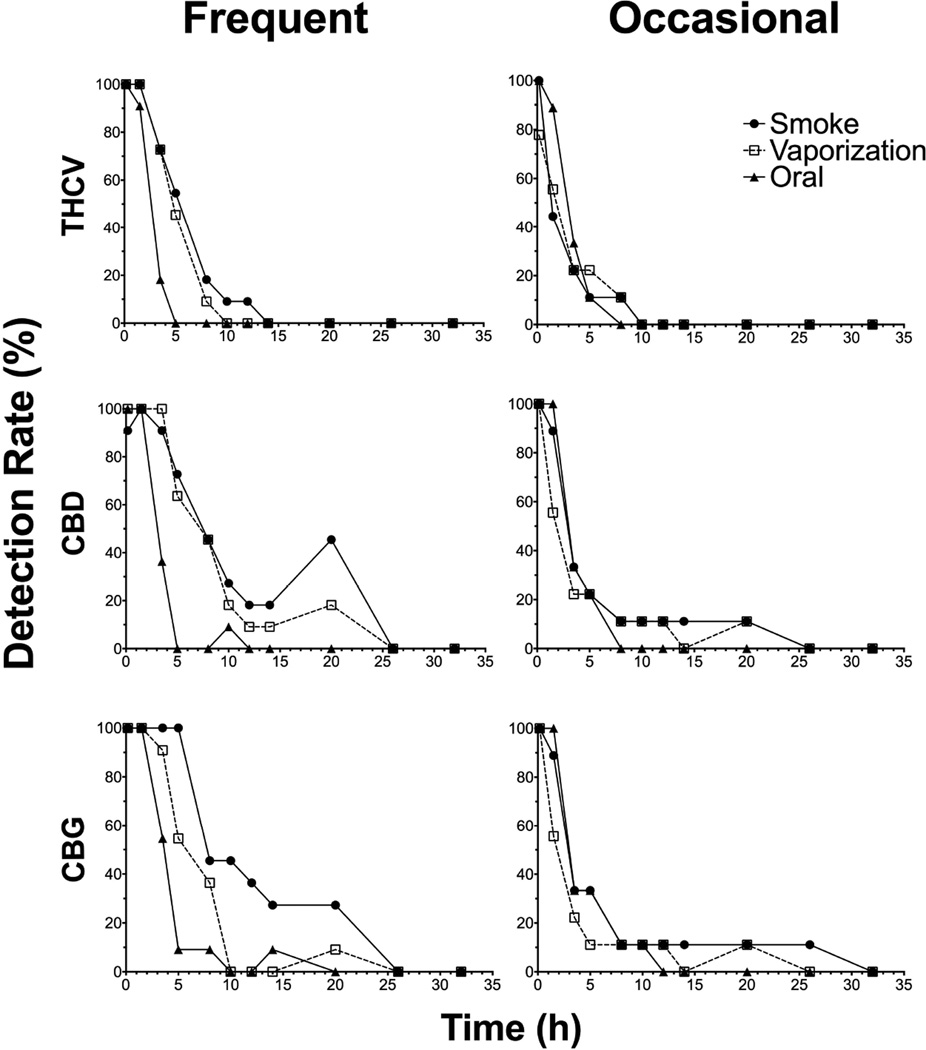
THCV, CBD and CBG detection rates at the LOQ (0.2µg/L) for frequent and occasional smokers are found in Figure 4. More frequent than occasional smokers were positive for the minor cannabinoids after all routes, since THCV was not detected after vaporization in some occasional smokers. At the LOQ, frequent smokers were no longer positive for THCV, CBD, and CBG at 14, 26, and 26h, respectively, and 10, 26, and 32h for occasional smokers. Frequent smokers’ detection rates were highest for the longest amount of time after smoked cannabis administration, as smoking produced slightly higher cannabinoid concentrations compared to vaporized and oral doses. Detection rates between frequent and occasional smokers were similar for minor cannabinoids following vaporized and oral administration.
Figure 4.
∆9-tetrahydrocannabivarin (THCV), cannabidiol (CBD), and cannabigerol (CBG) detection rates (%) up to 32h at the limits of quantification (0.2µg/L) for n=11 frequent (left) and n=9 occasional (right) smokers after smoked, vaporized, and oral cannabis (6.9% Δ9-tetrahydrocannabinol, THC; ~50.6mg THC) administration (0h)
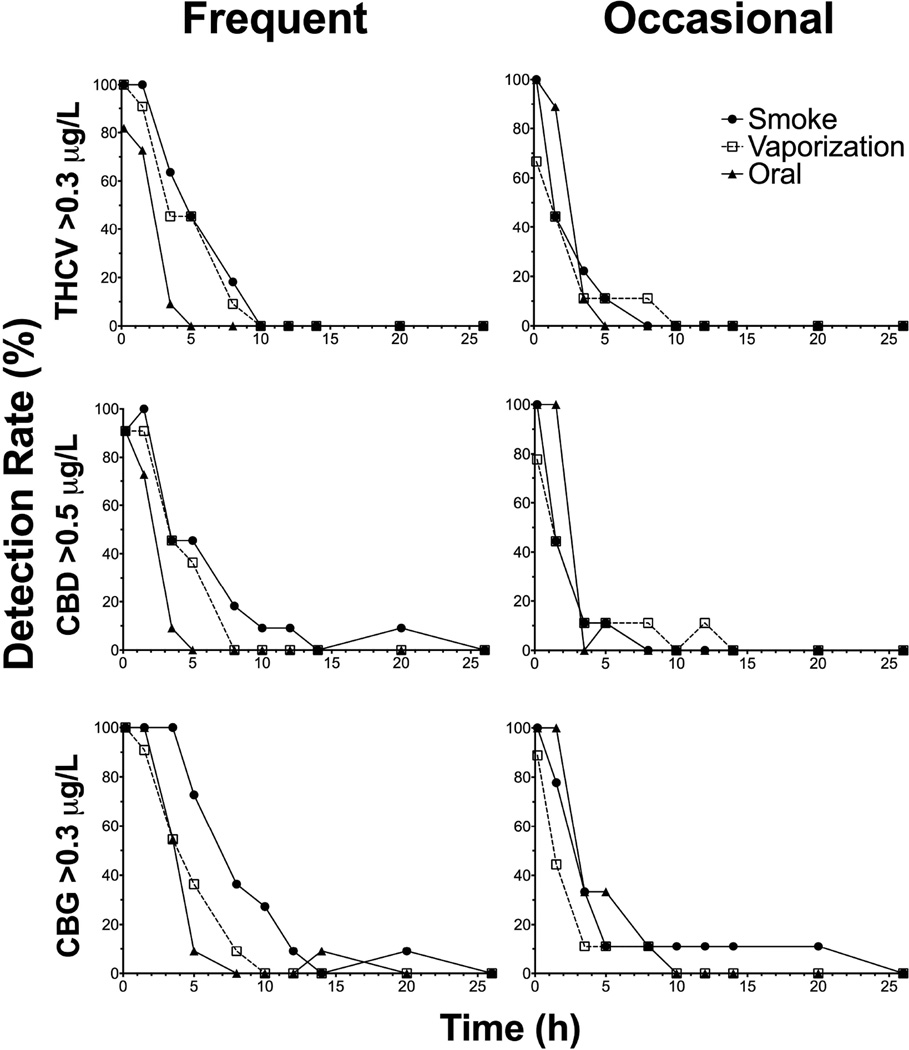
In order to establish detection windows reflecting use within the last 24h, different cutoffs were investigated for the minor cannabinoids. THCV, CBD, and CBG detection rates at proposed 0.3, 0.5, and 0.3µg/L cutoffs, respectively, for frequent and occasional smokers are found in Figure 5. At these cutoffs, THCV, CBD, and CBG were no longer detected in frequent smokers OF samples at 10, 26 and 26h, respectively, and 10, 14 and 26h for occasional smokers. Detection rates between frequent and occasional smokers were similar for minor cannabinoids at 0.3–0.5µg/L cutoffs.
Figure 5.
∆9-tetrahydrocannabivarin (THCV), cannabidiol (CBD), and cannabigerol (CBG) detection rates (%) up to 26h at the proposed cutoffs (0.3, 0.5, and 0.3µg/L, respectively) for n=11 frequent (left) and n=9 occasional (right) smokers after smoked, vaporized, and oral cannabis (6.9% Δ9-tetrahydrocannabinol, THC; ~50.6mg THC) administration (0h).
DISCUSSION
For the first time, we present parent cannabinoid (THC, THCV, CBD, and CBG) and metabolite (11-OH-THC and THCCOOH) disposition in OF following controlled smoked, vaporized, and oral (brownie) cannabis administration utilizing a within-subject study design for 11 frequent and 9 occasional cannabis smokers. Cannabinoid pharmacokinetics are well studied in OF following smoked[15–16, 28] administration of cannabis, while clinical data following vaporized[13] and edible[17, 21] cannabis are limited.
THC, THCV, CBD and CBG tmax indicate oral mucosa contamination from cannabis intake that is observed at the first OF collection time point. 11-OH-THC also appeared immediately (0.17h) in a few cases suggesting possible THC metabolism in the oral mucosa. Cytochrome P450 enzymes were identified in human oral tissue cells[29–32] and could contribute to the presence of metabolites in OF. 11-OH-THC was rarely detected after all administration routes and never beyond 1.5h post-dose. 11-OH-THC was detected more frequently after smoking in this study compared to previous studies due to our lower LOQ (0.2 vs. 0.5µg/L). Most observed 11-OH-THC concentrations would have been missed with previous analytical methods. THCCOOH appeared immediately (0.17h) in a few occasional smokers’ OF after inhaled routes but more frequently and for longer periods of time after oral intake. Among occasional smokers THCCOOH positive at 0.17h (4/9, 3/9, and 7/9 after smoked, vaporized, and oral doses, respectively), concentrations were greater than those at baseline in all but one case, suggesting THC metabolism in the oral mucosa and/or partitioning from blood. THCCOOH concentrations remained elevated in frequent smokers’ OF throughout the sessions, although the tmax also was delayed after oral administration, similar to the pattern observed in occasional smokers. A significantly later THCCOOH tmax also occurred in blood specimens from the same cohort following oral cannabis administration, supporting blood-OF partitioning. Similarly, Vandrey et al. observed delayed mean (range) OF THCCOOH tmax of 9.8 (3–30) and 17.4 (0–54)h following consumption of 25 and 50mg oral THC (brownie), respectively[21].
Frequent smokers’ THC Cmax after smoking a 6.9% THC cannabis cigarette in the present study were higher than those reported previously for similar potency cannabis[15–16], but our initial OF collection time post-dose (0.17 vs. 0.5h) was earlier. However, Toennes et al. collected OF 0.08h after smoking a 500µg THC/kg cannabis cigarette and observed higher median (range) THC Cmax of 6202 (387–71,147) and 1242 (397–6438)ng/g in frequent and occasional smokers, respectively[28]. Occasional smokers’ median THC Cmax in the present study were lower than those previously reported[15–16] but exhibited a wider range, which could be influenced by smoking history, topography, and possible titration. We observed lower median (range) THC concentrations for both groups of smokers compared to moderate smokers’ THC Cmax following vaporization of 500mg 6.7% THC ground cannabis[13]. Differences in THC Cmax after vaporization in this study could be due to differences in smoking history, inhalation topography, and titration. Additionally, less efficient cannabinoid vaporization can occur with increased plant material[33], as we vaporized ~750mg ground cannabis compared to 500mg in Hartman et al. Although no statistically significant differences in Cmax between smoking and vaporization were observed, differences in heating temperature could potentially release fewer cannabinoids during vaporization compared to smoking, although less pyrolysis of THC would be expected and there is no loss of THC in sidestream smoke as occurs during smoking. In addition, vaporization stores THC vapor in a plastic bag during heating, possibly losing small amounts of THC through absorption to the bag.
Frequent and occasional smokers’ THC Cmax following oral consumption were not significantly different, as this route is not amenable to self-titration. Our mean THC Cmax were lower than those reported by Vandrey et al. following 25 or 50mg oral (brownie) THC in drug-free users (n=6) but were more similar to those reported for 10mg oral THC in the same study[21]. Observed differences in oral THC concentrations could be due to our later collection time (0.17h post-inhalation dose, equating to 0.33h post-oral dose compared to their 0.2h time point). In a separate study, Niedbala et al. reported 2.2–7.1µg/L THC 1–2h after oral brownie consumption (20–25mg THC) in casual users (n=3)[17]. By 1.5h post-dose in our study, THC concentrations after oral cannabis were much greater (10.8–938 and 23.0–256µg/L in frequent and occasional smokers, respectively) than Niedbala’s reported THC concentrations at the same time post-dose; this may be due to a combination of different brownie preparations (i.e. how well the precursor Δ9-tetrahydrocannabinolic acid is converted to THC during baking) and our higher-potency THC variety. Compared to oral synthetic THC (dronabinol, Marinol®) administration, we observed increases in THC OF concentrations post-dose due to oral cavity contamination that did not occur with encapsulated synthetic oral THC (dronabinol)[34–35].
THC and CBG exhibited significantly higher concentrations in frequent smokers’ OF after inhaled routes than after oral cannabis dosing. Smoked and vaporized cannabis administration were previously reported to produce similar cannabinoid OF concentrations[13]; this was observed, except for a later CBG tlast after smoked cannabis compared to the vaporized dose. However, due to the greater CBG concentrations after smoked compared to vaporized cannabis, this could be expected. The same trend was observed with CBG disposition in whole blood and could be explained by inefficient CBG volatilization during vaporization. Significantly later tlast for minor cannabinoids in frequent smokers after inhaled cannabis could be due to the much greater concentrations achieved compared to brownie consumption. Differences in Cmax between the routes were not anticipated amongst occasional smokers as they often exhibit inefficient smoking/vaporization topography, leading to lower cannabinoid concentrations after inhalation similar to those following oral administration. Lower OF THC concentrations achieved after oral cannabis compared to inhaled cannabis could be due to oral intake mechanism (chewing and swallowing may not release as much THC as inhaling), ad libitum study design, conversion to CBN, degradation during baking, or possibly less efficient decarboxylation of acid to THC.
Cannabinoid metabolite detection and interpretation in OF can be complicated. 11-OH-THC is detected infrequently and only for a short period of time. While detection of 11-OH-THC in oral fluid is an indication of recent cannabis use, its absence does not preclude recent consumption. OF THCCOOH concentration variability observed in this study was also previously observed[13, 15–16].
For the first time, THCV and CBG disposition were characterized in OF for frequent and occasional smokers following controlled smoked, vaporized, and oral (brownie) cannabis consumption with similar detection rates between groups and routes. Previously, THCV and CBG disposition were only described in urine[36–38]. Mean THCV Cmax (tmax) were 17.5–40.2µg/L (0.17–0.29h) after inhalation routes and 3.2–4.5µg/L (0.47–0.53) after oral dosing among all participants, while mean CBG Cmax (tmax) were 87.4–244µg/L (0.17h) after inhaled routes and 11.9–17.0µg/L (0.41–0.47h) after oral dosing for all participants. In our cohort, both THCV and CBG were detected in 11/11 frequent and 7/9 occasional (THCV) and 11/11 frequent and 9/9 occasional (CBG) smokers after all administration routes for up to 26h at the LOQ of 0.2µg/L, making them applicable for identifying cannabis intake within about one day, as previously suggested by Desrosiers et al.[26]. THCV was not detected in 2 occasional smokers after vaporization with low THC concentrations (7.5 and 8.5 µg/L at 0.17h). CBD was previously investigated in OF following smoked[15] and vaporized[13] cannabis administration in different cohorts. While CBD Cmax after smoking in this study (0.17–0.29h) were slightly higher than those observed in frequent and occasional smokers 0.5h after smoking[15], our median occasional smokers’ CBD Cmax after vaporization were comparable to those reported for moderate smokers after the high THC dose without alcohol in Hartman et al. vaporization study[13]. To our knowledge, CBD was not previously investigated in OF following oral cannabis administration. CBD, while a useful marker of recent use in this study, cannot be thoroughly characterized until investigated at the higher-potency CBD cannabis material now available in the market.
DRUID and SAMHSA established THC OF confirmation cutoff guidelines of 1 and 2µg/L, respectively. However, frequent smokers’ THC concentrations remain well above these cutoffs for longer periods of time, making data interpretation difficult for estimating recent use. Occasional smokers’ OF THC concentrations are generally lower and may fall below the DRUID or SAMHSA cutoffs within a much shorter timeframe, making it difficult to capture recent use beyond several hours. OF THC concentrations after oral cannabis consumption are not as high as concentrations observed following inhaled routes and concentrations fall below DRUID and SAMHSA cutoffs much more quickly. Additionally, THC peak concentrations in OF following edible cannabis consumption occur prior to peak impairment, with no secondary peak after oral consumption, suggesting oral mucosa contamination rather than partitioning from blood.
In order to establish detection windows for capturing recent cannabis use, we previously investigated several combinations of cutoffs, including THC in combination with CBD, CBN, and/or THCCOOH[13, 15–16]. Our new expanded OF cannabinoid method incorporates THCV and CBG as additional analytes, although CBN could no longer be included as we could not chromatographically separate it from a matrix interference. Our low LOQs for minor cannabinoids, including THCV and CBG, allowed us to detect these analytes up to 26h following controlled cannabis administration. By applying a 0.3µg/L cutoff for THCV or CBG, detection windows were 8 and 20h, respectively, for both frequent and occasional smokers. Monitoring minor cannabinoids in OF offers the ability to detect recent cannabis use that is not achievable for THC and/or THCCOOH. However, minor cannabinoids (THCV, CBN, CBD, and CBG) were not detected in whole blood specimens collected from the same cohort following oral brownie administration (manuscript under review). While these data offer promising results for capturing recent cannabis use by increasing the cannabinoids analyzed in OF specimens, more research is necessary following other administration routes, including vape pens, dabs, waxes, THC oils, and other cannabis and/or THC products. Comparison to pharmacodynamic outcomes as well as on-site OF screening devices and other matrices would assist in further interpretation of OF cannabinoid data.
CONCLUSIONS
THC, metabolites, and minor cannabinoids were fully characterized in OF following controlled cannabis brownie consumption with direct comparison to smoked and vaporized administration in the same frequent and occasional smokers. The within-subject study design allowed for direct pharmacokinetic comparisons between the different administration routes. Few differences were observed between smoked and vaporized cannabis administrations. For the first time, THCV and CBG were characterized in OF after multiple routes and CBD was characterized for the first time after oral cannabis administration. Cannabinoid concentrations peaked immediately after cannabis consumption, regardless of route, as a result of oral mucosa contamination. As expected, greater THC concentrations were observed after smoked and vaporized cannabis compared to oral administration. Minor cannabinoids, including THCV, CBD, and CBG, were detected in 18/20, 20/20, and 20/20 study participants, respectively, after all three administration routes and up to 26h post-dose, indicating potential utilization as markers of cannabis use within 1 day that could be helpful in interpretation of clinical and forensic drug testing programs.
Acknowledgments
The authors would like to thank Dr. Sandrine Pirard for her contribution to study design and the contributions of the clinical staffs of the Intramural Research Program, National Institute on Drug Abuse, and the Clinical Research Unit, Johns Hopkins Bayview Medical Center. This study was registered on clinicaltrials.gov (NCT02177513). Quantisal devices and BG100 β-glucuronidase were provided by the manufacturers to NIH via Materials Transfer Agreements. This research was funded by the Intramural Research Program, National Institute on Drug Abuse, NIH. MNN acknowledges the Graduate Partnership Program, NIH.
REFERENCES
- 1.U. N. O. o. D. a. Crime. World Drug Report 2015. Journal. 2015;1 [Google Scholar]
- 2.Berning A, Compton R, Wochinger K. Results of the 2013–2014 National Roadside Survey of Alcohol and Drug Use by Drivers. Journal. 2015 [Google Scholar]
- 3.Asbridge M, Mann R, Cusimano MD, Trayling C, Roerecke M, Tallon JM, Whipp A, Rehm J. Cannabis and traffic collision risk: findings from a case-crossover study of injured drivers presenting to emergency departments. Int J Public Health. 2014;59:395. doi: 10.1007/s00038-013-0512-z. [DOI] [PubMed] [Google Scholar]
- 4.Li M-C, Brady JE, DiMaggio CJ, Lusardi AR, Tzong KY, Li G. Marijuana Use and Motor Vehicle Crashes. Epidemiologic Reviews. 2011 doi: 10.1093/epirev/mxr017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramaekers JG, Berghaus G, van Laar M, Drummer OH. Dose related risk of motor vehicle crashes after cannabis use. Drug Alcohol Depend. 2004;73:109. doi: 10.1016/j.drugalcdep.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Hartman RL, Huestis MA. Cannabis Effects on Driving Skills. Clin. Chem. 2013;59:478. doi: 10.1373/clinchem.2012.194381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van der Linden T, Legrand SA, Silverans P, Verstraete AG. DUID: oral fluid and blood confirmation compared in Belgium. J. Anal. Toxicol. 2012;36:418. doi: 10.1093/jat/bks038. [DOI] [PubMed] [Google Scholar]
- 8.Samyn N, De Boeck G, Verstraete AG. The use of oral fluid and sweat wipes for the detection of drugs of abuse in drivers. J. Forensic Sci. 2002;47:1380. [PubMed] [Google Scholar]
- 9.Strano-Rossi S, Castrignano E, Anzillotti L, Serpelloni G, Mollica R, Tagliaro F, Pascali JP, di Stefano D, Sgalla R, Chiarotti M. Evaluation of four oral fluid devices (DDS®, Drugtest 5000®, Drugwipe 5+® and RapidSTAT®) for on-site monitoring drugged driving in comparison with UHPLC-MS/MS analysis. Forensic Sci. Int. 2012;221:70. doi: 10.1016/j.forsciint.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Blencowe T, Pehrsson A, Lillsunde P, Vimpari K, Houwing S, Smink B, Mathijssen R, Van der Linden T, Legrand SA, Pil K, Verstraete A. An analytical evaluation of eight on-site oral fluid drug screening devices using laboratory confirmation results from oral fluid. Forensic Sci. Int. 2011;208:173. doi: 10.1016/j.forsciint.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 11.Vindenes V, Lund HM, Andresen W, Gjerde H, Ikdahl SE, Christophersen AS, Øiestad EL. Detection of drugs of abuse in simultaneously collected oral fluid, urine and blood from Norwegian drug drivers. Forensic Sci. Int. 2012;219:165. doi: 10.1016/j.forsciint.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Concheiro M, de Castro A, Quintela O, Cruz A, López-Rivadulla M. Confirmation by LC-MS of drugs in oral fluid obtained from roadside testing. Forensic Sci. Int. 2007;170:156. doi: 10.1016/j.forsciint.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 13.Hartman R, Anizan S, Jang M, Brown TL, Yun K, Gorelick D, Milavetz G, Spurgin A, Gaffney G, Huestis M. Cannabinoid disposition in oral fluid after controlled vaporizer administration with and without alcohol. Forensic Toxicol. 2015 [Google Scholar]
- 14.Schauer GL, King BA, Bunnell RE, Promoff G, McAfee TA. Toking, Vaping, and Eating for Health or Fun: Marijuana Use Patterns in Adults, U.S., 2014. Am. J. Prev. Med. 2016;50:1. doi: 10.1016/j.amepre.2015.05.027. [DOI] [PubMed] [Google Scholar]
- 15.Anizan S, Milman G, Desrosiers N, Barnes AJ, Gorelick DA, Huestis MA. Oral fluid cannabinoid concentrations following controlled smoked cannabis in chronic frequent and occasional smokers. Anal. Bioanal. Chem. 2013;405:8451. doi: 10.1007/s00216-013-7291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newmeyer MN, Desrosiers NA, Lee D, Mendu DR, Barnes AJ, Gorelick DA, Huestis MA. Cannabinoid disposition in oral fluid after controlled cannabis smoking in frequent and occasional smokers. Drug Test Anal. 2014;6:1002. doi: 10.1002/dta.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niedbala R, Kardos K, Fritch D, Kardos S, Fries T, Waga J, Robb J, Cone E. Detection of marijuana use by oral fluid and urine analysis following single-dose administration of smoked and oral marijuana. J Anal Toxicol. 2001;25:289. doi: 10.1093/jat/25.5.289. [DOI] [PubMed] [Google Scholar]
- 18.Toennes S, Ramaekers J, Theunissen E, Moeller M, Kauert G. Pharmacokinetic properties of Δ9-tetrahydrocannabinol in oral fluid of occasional and chronic users. J. Anal. Toxicol. 2010;34:216. doi: 10.1093/jat/34.4.216. [DOI] [PubMed] [Google Scholar]
- 19.Lee D, Schwope DM, Milman G, Barnes AJ, Gorelick DA, Huestis MA. Cannabinoid Disposition in Oral Fluid after Controlled Smoked Cannabis. Clin. Chem. 2012;58 doi: 10.1373/clinchem.2011.177881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee D, Vandrey R, Mendu DR, Murray JA, Barnes AJ, Huestis MA. Oral fluid cannabinoids in chronic frequent cannabis smokers during ad libitum cannabis smoking. Drug Test Anal. 2014 doi: 10.1002/dta.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell JM, Cone EJ, Flegel R, LoDico C, Herrmann ES, Bigelow GE, Vandrey R Oral Administration of Cannabis in Brownies. Journal. 2015 [Google Scholar]
- 22.S M Schulze H, Urmeew R, Auerbach K, Alvarez J, Bernhoft IM, de Gier H, Hagenzieker M, Houwing S, Knoche A, Pilgerstorfer M, Zlender B. Driving Under the Influence of Drugs, Alcohol and Medicines in Europe – finding from the DRUID project. Journal. 2012 [Google Scholar]
- 23.S. A. a. M. H. S. Administration. Mandatory Guidelines for Federal Workplace Drug Testing Programs. Federal Register. 2015;80:28054. [Google Scholar]
- 24.Lee D, Milman G, Schwope DM, Barnes AJ, Gorelick DA, Huestis MA. Cannabinoid Stability in Authentic Oral Fluid after Controlled Cannabis Smoking. Clin. Chem. 2012;58:1101. doi: 10.1373/clinchem.2012.184929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anizan S, Bergamaschi MM, Barnes AJ, Milman G, Desrosiers N, Lee D, Gorelick DA, Huestis MA. Impact of oral fluid collection device on cannabinoid stability following smoked cannabis. Drug Test Anal. 2014 doi: 10.1002/dta.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desrosiers NA, Scheidweiler KB, Huestis MA. Quantification of six cannabinoids and metabolites in oral fluid by liquid chromatography-tandem mass spectrometry. Drug Test Anal. 2015;7:684. doi: 10.1002/dta.1753. [DOI] [PubMed] [Google Scholar]
- 27.Moore C, Rana S, Coulter C, Day D, Vincent M, Soares J. Detection of conjugated 11-nor-Delta9-tetrahydrocannabinol-9-carboxylic acid in oral fluid. J. Anal. Toxicol. 2007;31:187. doi: 10.1093/jat/31.4.187. [DOI] [PubMed] [Google Scholar]
- 28.Toennes SW, Ramaekers JG, Theunissen EL, Moeller MR, Kauert GF. Pharmacokinetic Properties of 9-Tetrahydrocannabinol in Oral Fluid of Occasional and Chronic Users. Journal of analytical toxicology. 2010;34:216. doi: 10.1093/jat/34.4.216. [DOI] [PubMed] [Google Scholar]
- 29.Vondracek M, Xi Z, Larsson P, Baker V, Mace K, Pfeifer A, Tjälve H, Donato MT, Gomez-Lechon MJ, Grafström RC. Cytochrome P450 expression and related metabolism in human buccal mucosa. Carcinogenesis. 2001;22:481. doi: 10.1093/carcin/22.3.481. [DOI] [PubMed] [Google Scholar]
- 30.Yang S-P, Raner GM. Cytochrome P450 expression and activities in human tongue cells and their modulation by green tea extract. Toxicol Appl Pharmacol. 2005;202:140. doi: 10.1016/j.taap.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 31.Sacks PG, Zhao Z-L, Kosinska W, Fleisher KE, Gordon T, Guttenplan JB. Concentration dependent effects of tobacco particulates from different types of cigarettes on expression of drug metabolizing proteins, and benzo(a)pyrene metabolism in primary normal human oral epithelial cells. Food Chem Toxicol. 2011;49:2348. doi: 10.1016/j.fct.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou LX, Pihlstrom B, Hardwick JP, Park SS, Wrighton SA, Holtzman JL. Metabolism of phenytoin by the gingiva of normal humans: the possible role of reactive metabolites of phenytoin in the initiation of gingival hyperplasia. Clin. Pharmacol. Ther. 1996;60:191. doi: 10.1016/S0009-9236(96)90135-6. [DOI] [PubMed] [Google Scholar]
- 33.Hazekamp A, Ruhaak R, Zuurman L, van Gerven J, Verpoorte R. Evaluation of a vaporizing device (Volcano®) for the pulmonary administration of tetrahydrocannabinol. J. Pharm. Sci. 2006;95:1308. doi: 10.1002/jps.20574. [DOI] [PubMed] [Google Scholar]
- 34.Milman G, Barnes A, Schwope D, Schwilke E, Goodwin R, Kelly D, Gorelick D, Huestis M. Cannabinoids and metabolites in expectorated oral fluid after 8 days of controlled around-the-clock oral THC administration. Anal. Bioanal. Chem. 2011;401:599. doi: 10.1007/s00216-011-5066-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Milman G, Barnes AJ, Schwope DM, Schwilke EW, Darwin WD, Goodwin RS, Kelly DL, Gorelick DA, Huestis MA. Disposition of Cannabinoids in Oral Fluid after Controlled Around-the-Clock Oral THC Administration. Clin. Chem. 2010;56:1261. doi: 10.1373/clinchem.2009.141853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hidvegi E, Somogyi GP. Detection of cannabigerol and its presumptive metabolite in human urine after Cannabis consumption. Pharmazie. 2010;65:408. [PubMed] [Google Scholar]
- 37.ElSohly MA, Feng S, Murphy TP, Warrington AW, Ross S, Nimrod A, Mehmedic Z, Fortner N. Identification and quantitation of 11-nor-delta-tetrahydrocannabivarin-9-carboxylic acid, a major metabolite of delta-tetrahydrocannabivarin. J. Anal. Toxicol. 2001;25:476. doi: 10.1093/jat/25.6.476. [DOI] [PubMed] [Google Scholar]
- 38.Levin FR, Mariani JJ, Brooks DJ, Xie S, Murray KA. Δ9-Tetrahydrocannabivarin testing may not have the sensitivity to detect marijuana use among individuals ingesting dronabinol. Drug Alcohol Depend. 2010;106:65. doi: 10.1016/j.drugalcdep.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]