Abstract
The quantitative relationship between the catabolism of heme and the formation of bilirubin and carbon monoxide (CO) was studied in untreated rats and in animals treated with phenobarbital or the porphyrogenic drug, allylisopropylacetamide (AIA). A novel metabolic chamber permitting continuous collection of the bile and breath was utilized for quantitation of bilirubin-14C and 14CO after the administration of hematin-14C or glycine-14C.
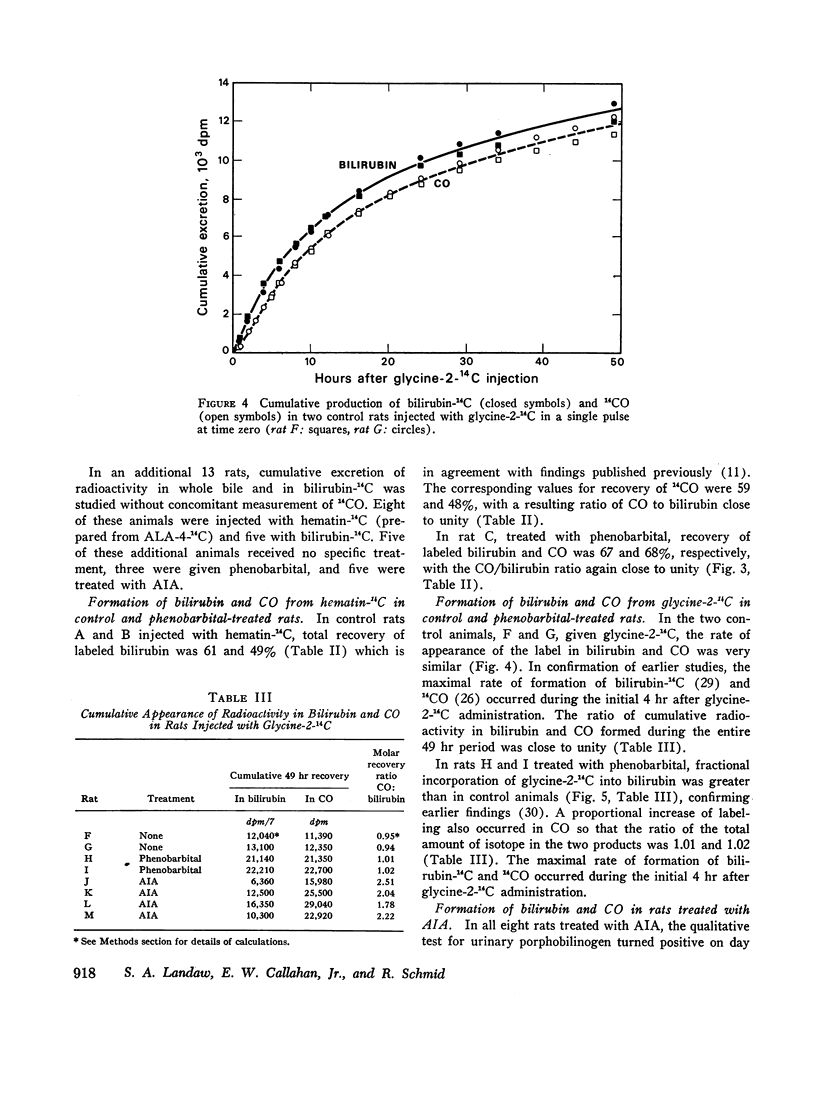
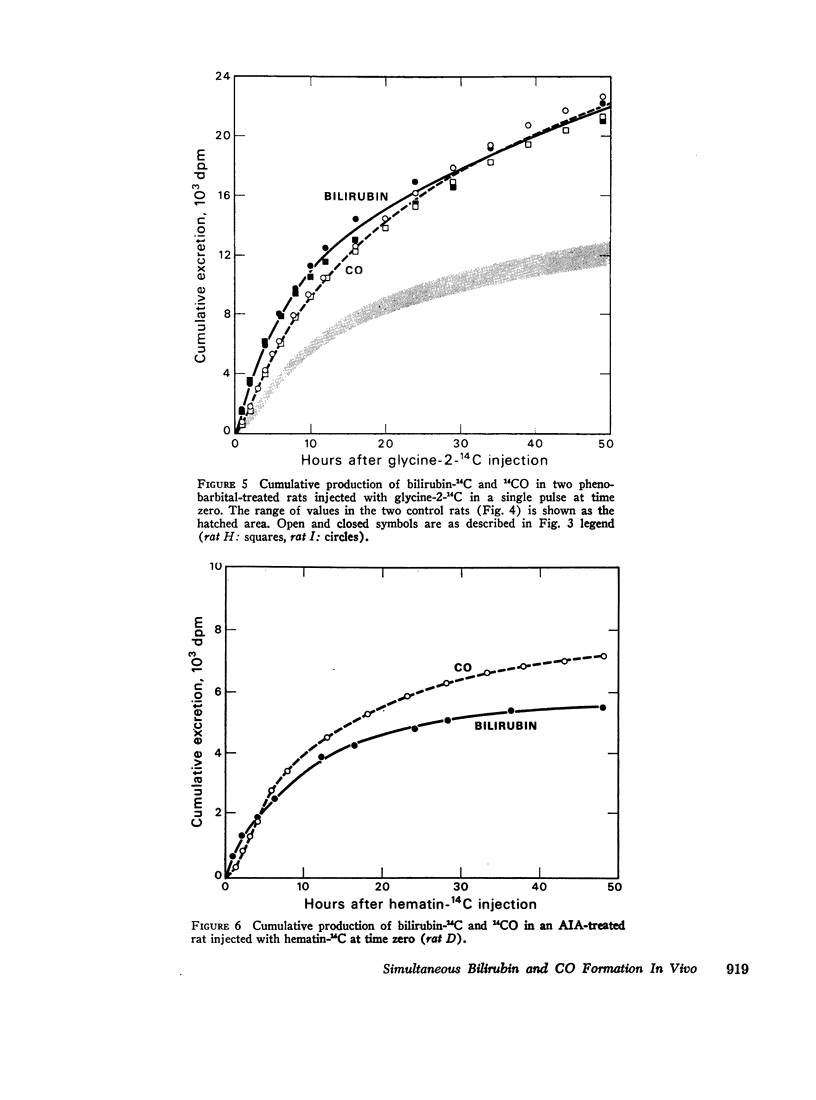
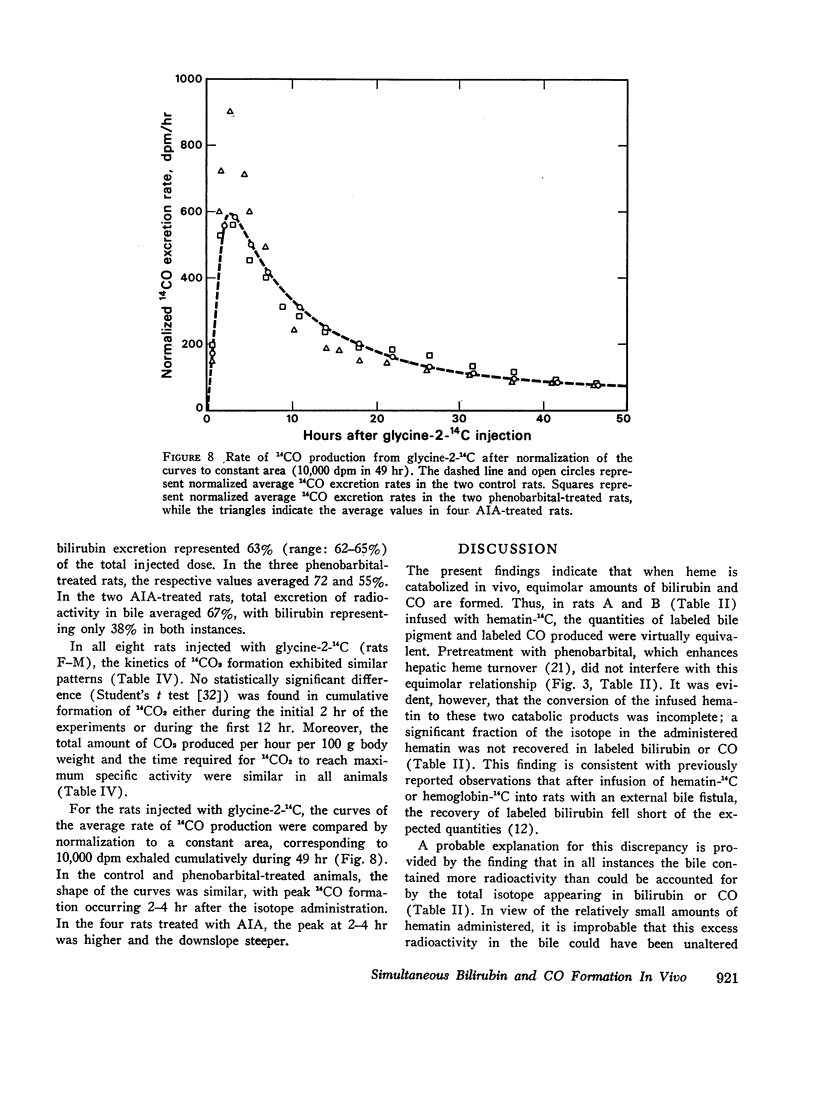
After intravenous infusion of hematin-14C, control and phenobarbital-treated rats produced equimolar amounts of labeled bilirubin and CO; a minor fraction of the infused radioactivity appeared in the bile in other metabolites. The equimolar relationship in the formation of bilirubin and CO was also observed after pulse-labeling with glycine-2-14C; in phenobarbital-treated rats both metabolites were formed at an increased rate as compared to controls. By contrast, AIA treatment reduced the fractional conversion of hematin-14C to bilirubin and CO; a major fraction of the infused radioactivity appeared in the bile in metabolites other than bilirubin. In addition, in AIA-treated animals the molar CO/bilirubin recovery ratio was consistently greater than 1.0. Comparable results were obtained in AIA-treated rats after pulse-labeling with glycine-2-14C.
These findings suggest that (a) in control and phenobarbital-treated rats infused hematin and heme formed in the liver are converted predominantly to bilirubin and CO, appearing in equimolar amounts; only a minor fraction of the hematin is degraded to other metabolites; (b) treatment with phenobarbital results in a proportional increase in the formation of both bilirubin and CO, reflecting increased heme synthesis and degradation in the liver; and (c) treatment with the porphyrogenic drug AIA shifts the CO/bilirubin ratio in favor of the gas, and enhances the formation of nonbilirubin metabolites.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bunn H. F., Jandl J. H. Exchange of heme among hemoglobins and between hemoglobin and albumin. J Biol Chem. 1968 Feb 10;243(3):465–475. [PubMed] [Google Scholar]
- COBURN R. F., BLAKEMORE W. S., FORSTER R. E. Endogenous carbon monoxide production in man. J Clin Invest. 1963 Jul;42:1172–1178. doi: 10.1172/JCI104802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn R. F., Williams W. J., White P., Kahn S. B. The production of carbon monoxide from hemoglobin in vivo. J Clin Invest. 1967 Mar;46(3):346–356. doi: 10.1172/JCI105536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELLER D. D., FEIST E. Conversion of glycine to fatty acids by adipose tissue. Proc Soc Exp Biol Med. 1962 Oct;111:18–21. doi: 10.3181/00379727-111-27693. [DOI] [PubMed] [Google Scholar]
- GOLDBERG A., RIMINGTON C. Experimentally produced porphyria in animals. Proc R Soc Lond B Biol Sci. 1955 Jan 27;143(911):257–279. doi: 10.1098/rspb.1955.0009. [DOI] [PubMed] [Google Scholar]
- Goldsmith J. R., Landaw S. A. Carbon monoxide and human health. Science. 1968 Dec 20;162(3860):1352–1359. doi: 10.1126/science.162.3860.1352. [DOI] [PubMed] [Google Scholar]
- Goldstein G. W., Hammaker L., Schmid R. The catabolism of Heinz bodies: an experimental model demonstrating conversion to non-bilirubin catabolites. Blood. 1968 Mar;31(3):388–395. [PubMed] [Google Scholar]
- Granick S. The induction in vitro of the synthesis of delta-aminolevulinic acid synthetase in chemical porphyria: a response to certain drugs, sex hormones, and foreign chemicals. J Biol Chem. 1966 Mar 25;241(6):1359–1375. [PubMed] [Google Scholar]
- Green M., Kench J. E. A study of the transfer of haem from haemoglobin to serum albumin. S Afr Med J. 1967 Sep 23;41(36):895–902. [PubMed] [Google Scholar]
- JACOB H. S., JANDL J. H. Effects of sulfhydryl inhibition on red blood cells. II. Studies in vivo. J Clin Invest. 1962 Jul;41:1514–1523. doi: 10.1172/JCI104607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANDL J. H. The Heinz body hemolytic anemias. Ann Intern Med. 1963 Apr;58:702–709. doi: 10.7326/0003-4819-58-4-702. [DOI] [PubMed] [Google Scholar]
- Kornfeld S., Chipman B., Brown E. B. Intracellular catabolism of hemoglobin and iron dextran by the rat liver. J Lab Clin Med. 1969 Feb;73(2):181–193. [PubMed] [Google Scholar]
- Kreimer-Birnbaum M., Pinkerton P. H., Bannerman R. M., Hutchison H. E. Dipyrrolic urinary pigments in congenital Heinz-body anaemia due to Hb köln and in thalassaemia. Br Med J. 1966 Aug 13;2(5510):396–396. doi: 10.1136/bmj.2.5510.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LABBE R. F., NISHIDA G. A new method of hemin isolation. Biochim Biophys Acta. 1957 Nov;26(2):437–437. doi: 10.1016/0006-3002(57)90033-1. [DOI] [PubMed] [Google Scholar]
- Landaw S. A., Winchell H. S. Endogenous production of carbon-14 labeled carbon monoxide: an in vivo technique for the study of heme catabolism. J Nucl Med. 1966 Sep;7(9):696–707. [PubMed] [Google Scholar]
- Levitt M., Schacter B. A., Zipursky A., Israels L. G. The nonerythropoietic component of early bilirubin. J Clin Invest. 1968 Jun;47(6):1281–1294. doi: 10.1172/JCI105820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marver H. S., Schmid R. Biotransformation in the liver: implications for human disease. Gastroenterology. 1968 Aug;55(2):282–289. [PubMed] [Google Scholar]
- Marver H. S., Schmid R., Schützel H. Heme and methemoglobin: naturally occurring repressors of microsomal cytochrome. Biochem Biophys Res Commun. 1968 Dec 30;33(6):969–974. doi: 10.1016/0006-291x(68)90408-7. [DOI] [PubMed] [Google Scholar]
- OSTROW J. D., HAMMAKER L., SCHMID R. The preparation of crystalline bilirubin-C14. J Clin Invest. 1961 Aug;40:1442–1452. doi: 10.1172/JCI104375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSTROW J. D., JANDL J. H., SCHMID R. The formation of bilirubin from hemoglobin in vivo. J Clin Invest. 1962 Aug;41:1628–1637. doi: 10.1172/JCI104620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RADIN N. S., RITTENBERG D., SHEMIN D. The rôle of glycine in the biosynthesis of heme. J Biol Chem. 1950 Jun;184(2):745–753. [PubMed] [Google Scholar]
- REMMER H., MERKER H. J. DRUG-INDUCED CHANGES IN THE LIVER ENDOPLASMIC RETICULUM: ASSOCIATION WITH DRUG-METABOLIZING ENZYMES. Science. 1963 Dec 27;142(3600):1657–1658. doi: 10.1126/science.142.3600.1657. [DOI] [PubMed] [Google Scholar]
- Robinson S. H. The origins of bilirubin. N Engl J Med. 1968 Jul 18;279(3):143–149. doi: 10.1056/NEJM196807182790306. [DOI] [PubMed] [Google Scholar]
- Robinson S. H., Tsong M., Brown B. W., Schmid R. The sources of bile pigment in the rat: studies of the "early labeled" fraction. J Clin Invest. 1966 Oct;45(10):1569–1586. doi: 10.1172/JCI105463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMID R., FIGEN J. F., SCHWARTZ S. Experimental porphyria. IV. Studies of liver catalase and other heme enzymes in sedormid porphyria. J Biol Chem. 1955 Nov;217(1):263–274. [PubMed] [Google Scholar]
- SCHMID R., HAMMAKER L. METABOLISM AND DISPOSITION OF C14-BILIRUBIN IN CONGENITAL NONHEMOLYTIC JAUNDICE. J Clin Invest. 1963 Nov;42:1720–1734. doi: 10.1172/JCI104858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SJOSTRAND T. The formation of carbon monoxide by the decomposition of haemoglobin in vivo. Acta Physiol Scand. 1952;26(4):338–344. doi: 10.1111/j.1748-1716.1952.tb00915.x. [DOI] [PubMed] [Google Scholar]
- SNYDER A. L., SCHMID R. THE CONVERSION OF HEMATIN TO BILE PIGMENT IN THE RAT. J Lab Clin Med. 1965 May;65:817–824. [PubMed] [Google Scholar]
- Schmid R., Marver H. S., Hammaker L. Enhanced formation of rapidly labelled bilirubin by phenobarbital: hepatic microsomal cytochromes as a possible source. Biochem Biophys Res Commun. 1966 Aug 12;24(3):319–328. doi: 10.1016/0006-291x(66)90158-6. [DOI] [PubMed] [Google Scholar]
- TOLBERT B. M., KIRK M., BAKER E. M. Continuous C14O2 and CO2 excretion studies in experimental animals. Am J Physiol. 1956 May;185(2):269–274. doi: 10.1152/ajplegacy.1956.185.2.269. [DOI] [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A. 1968 Oct;61(2):748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschudy D. P., Marver H. S., Collins A. A model for calculating messenger RNA half-life: short lived messenger RNA in the induction of mammalian delta-aminolevulinic acid synthetase. Biochem Biophys Res Commun. 1965 Dec 9;21(5):480–487. doi: 10.1016/0006-291x(65)90408-0. [DOI] [PubMed] [Google Scholar]
- White P., Coburn R. F., Williams W. J., Goldwein M. I., Rother M. L., Shafer B. C. Carbon monoxide production associated with ineffective erythropoiesis. J Clin Invest. 1967 Dec;46(12):1986–1998. doi: 10.1172/JCI105688. [DOI] [PMC free article] [PubMed] [Google Scholar]





