Abstract
In order to obtain direct evidence for the existence of a natriuretic hormone, dialysates and ultrafiltrates of plasma of dogs expanded with saline were tested for effects on sodium transport by the toad urinary bladder. Dialysate was obtained by dialysis of blood in vivo in a clinical dialyzer and by dialysis in vitro of small volumes of blood using a miniature model of the clinical dialyzer. Ultrafiltrates were prepared using selective molecular filters which permit passage of substances on the basis of molecular weight and three dimensional configuration.
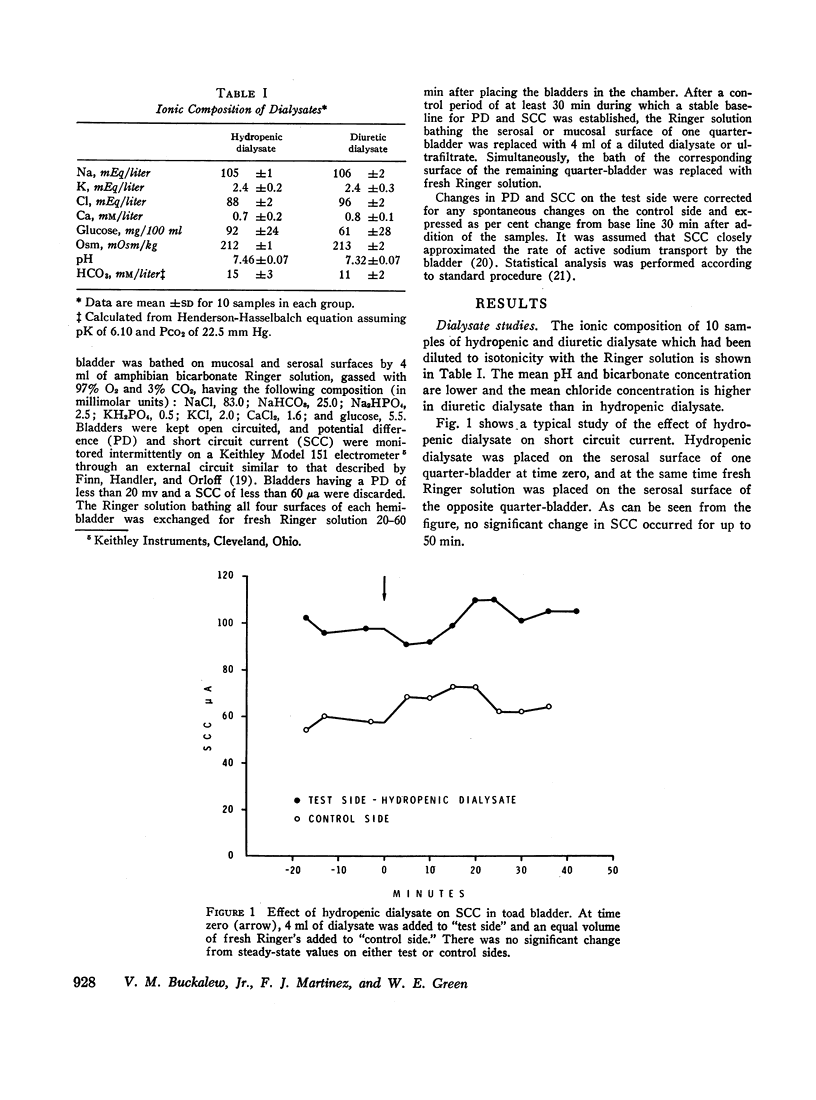
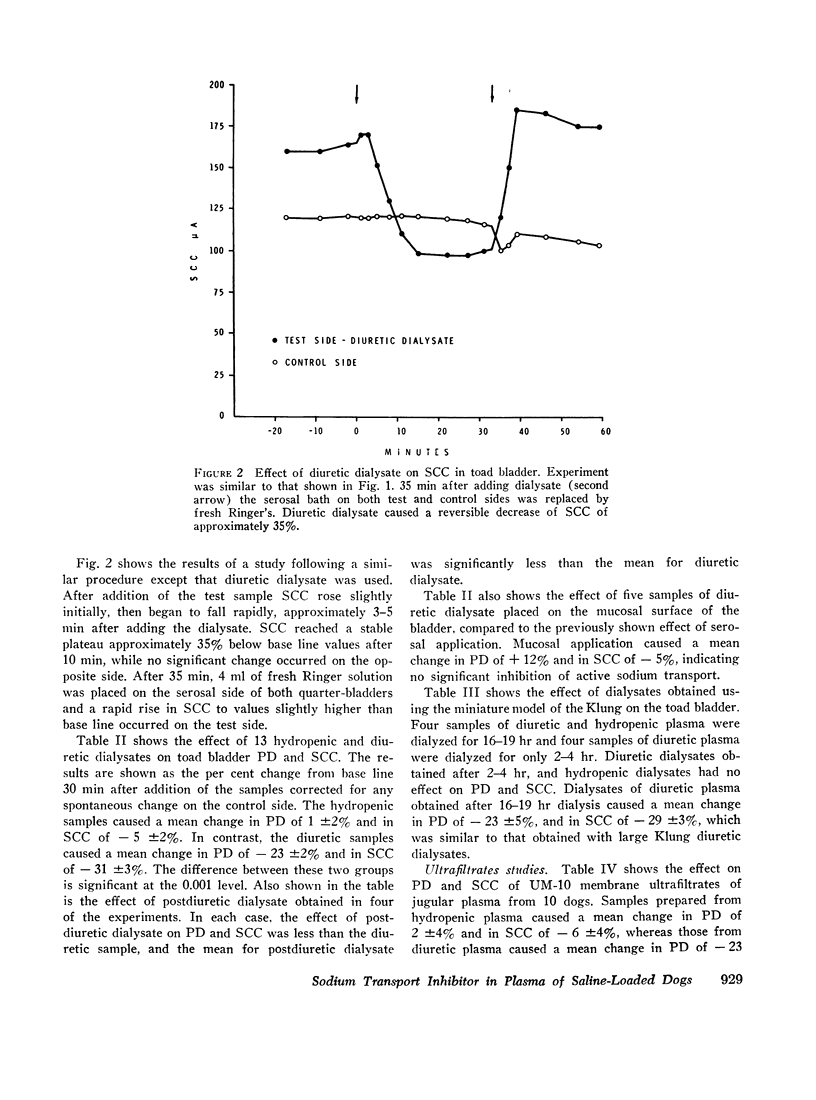
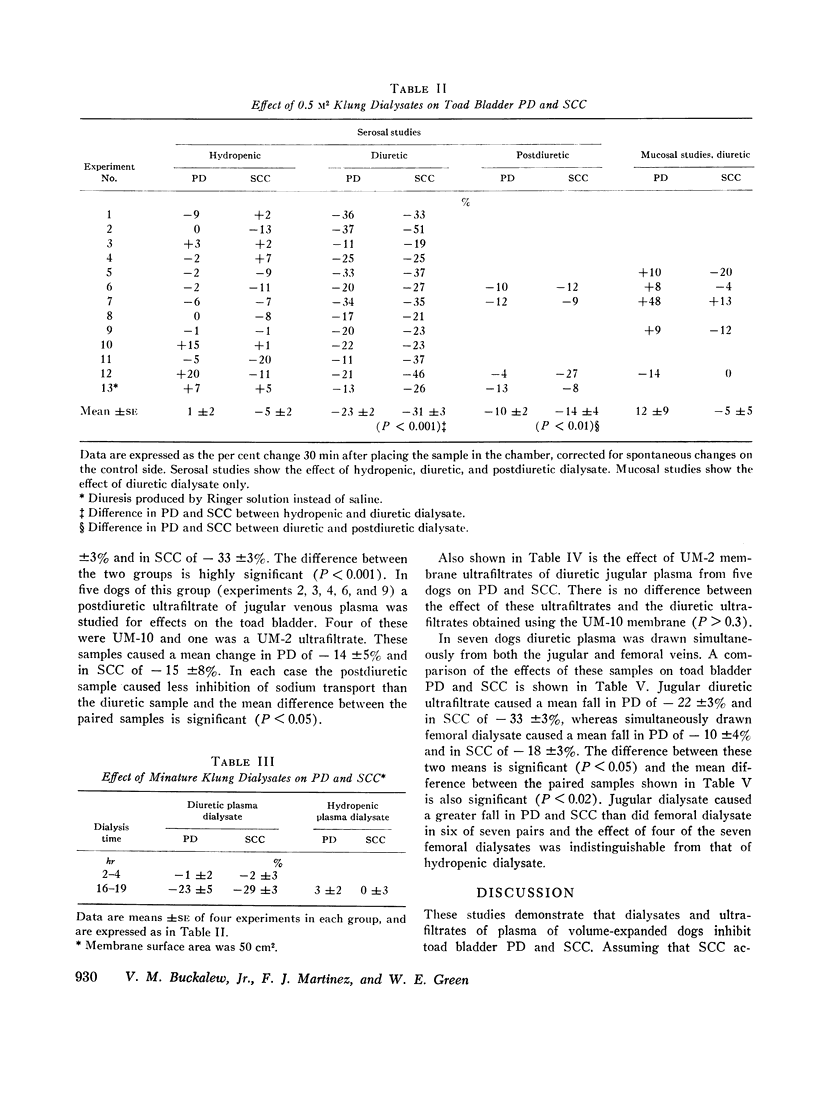
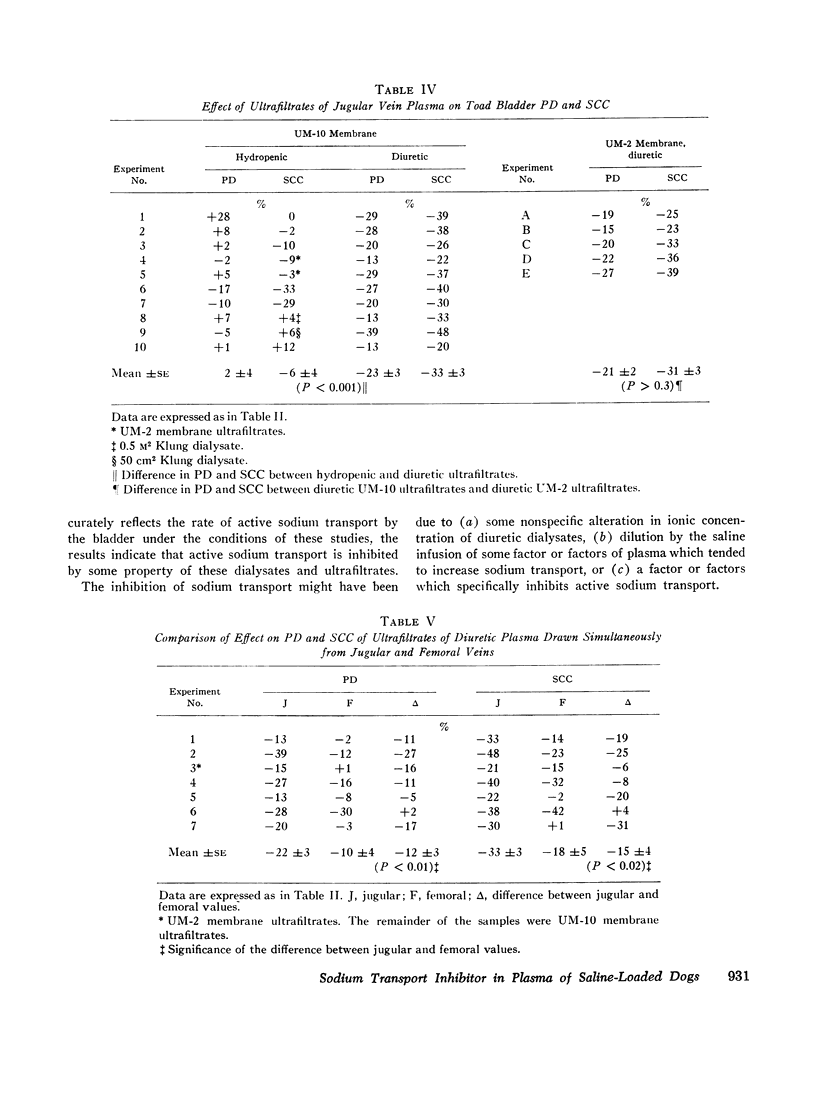
Dialysates and ultrafiltrates of hydropenic dogs caused a change in toad bladder potential difference of + 1% and in short circuit current of - 5%. In contrast, dialysates and ultrafiltrates from expanded dogs caused a change in potential difference of - 23% and in short circuit current of - 32%, a highly significant difference. Onset of reduction of short circuit current occurred within 3-5 min, reaching a maximum in 10-20 min. The effect was rapidly reversible, was specific for the serosal surface of the bladder, and could not be explained on the basis of nonspecific alterations in ionic composition or by dilutional effects. Ultrafiltrates of jugular vein plasma caused significantly more reduction of short circuit current than ultrafiltrates of femoral vein plasma. The data indicate the presence in plasma of saline-loaded dogs of a dialyzable inhibitor of toad bladder sodium transport. Ultrafiltrate studies using membranes of appropriate selectivity suggest the factor has a molecular weight of less than 3000.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER R. W., GALLETTI P. M. THE TRANSFER OF MACROMOLECULES IN AN ARTIFICIAL KIDNEY. Trans Am Soc Artif Intern Organs. 1965;11:95–98. doi: 10.1097/00002480-196504000-00019. [DOI] [PubMed] [Google Scholar]
- Bahlmann J., McDonald S. J., Ventom M. G., De Wardener H. E. The effect on urinary sodium excretion of blood volume expansion without changing the composition of blood in the dog. Clin Sci. 1967 Jun;32(3):403–413. [PubMed] [Google Scholar]
- Blatt W. F., Hudson B. G., Robinson S. M., Zipilivan E. M. Fractionation of protein solutions by membrane partition chromatography. Nature. 1967 Nov 4;216(5114):511–513. doi: 10.1038/216511b0. [DOI] [PubMed] [Google Scholar]
- Brenner B. M., Falchuk K. H., Keimowitz R. I., Berliner R. W. The relationship between peritubular capillary protein concentration and fluid reabsorption by the renal proximal tubule. J Clin Invest. 1969 Aug;48(8):1519–1531. doi: 10.1172/JCI106118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricker N. S., Klahr S., Purkerson M., Schultze R. G., Avioli L. V., Birge S. J. In vitro assay for a humoral substance present during volume expansion and uraemia. Nature. 1968 Sep 7;219(5158):1058–1059. doi: 10.1038/2191058a0. [DOI] [PubMed] [Google Scholar]
- CRABBE J. Stimulation of active sodium transport by the isolated toad bladder with aldosterone in vitro. J Clin Invest. 1961 Nov;40:2103–2110. doi: 10.1172/JCI104436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cort J. H., Dousa T., Pliska V., Lichardus B., Safárová J., Vranesić M., Rudinger J. Saluretic activity of blood during carotid occlusion in the cat. Am J Physiol. 1968 Oct;215(4):921–927. doi: 10.1152/ajplegacy.1968.215.4.921. [DOI] [PubMed] [Google Scholar]
- DE WARDENER H. E., MILLS I. H., CLAPHAM W. F., HAYTER C. J. Studies on the efferent mechanism of the sodium diuresis which follows the administration of intravenous saline in the dog. Clin Sci. 1961 Oct;21:249–258. [PubMed] [Google Scholar]
- DeHaven J. C., Shapiro N. Z. On the control of urine formation. Nephron. 1967;4(Suppl):1–63. [PubMed] [Google Scholar]
- Earley L. E., Daugharty T. M. Sodium metabolism. N Engl J Med. 1969 Jul 10;281(2):72–86. doi: 10.1056/NEJM196907102810205. [DOI] [PubMed] [Google Scholar]
- FRAZIER H. S., LEAF A. The electrical characteristics of active sodium transport in the toad bladder. J Gen Physiol. 1963 Jan;46:491–503. doi: 10.1085/jgp.46.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassina G., Carpenedo F., Santi R. Effect of prostaglandin E1 on isolated short-circuited frog skin. Life Sci. 1969 Feb 1;8(3):181–187. doi: 10.1016/0024-3205(69)90092-7. [DOI] [PubMed] [Google Scholar]
- Finn A. L., Handler J. S., Orloff J. Active chloride transport in the isolated toad bladder. Am J Physiol. 1967 Jul;213(1):179–184. doi: 10.1152/ajplegacy.1967.213.1.179. [DOI] [PubMed] [Google Scholar]
- Hunt J. R., Sadler J. H., Shinaberger J. H., Galletti P. M. Laboratory and clinical evaluation of a small countercurrent dialyzer, the Miniklung. Trans Am Soc Artif Intern Organs. 1968;14:109–113. [PubMed] [Google Scholar]
- Johnston C. I., Davis J. O. Evidence from cross circulation studies for a humoral mechanism in the natriuresis of saline loading. Proc Soc Exp Biol Med. 1966 Apr;121(4):1058–1063. doi: 10.3181/00379727-121-30965. [DOI] [PubMed] [Google Scholar]
- Johnston C. I., Davis J. O., Howards S. S., Wright F. S. Cross-circulation experiments on the mechanism of the natriuresis during saline loading in the dog. Circ Res. 1967 Jan;20(1):1–10. doi: 10.1161/01.res.20.1.1. [DOI] [PubMed] [Google Scholar]
- Johnston H. H., Herzog J. P., Lauler D. P. Effect of prostaglandin E1 on renal hemodynamics, sodium and water excretion. Am J Physiol. 1967 Oct;213(4):939–946. doi: 10.1152/ajplegacy.1967.213.4.939. [DOI] [PubMed] [Google Scholar]
- KESSLER G., WOLFMAN M. AN AUTOMATED PROCEDURE FOR THE SIMULTANEOUS DETERMINATION OF CALCIUM AND PHOSPHORUS. Clin Chem. 1964 Aug;10:686–703. [PubMed] [Google Scholar]
- LEAF A., ANDERSON J., PAGE L. B. Active sodium transport by the isolated toad bladder. J Gen Physiol. 1958 Mar 20;41(4):657–668. doi: 10.1085/jgp.41.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINSKY N. G., LALONE R. C. THE MECHANISM OF SODIUM DURESIS AFTER SALINE INFUSION IN THE DOG. J Clin Invest. 1963 Aug;42:1261–1276. doi: 10.1172/JCI104811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. B., Ferguson J. F. Prostaglandins and natriuresis: the effect of renal prostaglandins on PAH uptake by kidney cortex. Nature. 1969 Jun 21;222(5199):1185–1186. doi: 10.1038/2221185a0. [DOI] [PubMed] [Google Scholar]
- Levinsky N. G. Nonaldosterone influences on renal sodium transport. Ann N Y Acad Sci. 1966 Nov 22;139(2):295–303. doi: 10.1111/j.1749-6632.1966.tb41204.x. [DOI] [PubMed] [Google Scholar]
- Lichardus B., Pearce J. W. Evidence for a humoral natriuretic factor released by blood volume expansion. Nature. 1966 Jan 22;209(5021):407–409. doi: 10.1038/209407a0. [DOI] [PubMed] [Google Scholar]
- Martino J. A., Earley L. E. Demonstraton of a role of physical factors as determinants of the natriuretic response to volume expansion. J Clin Invest. 1967 Dec;46(12):1963–1978. doi: 10.1172/JCI105686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rector F. C., Jr, Martinez-Maldonado M., Kurtzman N. A., Sellman J. C., Oerther F., Seldin D. W. Demonstration of a hormonal inhibitor of proximal tubular reabsorption during expansion of extracellular volume with isotonic saline. J Clin Invest. 1968 Apr;47(4):761–773. doi: 10.1172/JCI105771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sealey J. E., Kirshman J. D., Laragh J. H. Natriuretic activity in plasma and urine of salt-loaded man and sheep. J Clin Invest. 1969 Dec;48(12):2210–2224. doi: 10.1172/JCI106187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright F. S., Brenner B. M., Bennett C. M., Keimowitz R. I., Berliner R. W., Schrier R. W., Verroust P. J., De Wardener H. E., Holzgreve H. Failure to demonstrate a hormonal inhibitor of proximal sodium reabsorption. J Clin Invest. 1969 Jun;48(6):1107–1113. doi: 10.1172/JCI106067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright F. S., Knox F. G., Howards S. S., Berliner R. W. Reduced sodium reabsorption by the proximal tubule of Doca-escaped dogs. Am J Physiol. 1969 Apr;216(4):869–875. doi: 10.1152/ajplegacy.1969.216.4.869. [DOI] [PubMed] [Google Scholar]



