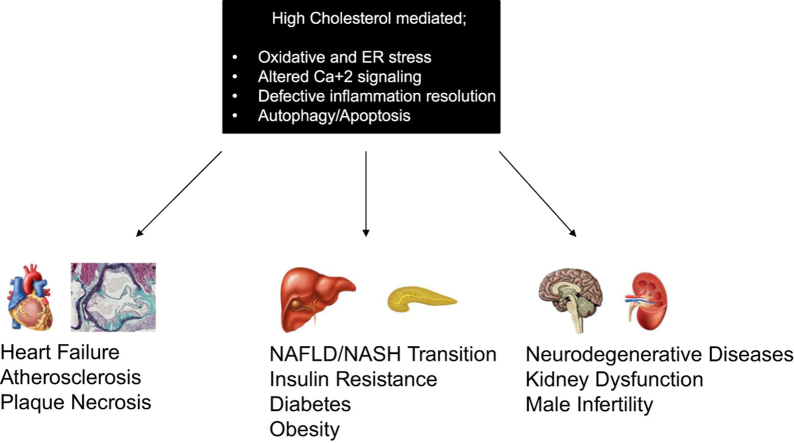
Abstract
Endoplasmic reticulum (ER) is the major site of protein folding and calcium storage. Beside the role of ER in protein homeostasis, it controls the cholesterol production and lipid-membrane biosynthesis as well as surviving and cell death signaling mechanisms in the cell. It is well-documented that elevated plasma cholesterol induces adverse effects in cardiovascular diseases (CVDs), liver disorders, such as non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatosis hepatitis (NASH), and metabolic diseases which are associated with oxidative and ER stress. Recent animal model and human studies have showed high cholesterol and ER stress as an emerging factors involved in the development of many metabolic diseases. In this review, we will summarize the crucial effects of hypercholesterolemia and ER stress response in the pathogenesis of CVDs, NAFLD/NASH, diabetes and obesity which are major health problems in western countries.
Abbreviations: ATF6, activating transcription factor 6; CHOP, C/EBP-homologous protein; CVD, cardiovascular disease; eIF2α, eukaryotic translation initiator factor 2α; ER, endoplasmic reticulum; GRP78, glucose regulated protein 78; IDL, intermediate-density lipoprotein; IRE1, inositol requiring kinase 1; JNK, c-Jun N-terminal kinase; LDL, low-density lipoprotein; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatosis hepatitis; Ox-LDL, oxidized-LDL; PERK, RNA-activated protein kinase-like endoplasmic reticulum kinase; ROS, reactive oxygen species; SREBP-1c, sterol regulatory element binding protein 1c; UPR, unfolded protein response; VLDL, very-low-density lipoprotein; XBP-1, X binding protein-1
Keywords: Endoplasmic reticulum stress, High cholesterol, Cardiovascular diseases, Non-alcoholic fatty liver disease, Non-alcoholic steatosis hepatitis
Graphical abstract
Highlights
-
•
Endoplasmic reticulum stress involves in various metabolic disease development.
-
•
Altered cholesterol metabolism is a well-documented inducer of ER stress.
-
•
ER stress mediated apoptosis leads many cardiovascular disorders.
-
•
UPR might lead NAFLD/NASH progression by enhancing inflammation and fibrosis.
1. Introduction to LDL cholesterol and ER stress
Cholesterol is a crucial component that maintains the fluidity and permeability of cell membrane in vertebrates. Cholesterol enters the circulation by two ways; i) intestinal absorption following digestion, ii) de novo synthesis of cholesterol which is primarily carried out by the liver [1]. Free cholesterol might be stored in liver by forming cholesterol esters or assembled into very-low-density lipoprotein (VLDL), together with triglycerides and apolipoproteins, followed by the secretion to blood circulation. During the circulation in blood, VLDL lose its triglyceride content, by lipoprotein lipase and hepatic lipase, and converted to intermediate-density lipoprotein (IDL), followed by low-density lipoprotein (LDL) [2]. Cholesterol enriched LDL particles are taken by LDL receptor which is highly expressed in liver hepatocytes [3].
It is well documented that oxidative stress, induced by reactive oxygen species (ROS), enhances the development of various diseases through damaging DNA, proteins, carbohydrates and lipids [4]. While nicotinamide adenine dinucleotide phosphate (NADPH) oxidases, cyclooxygenases, xanthine oxidase, peroxidases and mitochondrial respiratory chain are the enzymatic generators of ROS formation; catalase, superoxide dismutase (SOD), glutathione reductase, and heme oxygenase (HO) are belong to the antioxidant enzymatic mechanisms [5]. Excessive levels of ROS, described as oxidative stress, is derived by the imbalance between cellular ROS production and antioxidant systems. LDL oxidation, as a result of ROS exposure, induces the oxidized LDL (Ox-LDL) formation which then taken by macrophages via scavenger receptors and lead atherogenesis [6].
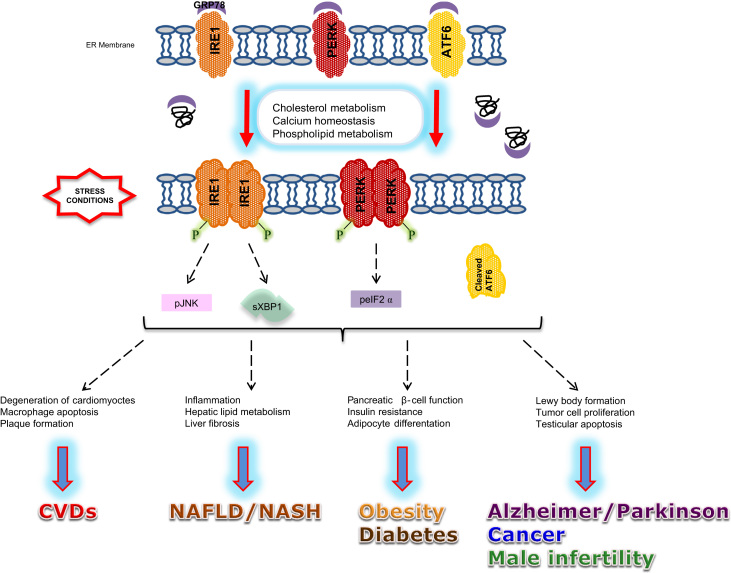
Endoplasmic reticulum (ER) is the major organelle for properly folding and post-translational modifications of proteins in addition to control production of cholesterol and lipid-membrane biosynthesis and acts as a major intracellular calcium reservoir in the cell, which maintains homeostasis of the organisms. Disruption of quality control mechanisms by a number of pathological and physiological reasons, which are mainly related with oxidative stress induction, results in accumulation of misfolded/unfolded proteins followed by increased ER stress. The high-fat diet was also identified as an activator of ER stress in liver [7]. Unfolded protein response (UPR) is complementary adaptive machinery, against ER stress, organized by three ER transmembrane receptor proteins; Inositol requiring kinase 1 (IRE1), double-stranded RNA-activated protein kinase (PKR)-like endoplasmic reticulum kinase (PERK) and activating transcription factor 6 (ATF6). Under unstressed conditions, glucose regulated protein 78 (GRP78) binds to these ER transmembrane receptor proteins and keeps them in an inactive state. Following the stress, unfolded/misfolded protein accumulation enhance the release of GRP78 from IRE1, PERK and ATF6 and leads their activation [8]. Each of these sensor proteins uses a unique mechanism to induce transcription factors and upregulate UPR target genes. As a first step, induction of chaperone molecules (GRP78, GRP94, PDI etc.) transcription occurs. Additionally, PERK activation leads eukaryotic translation initiator factor 2α (eIF2α) phosphorylation and inactivation declines mRNA translation and protein load on the ER. Due to the time of exposure and intensity of stress, aggregated proteins and damaged organelles might be removed by UPR-induced proteasome and autophagy [9]. If the stress is too severe and excess the capacity of these defense mechanisms, cells switch to autophagic or apoptotic cell death mechanisms (Fig. 1) [10], [11].
Fig. 1.
Mechanism of ER stress and UPR and its relation to metabolic diseases. Accumulation of unfolded/misfolded proteins, due to the altered cholesterol metabolism, induces ER stress, which then accelerates the release of GRP78 from IRE1, PERK and ATF6. In various cell types and different pathological conditions, release of GRP78 activates UPR, which is involved in the progression of metabolic disorders. ATF6, activating transcription factor 6; CVD, cardiovascular disease; peIF2α, phospho eIF2α; GRP78, glucose regulated protein 78; IRE1, inositol requiring kinase 1; pJNK, phospho JNK; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatosis hepatitis; PERK, RNA-activated protein kinase-like endoplasmic reticulum kinase; sXBP-1, spliced XBP1.
Hypercholesterolemia, a close form of hyperlipidemia, is a metabolic disorder described by increased cholesterol levels in circulation. In regard to clinical experiments, in developed countries, a significant number of adult population has hypercholesterolemia [12]. Various metabolic diseases, particularly cardiovascular diseases (CVDs), and non-alcoholic fatty liver diseases (NAFLD), is shown to be associated with stress conditions, including oxidative and ER stress, in both in vitro/in vivo and clinical studies. In this review we aim to summarize the current literature related to hypercholesterolemia mediated ER stress and leading metabolic diseases. Our discussion is focused on both in vivo and clinical data as a potential argument of ER stress modulation in CVDs, and NAFLD).
2. ER Stress related cardiovascular diseases
Cardiovascular system consisting, hematopoietic, vascular and cardiac components, is a transport system that transports nutrients and oxygen to all organs and collects metabolic waste through the venous and capillary network. CVDs are the leading cause of death, with highest mortality and morbidity rates worldwide. In regard to WHO report, an estimated 17.5 million people died from CVDs in 2012 that represents 31% of all global deaths [13]. High blood cholesterol levels are well recognized as a risk factor of CVD development. Epidemiological studies have shown that elevated LDL cholesterol levels, among genetic and environmental factors, can induce CVDs development even the absence of other risk factors [14]. In this section of our review, we aim to summarize the effect of ER stress on vascular and cardiac functions including heart failure, ischemia/reperfusion and atherosclerosis (Fig. 2).
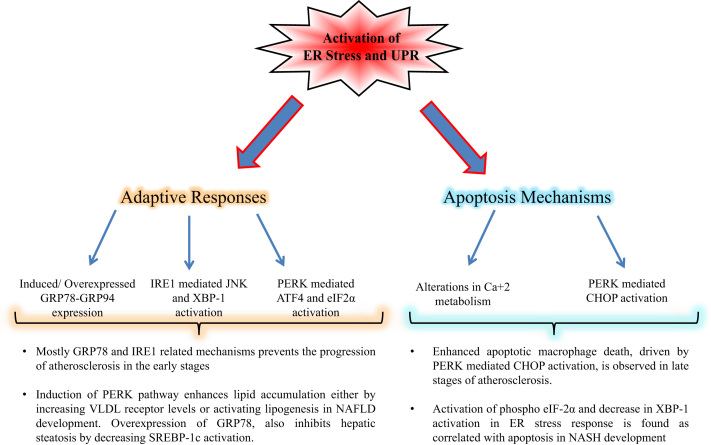
Fig. 2.
Fate of cell in response to ER stress involves in progression of cardiovascular and liver diseases. During basal stress conditions, adaptive response mechanisms of UPR control the defending of homeostasis against apoptosis. While increased of co-chaperone expressions, such as GRP78 and GRP94, maintains the true folding of proteins, activated IRE1 and PERK branches enhance autophagy and inhibition of translation respectively. Under severe stress, UPR mechanisms switches too apoptosis to eliminate irreversibly cell damage, mainly through CHOP activation.
2.1. In vitro/In vivo Experiments
We have previously reviewed the role of ER stress on CVDs [15], however the addition of recent literature in this field made this update essential. The correlation of CVDs and ER stress has been reported in various studies. It has been shown that Angiotensin II (AngII), well-documented cardiac hypertrophy mediator, might enhance ER stress related cell death signaling pathways by increasing C/EBP-homologous protein (CHOP) expression in rat cardiomyocytes [16]. Furthermore, it has been observed that deletion of p53-upregulated modulator of apoptosis (PUMA), an apoptosis initiator and an UPR gene product, inhibits cardiac myocyte death and improves cardiac function during ischemia/reperfusion periods [17].
On the other hand, detailed studies have revealed that overexpression of ER chaperones GRP78 and GRP94 protect cardiac muscle cells from UPR mediated death by different mechanisms. Using cultured cardiomyocytes, GRP94 overexpression is shown to reduce cell death following calcium overload [18], while GRP78 overexpression increase the viability by inhibiting CHOP levels and apoptosis [19]. Activation of ATF6 has been suggested as a protective signal for cardiomyocytes following damage in ischemia. Supporting in vivo studies identified that ATF6 overexpression results an increase in ventricular pump function [20], while ATF6 inhibition induces mortality rate [21].
Additionally, a number of in vitro and in vivo studies identified the role of ER stress response in atherosclerosis progression by disruption of ER function and development of smooth muscle cell, endothelial cell and macrophage death in arterial wall [22], [23]. Hyperhomocysteinemia mediated atherosclerotic lesion size increase has found to related with the induction of phospho PERK, GRP78 and GRP94 expressions in ApoE−/− mice [24]. Also, endothelial CHOP upregulation was found to be correlated to cell apoptosis and larger plaque size either in a hyperhomocysteinemic rabbit model [25] or following 7-ketocholesterol treatment [26].
2.2. Human Experiments
Proper synthesis and functional folding of proteins in ER plays crucial roles to maintain the function of heart, especially cardiomyocytes. ER-related functions are reported as leading factors for cardiac pathology and physiology through the increased ER tubule proliferation against stress conditions in cardiomyocytes [27]. Several studies showed that, both GRP78 and spliced XBP-1, well-known ER stress markers, were increased in the patients with heart failure which confirms the UPR induction in heart failure in humans [28], [29].
Atherosclerosis is one of the major CVDs which development occurs with increased ROS formation and ER stress response depending to early or late stage of the disease. While pro-survival mechanisms of UPR is activated in the early stage, cell death mechanisms, mainly driven PERK mediated CHOP activation, are observed in smooth muscle cells and macrophages during the late stages of atherosclerosis development [30]. In order to evaluate the interaction between ER stress and atherosclerotic plaque rupture, 152 human coronary artery autopsy samples were used and a significant induction in GRP78 and CHOP expressions were determined [31]. Additionally, another study by using 107 human carotid plaque samples, reported that while CHOP and other apoptosis-related genes were higher in the tissue around the necrotic core rather than periphery, both nuclear erythroid-related factor 2 (Nrf2)-related survival genes and mRNA/protein levels of PERK more expressed in periphery area compared to necrotic core [32].
3. ER Stress related non-alcoholic fatty liver disease
NAFLD, a chronic inflammation disease characterized with lipid accumulation, is becoming the most common liver disorder worldwide. Comparison of plasma and liver lipid levels demonstrated an increase of triglycerides, cholesterol and free fatty acids in patients with non-alcoholic steatosis hepatitis (NASH) compared to patients without NASH [33], [34]. Animal models and human studies suggested that high cholesterol levels also enhances ER stress induced NAFLD and NASH. Differently from our previous review [35], here we will discuss the role of ER stress response in the development of NAFLD/NASH in the light of cell culture, animal and clinical studies (Fig. 2).
3.1. In vitro/In vivo Experiments
In recent years, ER stress response has been identified as a crucial mechanism in many liver disorders, including NAFLD-NASH transition, by leading inflammation, lipid biosynthesis and apoptosis [7]. It has been shown that treatment of mice with pharmacologic ER stress inducers, such as thapsigargin and tunicamycin, leads lipid accumulation in liver. These data indicated that tunicamycin increases VLDL receptor expression, in mice, by mediating PERK-ATF4 branch of ER stress response [36]. On the other side, thapsigargin treatment leads to the activation of sterol regulatory element binding protein 1c (SREBP-1c) followed by an increase in hepatic lipogenesis [37]. Additionally, it is suggested that induction of PERK-peIF2α branch in response to ER stress enhances lipid accumulation through SREBP-1c activation in hepatocytes [38].
On the other hand, it has been demonstrated that both phosphorylation of c-Jun N-terminal kinase (JNK) and deficiency of hepatic XBP-1 are in correlation with NASH development which proves the role of ER stress response in the pathogenesis of disease [39]. Liver-specific XBP-1 deletion in mice has demonstrated a decrease in hepatic lipogenesis followed by inhibited lipid accumulation following lipogenic diets [40]. However, overexpression of GRP78, the major modulator of UPR, also inhibits hepatic steatosis by decreasing SREBP-1c activation, through inhibition, against ER stress response in ob/ob mice [41]. In another approach, caffeine treatment has shown its beneficial effect by decreasing UPR parameters, such as PERK, IRE1, GRP78 and CHOP, along with lipogenesis and inflammation associated markers following NAFLD development [42].
3.2. Human experiments
The effect of cholesterol metabolism alterations in NASH development has also implicated in a number of clinical studies. While the patients with NAFLD is characterized by hepatic fat accumulation (steatosis) without inflammation or fibrosis, 10–30% of NAFLD individuals shows the progression of NASH, which is defined by additional hepatic inflammation and fibrosis in addition to steatosis. Increased levels of NLRP3, a protein involves in inflammasome formation, is determined in liver biopsies of patients with NASH compared to patients without NASH [43]. Although, there is still need further studies to establish a certain mechanism between ER stress response and inflammasome in the liver.
It is reported that, a variety of UPR components is induced in the liver of NAFLD/NASH patients. Whereas, phospho eIF-2α branch of ER stress response is induced in both NAFLD and NASH patients, related activating transcription factor 4 (ATF4), CHOP, and GADD34 expressions were not effected in most subjects. Additionally, NASH development that is associated with a decrease in XBP-1 activation is found as correlated with an activation of JNK via IRE1 pathway followed by apoptosis [44]. In another study, human liver biopsies are classified as normal, steatosis and NASH, an induction in ER stress response related autophagy and apoptosis genes are also observed, which may regulate the transition from steatosis to NASH, as well as protein expression of spliced XBP-1 and nuclear localization of total XBP-1, while no change observed in ER stress response proteins such as ATF4, CHOP, phospho JNK and phospho eIF2α in patients with NASH compared to normal subjects [45].
4. ER Stress related insulin resistance, obesity, and diabetes
Increased prevalence of metabolism affections, including high cholesterol, gathered into the “Metabolic Syndrome” term, which is associated with insulin resistance (decreased glucose transport inside the cell in regard to the degenerated insulin signaling), obesity (fat accumulation in adipose tissue), and diabetes (altered secretion of insulin in pancreas). This part of our review summarizes the current literature of UPR progression in the development of insulin resistance, obesity and diabetes.
UPR is also crucial for proper function of secretory cells such as insulin producing β-cells that contains high protein synthesis levels. In regard to the high insulin requirement, pancreatic β-cells contains plenty of ER to ensure proper synthesis/folding of pro-insulin. Related studies in type 2 diabetes have shown the importance of UPR in pancreatic β-cell death. It has determined that PERK deficient mice models and pancreatic β-cells are more sensitive to develop neonatal hyperglycemia which is associated with islet proliferation defects by increased apoptosis [46]. Additionally, elF2α phosphorylation failure in mice is defined in the development of diabetes in regard to the improper translation of pro-insulin, increased ROS, reduced expression of β-cell-specific genes, and apoptosis [47].
ER stress response, following high fat diet supplementation, has also been observed in insulin resistance mechanisms. Genetic deletion of XBP-1 in mouse models resulted with an increased sensitivity to ER stress followed by insulin resistance, and insulin receptor substrate-1 (IRS-1) degradation, which inhibits the insulin receptor signaling [48]. It has been also shown that, enhanced ER stress activation leads to serine phosphorylation of IRS-1, followed by promoting insulin resistance through disassociating IRS-1 from insulin receptor signaling in high fat diet mediated obesity [49].
Oxidative stress and related UPR development has been determined in the etiology of insulin resistance in obesity. In obese patients compared to individuals with normal weight, due to dysregulated antioxidant capacity such as decreased activities of SOD, glutathione peroxidase and catalase, increased amount of ROS in observed [50]. Chronic activation of UPR has been also implicated in adipose tissue of genetic and dietary murine models of obesity together with obese individuals [7], [51], [52]. GRP78 overexpression in the liver of ob/ob mice is shown to reduce hepatic steatosis followed by impaired insulin signaling [41], in addition to up-regulated ERAD and chaperone proteins including calnexin, calreticulin and GRP94 in adipose tissue [53], which proves UPR malfunction in the liver, and adipose tissue of obese mice. Contrary, feeding healthy men with a regular U.S diet (50% carbohydrate, 35% fat, and 15% protein) for one week resulted in an enhanced insulin resistance following oxidative stress mediated GLUT4 carbonylation in adipose tissue together with no activation on ER stress response [54].
5. ER Stress related other diseases
ER stress and related UPR is also a hallmark for various diseases such as neurodegenerative diseases, cancer, kidney injury and male infertility. Below, we will briefly discuss the effect of ER stress response on these disease models.
It has been reported that β-amyloid plaques and α-synuclein accumulation, well-defined parameters of Alzheimer's and Parkinson's disease, is a critical inducer of ER stress. By using dopaminergic neurons from Parkinson's disease patients, phosphorylated forms of PERK and eIF2α were shown as co-localized with α-synuclein, which is the major component in formation of Lewis bodies [55]. Pathological studies using brains of Alzheimer's patients have revealed the presence of spliced XBP-1 and phosphorylated IRE1α [56] whereas in vitro and in vivo studies proved β-amyloid mediated CHOP induction [57]. Additionally, we have reviewed the involvement of ER stress in the progression of ALS in the light of proteasomal degradation, autophagy and apoptosis [58].
Nutrient deprivation, low oxygen supply and acidic pH, well-known markers of tumor development, are also defined as an activator of ER stress. IRE1α and PERK, two main signaling branch of UPR, are described as a critical factor for tumor cell survival and proliferation [59], [60]. Additionally, increased GRP78 levels are observed in a number of malignancies, which is correlated with survival mechanisms followed by chemoresistance to therapy in cancer cell lines and clinical tumor samples [61].
It is determined that increased aggregation of oxidative carbonylated GRP78 and PDI, with age, might result with ER associated kidney dysfunction [62]. Investigating ER stress response in human diabetic kidneys reports elevated GRP78, XBP-1 and calnexin mRNA levels in patients with established diabetes compared to basal diabetes [63]. Additionally, ER-resident chaperone GRP78 was found as highly expressed in the samples of renal disease patients [64]. Also, by using human kidney transplant biopsies, GRP78 was observed in co-expression with inflammatory transcription factor NF-κB which suggests the role of ER stress in tubular inflammation activation [65]. On the other hand, ER stress mediated autophagic activity might be described as a protective mechanism against ER stress mediated apoptotic death of renal cells. Tomino et al. [66] indicated that podocyte-specific Atg7 deficient mice, demonstrated high levels of proteinuria together with an induction in ER stress response.
Male infertility contains approximately 50% of infertility issues, and elevated plasma cholesterol levels might enhance male infertility by decreasing semen quality and sperm concentration. By using both mouse and human testis tissues, high GRP78 staining has reported in postmeiotic germ cells and spermatocytes with no staining in spermatogonium [67]. Additionally, while one cycle of hyperthermia induces survival mechanisms of UPR by activates PERK/eIF2α and IRE1α/XBP-1 branches, repetitive cycles of hyperthermia promote ER stress-mediated apoptosis through CHOP, phospho JNK, and caspase-3 activation [68].
6. Future directions and conclusion
Investigation of ER stress and UPR signaling has a number of difficulties. Since brain, heart, vessels, kidney etc. have multiple cell types, different branches of UPR is activated, in response to stress to restore homeostasis. Further difficulties occur when studying on UPR, such as determining to quantify mRNA or protein levels of ER stress markers, although, the best way is to measure both mRNA and protein levels. Table 1 shows the well-known ER stress response genes that have been identified to be associated with a wide range of metabolic/non-metabolic disorders.
Table 1.
Role of ER stress response genes in various disorders.
| Genes | Samples/Disease | Finding | Reference |
|---|---|---|---|
| GRP78/GRP94 | |||
| Cultured cardiomyocytes | Individually, GRP78 and GRP94 overexpression increased viability by reducing apoptosis | [18], [19] | |
| Patients with heart failure | Increased GRP78 expression and sXBP-1 induction was determined | [28], [29] | |
| ob/ob mice | Overexpression of GRP78 showed protective affects against hepatic steasosis | [41], [53] | |
| Cancer cell lines and human tumor samples | Induced GRP78 levels confers chemoresistance by increasing cell viability | [61] | |
| Human diabetic kidneys | Elevated GRP78 levels were detected in patients with established diabetes compared to basal diabetes individuals | [63], [64] | |
| IRE1/XBP-1 | |||
| Liver specific XBP-1 deficient mice | XBP-1 deficiency resulted a decrease in hepatic lipogenesis and lipid accumulation | [40] | |
| Human liver samples | Induction of IRE1 and sXBP-1 expressions was observed in patients with NASH | [44], [45] | |
| Mice testis samples | IRE1/XBP-1 branch acts as a survival response of ER stress against one cycle of hyperthermia | [68] | |
| PERK/ATF4–eIF2α | |||
| Human carotid plaque samples | PERK expression was found more expressed in periphery compared to necrotic core | [32] | |
| Primary hepatocytes | PERK related signaling pathways induces VLDL receptor expression and lipid accumulation | [36], [38] | |
| Type 2 diabetes | Individually, PERK deficiency and elF2α phosphorylation failure was resulted in increased apoptosis in pancreatic β-cells | [46], [47] | |
| Dopaminergic neurons of Parkinson's patients | Phospho PERK and eIF2α is shown to involve in Lewis body formation | [55] | |
| CHOP | |||
| Cultured cardiomyocytes | Angiotensin II induces apoptosis by increasing CHOP expression | [16] | |
| Atherosclerosis development in mice | CHOP upregulation was found to be correlated with endothelial cell apoptosis | [25], [26] | |
| Human coronary artery samples | Enhanced CHOP and GRP78 expression was observed | [31] | |
| Mice testis tissues | ER stress related apoptosis mechanisms, such as CHOP, were induced by repetitive cycles of hyperthermia | [68] | |
Researches to reveal the relationship between hypercholesterolemia and ER stress continues to expand and build on a theory regarding its role in various metabolic diseases. Here we have focused on hypercholesterolemia mediated ER stress, and further support with cell culture, animal and clinical studies. Despite the increased number of studies on this area, further studies are still needed to identify the underlying signaling pathways and define efficient therapeutics.
Acknowledgment
The authors acknowledge funding from The Scientific and Technological Research Council of Turkey (TUBITAK) 115S464 and Marmara University Research Fund SAG-C-DRP-130515-0164.
References
- 1.Ikonen E. Cellular cholesterol trafficking and compartmentalization. Nat. Rev. Mol. Cell Biol. 2008;9(2):125–138. doi: 10.1038/nrm2336. [DOI] [PubMed] [Google Scholar]
- 2.Hussain M.M. Intestinal lipid absorption and lipoprotein formation. Curr. Opin. Lipido. 2014;25(3):200–206. doi: 10.1097/MOL.0000000000000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstein J.L., Brown M.S. The LDL receptor. Arterioscler Thromb. Vasc. Biol. 2009;29(4):431–438. doi: 10.1161/ATVBAHA.108.179564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valko M. Free radicals and antioxidants in normal physiological functions and human disease. Int J. Biochem Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Droge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82(1):47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 6.Tabas I., Bornfeldt K.E. Macrophage phenotype and function in different stages of atherosclerosis. Circ. Res. 2016;118(4):653–667. doi: 10.1161/CIRCRESAHA.115.306256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ozcan U. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306(5695):457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 8.Ron D., Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007;8(7):519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 9.Kroemer G., Marino G., Levine B. Autophagy and the integrated stress response. Mol. Cell. 2010;40(2):280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basseri S., Austin R.C. Endoplasmic reticulum stress and lipid metabolism: mechanisms and therapeutic potential. Biochem Res Int. 2012;2012:841362. doi: 10.1155/2012/841362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gotoh T., Endo M., Oike Y. Endoplasmic reticulum stress-related inflammation and cardiovascular diseases. Int J. Inflam., 2011. 2011:259462. doi: 10.4061/2011/259462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuklina E.V., Yoon P.W., Keenan N.L. Trends in high levels of low-density lipoprotein cholesterol in the United States, 1999–2006. JAMA. 2009;302(19):2104–2110. doi: 10.1001/jama.2009.1672. [DOI] [PubMed] [Google Scholar]
- 13.Mendis S., Davis S., Norrving B. Organizational update: the world health organization global status report on noncommunicable diseases 2014; one more landmark step in the combat against stroke and vascular disease. Stroke. 2015;46(5):e121–e122. doi: 10.1161/STROKEAHA.115.008097. [DOI] [PubMed] [Google Scholar]
- 14.Yancy C.W. ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):1810–1852. doi: 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 15.Sozen E., Karademir B., Ozer N.K. Basic mechanisms in endoplasmic reticulum stress and relation to cardiovascular diseases. Free Radic. Biol. Med. 2015;78:30–41. doi: 10.1016/j.freeradbiomed.2014.09.031. [DOI] [PubMed] [Google Scholar]
- 16.Okada K. Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation. 2004;110(6):705–712. doi: 10.1161/01.CIR.0000137836.95625.D4. [DOI] [PubMed] [Google Scholar]
- 17.Toth A. Targeted deletion of Puma attenuates cardiomyocyte death and improves cardiac function during ischemia-reperfusion. Am. J. Physiol. Heart Circ. Physiol. 2006;291(1):H52–H60. doi: 10.1152/ajpheart.01046.2005. [DOI] [PubMed] [Google Scholar]
- 18.Vitadello M. Overexpression of the stress protein Grp94 reduces cardiomyocyte necrosis due to calcium overload and simulated ischemia. FASEB J. 2003;17(8):923–925. doi: 10.1096/fj.02-0644fje. [DOI] [PubMed] [Google Scholar]
- 19.Fu H.Y. Overexpression of endoplasmic reticulum-resident chaperone attenuates cardiomyocyte death induced by proteasome inhibition. Cardiovasc Res. 2008;79(4):600–610. doi: 10.1093/cvr/cvn128. [DOI] [PubMed] [Google Scholar]
- 20.Martindale J.J. Endoplasmic reticulum stress gene induction and protection from ischemia/reperfusion injury in the hearts of transgenic mice with a tamoxifen-regulated form of ATF6. Circ. Res. 2006;98(9):1186–1193. doi: 10.1161/01.RES.0000220643.65941.8d. [DOI] [PubMed] [Google Scholar]
- 21.Toko H. ATF6 is important under both pathological and physiological states in the heart. J. Mol. Cell Cardiol. 2010;49(1):113–120. doi: 10.1016/j.yjmcc.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 22.Kedi X. Free cholesterol overloading induced smooth muscle cells death and activated both ER- and mitochondrial-dependent death pathway. Atherosclerosis. 2009;207(1):123–130. doi: 10.1016/j.atherosclerosis.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 23.Moore K.J., Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145(3):341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou J. Association of multiple cellular stress pathways with accelerated atherosclerosis in hyperhomocysteinemic apolipoprotein E-deficient mice. Circulation. 2004;110(2):207–213. doi: 10.1161/01.CIR.0000134487.51510.97. [DOI] [PubMed] [Google Scholar]
- 25.Zulli A. High dietary taurine reduces apoptosis and atherosclerosis in the left main coronary artery: association with reduced CCAAT/enhancer binding protein homologous protein and total plasma homocysteine but not lipidemia. Hypertension. 2009;53(6):1017–1022. doi: 10.1161/HYPERTENSIONAHA.109.129924. [DOI] [PubMed] [Google Scholar]
- 26.Pedruzzi E. NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol. Cell Biol. 2004;24(24):10703–10717. doi: 10.1128/MCB.24.24.10703-10717.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maron B.J., Ferrans V.J., Roberts W.C. Ultrastructural features of degenerated cardiac muscle cells in patients with cardiac hypertrophy. Am. J. Pathol. 1975;79(3):387–434. [PMC free article] [PubMed] [Google Scholar]
- 28.Dally S. Compartmentalized expression of three novel sarco/endoplasmic reticulum Ca2+ATPase 3 isoforms including the switch to ER stress, SERCA3f, in non-failing and failing human heart. Cell Calcium. 2009;45(2):144–154. doi: 10.1016/j.ceca.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Sawada T. X-box binding protein 1 regulates brain natriuretic peptide through a novel AP1/CRE-like element in cardiomyocytes. J. Mol. Cell Cardiol. 2010;48(6):1280–1289. doi: 10.1016/j.yjmcc.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Zhou A.X., Tabas I. The UPR in atherosclerosis. Semin Immunopathol. 2013;35(3):321–332. doi: 10.1007/s00281-013-0372-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myoishi M. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation. 2007;116(11):1226–1233. doi: 10.1161/CIRCULATIONAHA.106.682054. [DOI] [PubMed] [Google Scholar]
- 32.Garbin U. Do oxidized polyunsaturated Fatty acids affect endoplasmic reticulum stress-induced apoptosis in human carotid plaques? Antioxid. Redox Signal. 2014;21(6):850–858. doi: 10.1089/ars.2014.5870. [DOI] [PubMed] [Google Scholar]
- 33.Puri P. The plasma lipidomic signature of nonalcoholic steatohepatitis. Hepatology. 2009;50(6):1827–1838. doi: 10.1002/hep.23229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Puri P. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46(4):1081–1090. doi: 10.1002/hep.21763. [DOI] [PubMed] [Google Scholar]
- 35.Bozaykut P. Endoplasmic reticulum stress related molecular mechanisms in nonalcoholic steatohepatitis. Mech. Ageing Dev. 2016;157:17–29. doi: 10.1016/j.mad.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Jo H. Endoplasmic reticulum stress induces hepatic steatosis via increased expression of the hepatic very low-density lipoprotein receptor. Hepatology. 2013;57(4):1366–1377. doi: 10.1002/hep.26126. [DOI] [PubMed] [Google Scholar]
- 37.Lee J.N., Ye J. Proteolytic activation of sterol regulatory element-binding protein induced by cellular stress through depletion of Insig-1. J. Biol. Chem. 2004;279(43):45257–45265. doi: 10.1074/jbc.M408235200. [DOI] [PubMed] [Google Scholar]
- 38.Bobrovnikova-Marjon E. PERK-dependent regulation of lipogenesis during mouse mammary gland development and adipocyte differentiation. Proc. Natl. Acad. Sci. USA. 2008;105(42):16314–16319. doi: 10.1073/pnas.0808517105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chapados N.A., Lavoie J.M. Exercise training increases hepatic endoplasmic reticulum (er) stress protein expression in MTP-inhibited high-fat fed rats. Cell Biochem. Funct. 2010;28(3):202–210. doi: 10.1002/cbf.1643. [DOI] [PubMed] [Google Scholar]
- 40.Lee A.H. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320(5882):1492–1496. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kammoun H.L. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J. Clin. Invest. 2009;119(5):1201–1215. doi: 10.1172/JCI37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng X. Caffeine reduces hepatic lipid accumulation through regulation of lipogenesis and ER stress in zebrafish larvae. J. Biomed. Sci. 2015;22:105. doi: 10.1186/s12929-015-0206-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wree A. NLRP3 inflammasome activation is required for fibrosis development in NAFLD. J. Mol. Med (Berl.) 2014;92(10):1069–1082. doi: 10.1007/s00109-014-1170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Puri P. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology. 2008;134(2):568–576. doi: 10.1053/j.gastro.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 45.Lake A.D. The adaptive endoplasmic reticulum stress response to lipotoxicity in progressive human nonalcoholic fatty liver disease. Toxicol. Sci. 2014;137(1):26–35. doi: 10.1093/toxsci/kft230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harding H.P. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell. 2000;5(5):897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- 47.Back S.H. Translation attenuation through eIF2alpha phosphorylation prevents oxidative stress and maintains the differentiated state in beta cells. Cell Metab. 2009;10(1):13–26. doi: 10.1016/j.cmet.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hage Hassan R. Endoplasmic reticulum stress does not mediate palmitate-induced insulin resistance in mouse and human muscle cells. Diabetologia. 2012;55(1):204–214. doi: 10.1007/s00125-011-2328-9. [DOI] [PubMed] [Google Scholar]
- 49.Kawasaki N. Obesity-induced endoplasmic reticulum stress causes chronic inflammation in adipose tissue. Sci. Rep. 2012;2:799. doi: 10.1038/srep00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amirkhizi F. Impaired enzymatic antioxidant defense in erythrocytes of women with general and abdominal obesity. Obes. Res Clin. Pr. 2014;8(1):e26–e34. doi: 10.1016/j.orcp.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 51.Boden G. Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes. 2008;57(9):2438–2444. doi: 10.2337/db08-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sharma N.K. Endoplasmic reticulum stress markers are associated with obesity in nondiabetic subjects. J. Clin. Endocrinol. Metab. 2008;93(11):4532–4541. doi: 10.1210/jc.2008-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ye R. Grp78 heterozygosity promotes adaptive unfolded protein response and attenuates diet-induced obesity and insulin resistance. Diabetes. 2010;59(1):6–16. doi: 10.2337/db09-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boden G. Excessive caloric intake acutely causes oxidative stress, GLUT4 carbonylation, and insulin resistance in healthy men. Sci. Transl. Med. 2015;7(304):304re7. doi: 10.1126/scitranslmed.aac4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sugeno N. Serine 129 phosphorylation of alpha-synuclein induces unfolded protein response-mediated cell death. J. Biol. Chem. 2008;283(34):23179–23188. doi: 10.1074/jbc.M802223200. [DOI] [PubMed] [Google Scholar]
- 56.Lee J.H. Induction of the unfolded protein response and cell death pathway in Alzheimer's disease, but not in aged Tg2576 mice. Exp. Mol. Med. 2010;42(5):386–394. doi: 10.3858/emm.2010.42.5.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prasanthi J.R. Silencing GADD153/CHOP gene expression protects against Alzheimer's disease-like pathology induced by 27-hydroxycholesterol in rabbit hippocampus. PLoS One. 2011;6(10):e26420. doi: 10.1371/journal.pone.0026420. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58.Karademir B., Corek C., Ozer N.K. Endoplasmic reticulum stress and proteasomal system in amyotrophic lateral sclerosis. Free Radic. Biol. Med. 2015;88(Pt A):42–50. doi: 10.1016/j.freeradbiomed.2015.05.038. [DOI] [PubMed] [Google Scholar]
- 59.Romero-Ramirez L. X box-binding protein 1 regulates angiogenesis in human pancreatic adenocarcinomas. Transl. Oncol. 2009;2(1):31–38. doi: 10.1593/tlo.08211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koumenis C. Regulation of protein synthesis by hypoxia via activation of the endoplasmic reticulum kinase PERK and phosphorylation of the translation initiation factor eIF2alpha. Mol. Cell Biol. 2002;22(21):7405–7416. doi: 10.1128/MCB.22.21.7405-7416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li X., Zhang K., Li Z. Unfolded protein response in cancer: the Physician's perspective. J. Hematol. Oncol. 2011;4:8. doi: 10.1186/1756-8722-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Naidoo N. The endoplasmic reticulum stress response and aging. Rev. Neurosci. 2009;20(1):23–37. doi: 10.1515/revneuro.2009.20.1.23. [DOI] [PubMed] [Google Scholar]
- 63.Lindenmeyer M.T. Proteinuria and hyperglycemia induce endoplasmic reticulum stress. J. Am. Soc. Nephrol. 2008;19(11):2225–2236. doi: 10.1681/ASN.2007121313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Adam J. Endoplasmic reticulum stress in UMOD-related kidney disease: a human pathologic study. Am. J. Kidney Dis. 2012;59(1):117–121. doi: 10.1053/j.ajkd.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 65.Fougeray S. Metabolic stress promotes renal tubular inflammation by triggering the unfolded protein response. Cell Death Dis. 2011:2. doi: 10.1038/cddis.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oliva Trejo J.A. Transient increase in proteinuria, poly-ubiquitylated proteins and ER stress markers in podocyte-specific autophagy-deficient mice following unilateral nephrectomy. Biochem Biophys. Res Commun. 2014;446(4):1190–1196. doi: 10.1016/j.bbrc.2014.03.088. [DOI] [PubMed] [Google Scholar]
- 67.Huo R. Differential expression of glucose-regulated protein 78 during spermatogenesis. Cell Tissue Res. 2004;316(3):359–367. doi: 10.1007/s00441-004-0885-7. [DOI] [PubMed] [Google Scholar]
- 68.Kim J.H. Testicular hyperthermia induces Unfolded Protein Response signaling activation in spermatocyte. Biochem Biophys. Res Commun. 2013;434(4):861–866. doi: 10.1016/j.bbrc.2013.04.032. [DOI] [PubMed] [Google Scholar]