Abstract
Introduction
Changes in cerebrospinal fluid (CSF) tau and amyloid β (Aβ)42 accompany development of Alzheimer's brain pathology. Robust tau and Aβ42 immunoassays were developed to establish a tau/Aβ42 cutoff distinguishing mild-to-moderate Alzheimer's disease (AD) subjects from healthy elderly control (HC) subjects.
Methods
A CSF tau/Aβ42 cutoff criteria was chosen, which distinguished the groups and maximized concordance with amyloid PET. Performance was assessed using an independent validation cohort.
Results
A tau/Aβ42 = 0.215 cutoff provided 94.8% sensitivity and 77.7% specificity. Concordance with PET visual reads was estimated at 86.9% in a ∼50% PET positive population. In the validation cohort, the cutoff demonstrated 78.4% sensitivity and 84.9% specificity to distinguish the AD and HC populations.
Discussion
A tau/Aβ42 cutoff with acceptable sensitivity and specificity distinguished HC from mild-to-moderate AD subjects and maximized concordance to brain amyloidosis. The defined cutoff demonstrated that CSF analysis may be useful as a surrogate to imaging assessment of AD pathology.
Keywords: Alzheimer's disease, Diagnostic test assessment, PET, Amyloid β42, Tau
1. Introduction
Amyloid β (Aβ) and tau pathology precede clinical symptoms in Alzheimer's disease (AD) by many years [1], [2], [3], [4]. Cerebrospinal fluid (CSF) tau and Aβ [5], [6], [7], [8], [9], [10] correlate with brain pathology and AD diagnosis [11], [12], [13]. Intensive efforts have focused on earlier identification of patients at risk for developing AD, such as patients with mild cognitive impairment (MCI), and demonstrate that biomarkers related to AD pathology play an important role [14], [15].
Amyloid PET ligands are approved by regulatory agencies to estimate Aβ neuritic plaque density in patients with cognitive impairment being evaluated for AD, along with other diagnostic evaluations. Although PET ligands provide an early biomarker, cost, limited access, and lack of treatment options have limited use [16]. CSF biomarkers could overcome cost and access issues, but unlike PET ligands, no CSF assay is approved by regulatory agencies as a diagnostic tool. Although CSF assays are available with a Conformité Européene (European Conformity, CE) mark and documented analytical validation, these assays are not accompanied by a universal cutpoint to enable an AD diagnosis. Robust assays with good reliability and reproducibility have been difficult to develop.
There are several challenges in developing robust CSF assays that meet regulatory requirements. First, collection methods have varied and may be confounded by a significant loss of Aβ42 because of binding to test tubes [17]. Second, there are concerns about intralaboratory and interlaboratory variability [18]. Third, most studies reporting cutoffs used single cohorts for distinguishing AD from healthy elderly control (HC) subjects, although not confirming sensitivity and specificity with replication samples, possibly overestimating key performance characteristics. In addition, understanding the correlation between CSF results and amyloid PET imaging is important for their clinical use. To address this gap, investigational use tau and Aβ42 assays were developed using the Luminex xMAP platform and validated to Clinical Laboratory Improvement Amendments' standards [19] to address interlaboratory and intralaboratory variability (full methods/results in Supplementary Data). A cutoff ratio of tau/Aβ42 was determined, which distinguished patients with mild-to-moderate Alzheimer's disease (mmAD) from HC subjects across four countries and five sites. Among candidate ratios, the cutoff was set by optimizing concordance with amyloid PET imaging. This cutoff was confirmed in a validation study of HC and mmAD subjects across a wider geographic range. Finally, correlation with amyloid PET imaging was assessed using both 18F-flutemetamol and Pittsburgh Compound B (PiB).
2. Methods
2.1. Study design
The cutoff-setting study (Merck Protocol 290) was conducted at five sites (United States, Netherlands, Norway, and Australia [two sites]). CSF samples collected between 2009 and 2013 used a convenience sampling approach, allowing each site to use site-specific noninterventional study protocols for local Institutional Review Board approval. Subjects were enrolled per each site's protocol/work plan; each subject signed informed consent before study procedures were conducted. The validation study (Merck Protocol 261) was conducted at nine sites in six countries (United States, Canada, Argentina, Spain, Germany, and UK).
2.2. Subjects
The cutoff-setting study enrolled subjects ≥50 to ≤85 years with a clinical diagnosis of AD, HC, MCI, or non-AD dementia. Subjects clinically diagnosed as AD or MCI had a Mini-Mental State Examination (MMSE) score ≥12 and <28, and HC subjects had a score ≥28 and were free of cognitive deficits based on site-specific neuropsychological testing. Standard of care medications for AD were allowed; however, subjects were excluded if they participated in a clinical trial within the last 2 months or had ever received an AD vaccine. Before CSF testing, clinical results were reviewed by a central monitoring team to prespecify subjects to include in each analysis. PET images were ±180 days from the date of CSF collection and used 18F-flutemetamol or C-11 PiB (11C-PiB) tracers. The PET imaging data for subjects clinically diagnosed with mmAD, amnestic MCI, and HC were analyzed and standardized uptake value ratios (SUVRs) were computed and included in the concordance analyses.
For the validation study, subjects completed the MMSE and Clinical Dementia Rating Scale–Sum of Boxes at screening to determine cognitive health (HC population) or probable mmAD based on the 1984 National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association criteria and Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Patients with AD required an MMSE score ≥15 and ≤26. Patients with AD were required to have a magnetic resonance imaging scan within 1 year of screening to rule out non-AD conditions. HC subjects required an MMSE score ≥28, a Clinical Dementia Rating Scale–Sum of Boxes of 0, and a modified Hachinski score of ≤4.
2.3. Procedures
Details of the CSF collection procedures are described in the Supplementary Data.
2.4. PET methods
PET imaging was conducted for the cutoff-setting study at sites 2, 3, and 4. 18F-Flutemetamol dose was administered as a single intravenous bolus of 185 MBq [5 millicuries (mCi)] in a maximum dose volume of 10 mL, within 40 seconds, followed by an intravenous flush of 5 to 15 mL of 0.9% sterile sodium chloride injection. A 20-minute PET image was acquired starting 90 minutes after 18F-flutemetamol injection, using a PET scanner in three-dimensional (3D) mode with appropriate data corrections, patient head positioning to reduce movement and image acquisition. PET images with 11C-PIB were acquired for 30 minutes starting 40 minutes after an intravenous injection of 370 MBq of the radiotracer [4].
After PET image reconstruction and coregistration with magnetic resonance imaging anatomic images, PET images were scored (±) by readers blinded to patient's diagnosis following recommended methods or using approved visual read methodology. 18F-Flutemetamol PET images from sites 3 and 4 were scored at Bioclinica, Inc (San Francisco, CA), whereas images from site 2 subjects were scored by a local radiologist. If any one of the brain regions systematically reviewed for 18F-flutemetamol uptake was positive in either brain hemisphere, the scan was considered positive. Otherwise, it was considered negative. For 11C-PIB, the images were processed and SUVR determined [4]. The cerebellar cortex was used as the reference region and the cortical composite index was the average SUVR of the area-weighted mean of frontal, superior parietal, lateral temporal, lateral occipital, and anterior and posterior cingulate regions. An SUVR cutoff value of 1.5 was applied to differentiate positive from negative scans [4].
2.5. Luminex xMAP tau/Aβ42 assay
The Aβ42 sandwich immunoassay comprised clones 1-11-3 and 6E10 (both from Covance, Catalog# SIG-39169, and SIG-39320, respectively). Antibody 1-11-3 is specific for the free carboxyl terminus of Aβ42, whereas antibody 6E10 recognizes an amino terminal epitope within amino acids 3 to 8 of beta amyloid (EFRHDS). The tau sandwich immunoassay was comprised of clones 10H8 and 19G10. These antibodies were made by Merck & Co., Inc., and (based on Tau441 isoform) recognize amino acid sequences 220-224 (TREPK), and 188-194 (PPKSGDR), respectively. For more details of the assay, including linear ranges, precision profiles, lot-to-lot and site-to-site precision, analytic reactivity, sensitivity, hook effect and carry over, and effect of interfering substances, see Supplementary Data.
CSF samples and control subjects were diluted 1:2 for tau and 1:20 for Aβ42 analysis to prepare 50 μL/well final volume. A bead mix (50 μL) containing beads coated with capture antibody to either tau or Aβ42 was added to each well. After aspirating the bead carrier liquid from the wells, 50 μL of each diluted CSF or control sample was added and incubated for 2 hours. Liquid was removed and two washes with 150 μL of 1× wash buffer were completed, followed by 1 hour of incubation with 50 μL of tau or Aβ42 detection antibody. Liquid was removed from the wells, two bead washes with 150 μL of 1× wash buffer were completed, followed by 30 minutes of incubation in 50 μL of Reporter solution. Liquid was removed, two washes with 150 μL of 1× wash buffer were completed, and samples were resuspended with 100 μL of wash buffer followed by detection on a FlexMap 3D instrument. All incubations occurred at 25°C in the dark on an orbital shaker at 800 rpm.
2.6. Statistical methods
The main objective of the statistical analysis was to set a cutoff for the CSF tau/Aβ42 ratio that met or exceeded prespecified performance criteria of sensitivity ≥80% and specificity ≥60% for the discrimination of disease status, AD versus HC, and maximized concordance with amyloid PET tracer visual read results (18F-flutemetamol tracer). Results for tau and Aβ42 alone are also presented. For tau/Aβ42, the set of cutoffs with one-sided 95% confidence limit [20] for specificity >60% and for sensitivity >80% was identified. For possible cutoffs in this window, nonparametric kernel density estimators of concordance with amyloid PET 18F-flutemetamol visual read values were used to identify the value with the highest total agreement [21]. Bootstrap resampling was used to adjust for bias and construct confidence intervals (CIs) for agreement parameters along with corresponding significance tests. For analyses of correlations between PET and CSF measures, all subjects with PET scans were used, including subjects with MCI (N = 15) and indeterminate diagnosis (N = 5).
For the validation analysis, assuming the tau/Aβ42 cutoff established in the cutoff-setting study had a true sensitivity of 0.8 and a true specificity of 0.6, simulations indicated that 51 patients with AD provided 93% power (α = 0.05, one-sided) to show that the true sensitivity was >0.6. Similarly, with 51 control subjects there was 88% power (α = 0.05, one-sided) to show that the true specificity was >0.4. Therefore, the power to show both a true sensitivity >0.6 and a true specificity >0.4 was >80% (93% × 88% = 81.8%).
3. Results
3.1. Subject demographics and cutoff-setting study disposition
The AD and HC cohorts were well matched for gender and age (Table 1). The cohort used to estimate concordance had almost equal gender representation (49% male), ages ranged from 52 to 85 years, and MMSE scores ranged from 17 to 30. Supplementary Table 1 gives similar demographic information for samples used in the amyloid PET 18F-flutemetamol concordance and 11C-PiB SUVR and CSF analyses.
Table 1.
Demographics in subjects analyzed for the cutoff-setting study
| Diagnosis | N | Female/male (% male) | Mean age (y) | MMSE |
|---|---|---|---|---|
| Sensitivity and specificity analysis | ||||
| HC | 188 | 104/84 (45%) | 67 (50, 84) | 29 (28, 30) |
| AD | 155 | 86/69 (45%) | 64 (52, 84) | 22 (12, 27) |
| All subjects | 343 | 190/153 (45%) | 66 (50, 84) | 28 (12, 30) |
| Concordance analysis (flutametamol visual read vs. CSF) | ||||
| HC | 44 | 24/20 (45%) | 71 (53, 82) | 29 (28, 30) |
| AD | 33 | 16/17 (52%) | 63 (52, 78) | 22 (17, 27) |
| MCI | 13 | 6/7 (54%) | 69 (56, 85) | 27 (21, 30) |
| IND | 5 | 2/3 (60%) | 63 (59, 74) | 27 (27, 29) |
| All subjects | 95 | 48/47 (49%) | 67 (52, 85) | 28 (17, 30) |
| PiB versus CSF analysis | ||||
| HC | 15 | 10/5 (33%) | 75 (65, 84) | 29 (28, 30) |
| AD | 4 | 4/0 (0%) | 74 (57, 77) | 20 (12, 22) |
| MCI | 2 | 2/0 (0%) | 72 (68, 77) | 27 (25, 29) |
| All subjects | 21 | 16/5 (24%) | 75 (57, 84) | 29 (12, 30) |
Abbreviations: AD, Alzheimer's disease; CSF, cerebrospinal fluid; HC, healthy control subjects; IND, indeterminate; MCI, mild cognitive impairment; PiB, Pittsburgh Compound B.
NOTE. Summary statistics for age and Mini-Mental State Examination (MMSE) are median (min, max). Also, HC and AD subjects in concordance analysis were used in the sensitivity/specificity analysis. HC and AD subjects in PiB versus CSF Analysis were used in the sensitivity/specificity analysis.
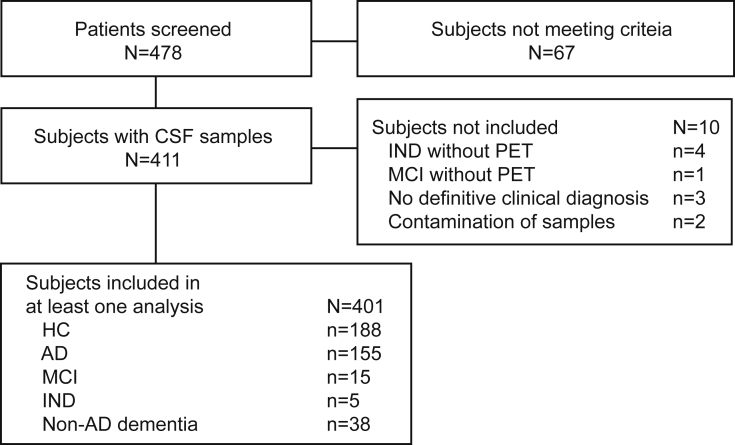
Overall, 478 subjects were screened; 67 did not meet entry criteria (Fig. 1). An additional 10 subjects were excluded from all analyses: four had an indeterminate (IND) clinical diagnosis (within the HC-MCI-AD spectrum but not clear which category) and did not have a PET measurement, one subject had an MCI diagnosis and no PET measurement, three subjects did not have a definitive clinical diagnosis, and two subjects' CSF samples had blood contamination. Overall, 401 subjects were included in at least one analysis.
Fig. 1.
Patient disposition for cutoff-setting study. Abbreviations: AD, Alzheimer's disease; CSF, cerebrospinal fluid; HC, healthy control subjects; MCI, mild cognitive impairment; IND, indeterminate.
3.2. Cutoff setting and performance estimates
The mean (standard deviation) in HC and AD subjects for CSF Aβ42 and tau is indicated in Table 2. In AD versus HC CSF, the mean Aβ42 concentration was 2.2-fold lower and mean tau concentration was 1.7-fold higher, which is consistent with prior findings [10], [12], [22], [23].
Table 2.
Summary statistics for CSF Aβ42 and tau measures used for cutoff setting and non-AD dementia samples
| Clinical diagnosis | N | Aβ42 (pg/mL) mean (SD) | Tau (pg/mL) mean (SD) | Ratio tau/Aβ42 |
|---|---|---|---|---|
| HC | 188 | 823 (303) | 126 (39) | 0.175 (0.096) |
| AD | 155 | 379 (155) | 208 (83) | 0.613 (0.302) |
| MCI | 13 | 500 (207) | 171 (78) | 0.421 (0.281) |
| IND | 5 | 868 (384) | 136 (68) | 0.201 (0.188) |
| NON | 38 | 540 (241) | 127 (41) | 0.308 (0.244) |
Abbreviations: AD, Alzheimer's disease; CSF, cerebrospinal fluid; HC, healthy control subjects; MCI, mild cognitively impaired; IND, indeterminate; NON; non-Alzheimer's dementia; SD, standard deviation.
NOTE. CSF: negative = CSF tau/Aβ42 < cutoff (0.215); positive = CSF tau/Aβ42 ≥ cutoff (0.215). Values in table are number of individuals.
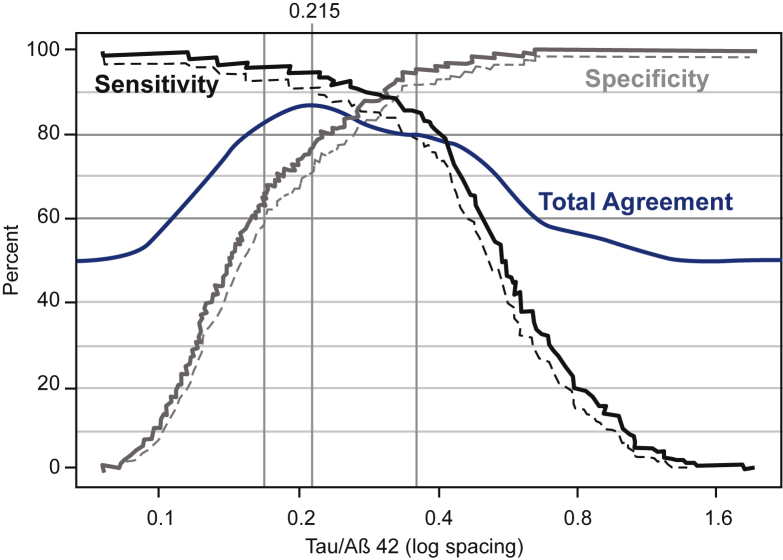
Fig. 2 shows sensitivity and specificity estimates to discriminate AD from HC subjects with 95% lower confidence bound (CL) for each level of tau/Aβ42. The interval (0.169, 0.360) yielded a window with 95% CL for sensitivity of ≥80% and the 95% CL for specificity of ≥60%. Total agreement with PET estimates (blue curve) were maximized within this window at tau/Aβ4 = 0.215 using the rule that samples are considered positive when tau/Aβ42 ≥0.215. As selected, the estimated sensitivities and specificities demonstrated general consistency across sites (data not shown).
Fig. 2.
Cutoff plot: estimates of sensitivity, specificity, and total agreement with PET versus CSF tau/Aβ42. The cutoff plot displays estimates of sensitivity, specificity, and total agreement with PET (flutemetamol visual read) versus prospective cutoffs for CSF tau/Aβ42 using log scaling. Sensitivity (solid line, black) and specificity (solid line, gray) are displayed along with 95% lower confidence limits (dashed lines). The estimate of total agreement (solid line, blue) is based on nonparametric density estimation. Vertical lines (gray) show the CSF window (0.169, 0.360) that achieves the acceptable sensitivity and specificity performance. The value that maximizes total agreement within this window (0.215) is also shown with a vertical line and identified on the top axis. Abbreviation: CSF, cerebrospinal fluid.
Table 3 summarizes sensitivity, specificity, and agreement estimates with 18F-flutemetamol PET visual read based on the proposed cutoff (also see Supplementary Figs. 1 and 2). For the rule based on the tau/Aβ42 ratio, the initial estimate of total agreement was 87.1%. Bootstrap estimation, which reduces bias and estimates precision, yielded a total agreement estimate of 88.0% with 95% CI (82.3%, 92.6%). Subtracting a bias estimate because of the optimization over prospective cutoffs in the window yielded a final total agreement estimate of 86.9% with 95% CI (80.7%, 91.9%). As employed in the cutoff cohort, the estimated sensitivity for AD detection using tau/Aβ42 with the 0.215 cutoff was 94.8% (95% CL = 91.1%) and estimated specificity was 77.7% (95% CL = 72.3%).
Table 3.
CSF proposed cutoff and performance estimates at cutoff
| Ratio tau/Aβ42 | Aβ42 (pg/mL) | Tau (pg/mL) | |
|---|---|---|---|
| Cutoff | 0.215 | 663 | 184 |
| Window: sensitivity ≥80% and specificity ≥60% | (0.169, 0.360) | (469, 663) | No window |
| Sensitivity and specificity to discriminate AD from HC | |||
| Specificity (%, 95% CL) | 77.7 (72.3, 100) | 66.0 (60.1, 100) | 92.6 (88.8, 100) |
| Sensitivity (%, 95% CL) | 94.8 (91.1, 100) | 94.8 (91.1, 100) | 56.8 (50.2, 100) |
| Concordance with PET (flutemetamol visual read) | |||
| Initial estimates | |||
| Total agreement (%) | 87.1 | 78.5 | 73.7 |
| Negative agreement (%) | 80.2 | 64.3 | 90.8 |
| Positive agreement (%) | 94.0 | 92.8 | 56.6 |
| Bootstrap estimates adjusted for optimization bias | |||
| Total agreement (%), (95% CI) | 86.9 (80.7, 91.9) | 77.9 (71.1, 83.9) | 72.9 (66.7, 79.1) |
| Negative agreement (%), (95% CI) | 80.1 (68.8, 89.0) | 67.9 (55.3, 81.1) | 83.1 (51.9, 95.6) |
| Positive agreement (%), (95% CI) | 94.0 (86.9, 98.0) | 89.4 (74.9, 95.3) | 69.1 (45.9, 90.0) |
Abbreviations: AD, Alzheimer's disease; CI, confidence interval; CL, lower confidence bound; HC, healthy control subjects.
NOTE. Estimates of concordance were based on nonparametric kernel density estimation. Initial estimates were used to determine the cutoff. Estimates at the proposed cutoff were based on 5000 bootstrap samples. Optimization bias was estimated using bootstrap resampling. Total agreement defined as 0.5*negative agreement + 0.5*positive agreement. The response bootstrapped was the logit transformation of agreement, and CIs were based on percentiles of the bootstrap.
For Aβ42 measure alone, the value maximizing concordance within the sensitivity/specificity window was the right end point of the window (Table 3) at 663 pg/mL. Agreement with PET for this cutoff was estimated to be 77.9%, which is lower than for tau/Aβ42 (P < .01). For tau, no window existed that met the prespecified sensitivity and specificity performance criteria. Agreement statistics for tau correspond to the value maximizing total agreement with PET overall (184 pg/mL). However, at this cutoff, the estimated sensitivity to discriminate AD from HC subjects was 56.8%, and the estimate of total agreement with PET was the lowest at 72.9%.
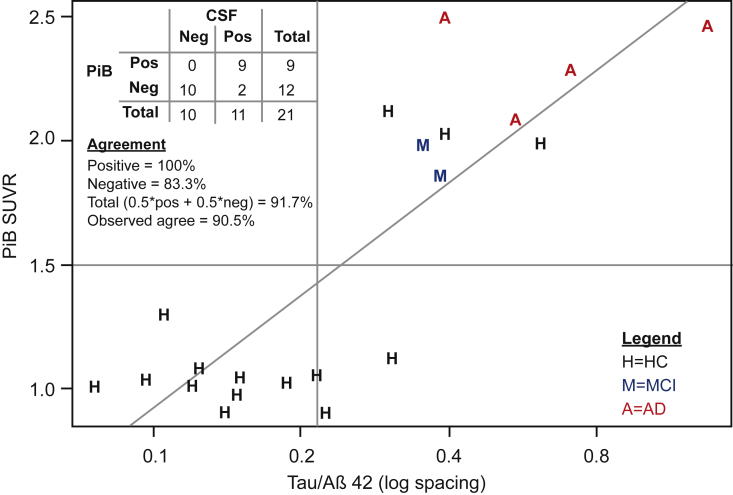
The tau/Aβ42 cutoff was also examined in 21 HC, MCI, and AD subjects with 11C-PiB SUVR data. Using a PiB SUVR cutoff of 1.5, estimated total agreement was 91.7% for classifying brain amyloid with the tau/Aβ42 cutoff (Fig. 3).
Fig. 3.
Plot of PiB SUVR values versus CSF tau/Aβ42 and agreement statistics. PiB SUVR values are plotted versus CSF tau/Aβ42. Reference lines are drawn at the PiB SUVR cutoff (1.5) used by sites 3 and 4, and the CSF tau/Aβ42 proposed in this study. A least squared regression line is also plotted. The plotting symbols code for the clinical diagnoses. Abbreviations: AD, Alzheimer's disease; CSF, cerebrospinal fluid; HC, healthy control subjects; MCI, mild cognitive impairment; PiB, Pittsburgh Compound B; SUVR, standardized uptake value ratio.
3.3. Validation of the tau/Aβ42 cutoff
For patients with AD in the validation study, 40 of 51 patients had tau/Aβ42 ≥0.215 (Supplementary Table 3). Estimated sensitivity was 78.4%, with a one-sided 95% CI lower bound = 66.8%, supporting the first coprimary hypothesis, because the lower bound on sensitivity was >60%. For HC subjects, 45 of 53 subjects had tau/Aβ42 <0.215. The estimated specificity was 84.9%, with a one-sided 95% CL = 74.4%, supporting the second coprimary hypothesis, because the lower bound on specificity was >40% (Supplementary Table 3). As in the training set, the estimated sensitivities and specificities by site demonstrated general consistency across sites (Supplementary Table 3).
4. Discussion
Amyloid PET tracers have achieved regulatory approval to aid in AD evaluation. Diagnostic CSF assays are not available, although they would represent a more accessible and cost-effective option. The tau and Aβ CSF assays reported herein demonstrated performance characteristics required of an AD diagnostic aid, including acceptable lot-to-lot and site-to-site variability. The cutoff-setting study demonstrated that a tau/Aβ42 = 0.215 cutoff provides 94.8% (91.1%, 100%) sensitivity and 77.7% (72.3%, 100%) specificity in discriminating AD from HC subjects. The cutoff selected from a range of values maximized concordance with flutemetamol PET scan visual reads, giving an estimate of 86.9% concordance. The agreement of 91.7% with a separate group of subjects and PiB SUVR confirms that the cutoff likely detects brain amyloid deposition. The cutoff was validated in an independent cohort of AD and HC subjects from different sites and countries, showing an estimated 78.4% sensitivity and 84.9% specificity in distinguishing AD from HC subjects.
This study is notable for several reasons. The good concordance between amyloid PET and CSF tau/Aβ42 suggests that the CSF measure may be used, similar to amyloid PET tracers, for detecting amyloid pathology in the brain of adult patients with cognitive impairment being evaluated for potential inclusion in Alzheimer's dementia trials [24], [25], [26]. The amyloid PET tracers were approved, in part, as they demonstrated good concordance with postmortem amyloid pathology, including modified Bielschowsky silver staining of plaque. For an alternate method to detect brain amyloid as a registered diagnostic device, a CSF device would most likely need to demonstrate similar concordance to postmortem plaque, or perhaps an amyloid PET tracer could be considered as a new “truth standard.” Other studies using different PET tracers and CSF assays also report relatively high concordance between CSF analytes and amyloid PET deposition [22], [27], [28], [29], [30], [31]. Here we demonstrated 86.9% and 91.7% concordance between CSF tau/Aβ42 and either flutemetamol or PiB, respectively.
Prior studies comparing CSF and amyloid PET measures used SUVR cutoffs for PET amyloid classification. Our study used the visual read method approved by regulatory agencies for PET amyloid classification and showed a concordance rate of 86.9%, comparable to rates in two recent large studies (tau/Aβ42 ratios) of 86% and 92% [30], [31] and in a recent phase 2 clinical trial in prodromal AD (87%) [27]. In our cutoff-setting study, concordance rates with 18F-flutemetamol PET using SUVR cutoffs from two different methods were similar to the overall concordance rate using visual reads. In addition, in a smaller subgroup of subjects assessed with PiB PET SUVR, the concordance rate was consistent at 91.7%. Taken together, the high agreement between the cutoff ratio and different PET tracers using different classification methods suggests that tau/Aβ42 can accurately identify subjects with brain amyloidosis. The correlation is not perfect; however, the lower bound of the 95% CI for most studies falls well <90%.
An important issue is whether tau, Aβ42, or the ratio tau/Aβ42 shows superior performance characteristics. Palmqvist et al. [31] found the agreement in MCI subjects between CSF Aβ42 and 18F-flutemetamol PET with SUVR was 92%. Tau/Aβ42 did not improve concordance. Our study showed Aβ42 concordance with 18F-flutemetamol PET visual reads of 77.9%; tau/Aβ42 increased the concordance to 86.9%. This difference may be because of many factors including the use of different assays (INNOTEST enzyme-linked immunosorbent assay), visual reads versus SUVR, and different patient cohorts (MCI vs. AD and HC) [31]. As visual read is the method approved for clinical practice, future studies may be needed to replicate the results. Interestingly, in a recent head-to-head comparison of amyloid PET and CSF biomarkers in MCI subjects who later developed dementia (MCI-AD) compared with HC subjects, tau/Aβ42 had better diagnostic accuracy for distinguishing MCI-AD from HC subjects than Aβ42 alone and was comparable to amyloid PET measures [14]. As amyloid PET tracers detect neuritic plaque with a surrounding of tau-containing abnormal neurites, and not diffuse amyloid, perhaps the increased CSF tau along with the reduced amyloid is more reflective of this lesion compared with CSF amyloid alone.
Prior studies attempting to define tau/Aβ42 distinguishing AD and HC subjects using other assay methods have reported similar results [5], [32], [33]. A review of nine studies reported sensitivities between 71% and 90% and specificities between 80% and 100% [33]. In a meta-analysis of 11 studies, average sensitivities were 89% (95% CI: 84%, 92%) and specificities were 87% (95% CI: 83%, 90%) for the combination of tau with Aβ42 to distinguish HC from AD subjects [32]. A third review, including 16 studies, each with sample sizes ≥100, reported mean sensitivity of 88.7% (95% CI: 84.0%, 93.5%) and mean specificity of 88.7% (95% CI: 85.6%, 91.8%) [5]. Most prior studies compared tau and Aβ42 separately as well as the ratio, and in general, meta-analyses indicate that the ratio performs better than either one alone. Given that establishing and assessing a cutoff in the same cohort will likely produce higher sensitivities and specificities compared with replication cohorts using a prespecified cutoff, the published literature to date may tend to overestimate sensitivity and specificity. In this study, we selected >60% for specificity based on prior reports of means and CIs and a feasible sample size. Failure to hit this target would suggest that this assay was inadequate for differentiating patients with AD from HC subjects.
Studies of MCI subjects demonstrated that tau/Aβ42 also predicts which subjects develop AD dementia in the next 2 to 5 years [34]. These studies reported sensitivities between 64% and 95% and specificities between 53% and 97% [34]. A meta-analysis of 10 studies reported overall sensitivity of 87% (95% CI: 80%, 95%), specificity of 70% (95% CI: 57%, 83%), and positive predictive value of 65% (95% CI: 53%, 77%) [34]. These results along with those from the present studies suggest the assay cutoff may be useful in identifying MCI subjects at risk of progressing to dementia.
4.1. Limitations
One potential limitation of a cutoff-setting study is the concern that a CSF cutoff appropriate in one region may not be appropriate for others. Here, the cutoff-setting study included sites from the United States, Norway, the Netherlands, and Australia, and the validation study was conducted in six countries. Despite regional differences in both study populations, there were neither noticeable differences in CSF ratios between sites for HC or AD subjects nor any apparent difference in cutoff performance in the present study. This study was, however, limited by the CSF cutoffs that could be used to distinguish subjects with AD versus non-AD dementia. In the present study, 50% of the non-AD dementia subjects tested positive using the CSF ratio (data not shown), which is consistent with prior studies indicating lower specificity relative to HC subjects [14]. Although this may reflect the assay, it may also be that some of the patients with AD were misclassified and that some of the control subjects have preclinical AD. Furthermore, negative agreement of tau/Aβ42 and 18F-flutemetamol PET was 80.1%, indicating that a subject with a negative PET scan had a ∼20% chance of a positive CSF result. The negative agreement (80.1%) was lower than the positive agreement (94%) and may have been because of assay variability or could instead suggest that the CSF measures detected amyloid pathology before the stage of neuritic amyloid plaque formation. [3], Finally, given that the underlying amyloid and tau pathologies are continuous and not discrete, the use of a cutpoint may not be optimal. Further analyses may provide alternative, clinically useful approaches to tau and Aβ42 measures, which could include “gray zones.”
In conclusion, this study demonstrates a robust tau/Aβ42 measure that distinguished AD subjects from HC subjects and identified subjects with brain amyloidosis. This cutoff was validated in a second cohort. Our results support the view that CSF tau/Aβ42 measures are useful surrogates to amyloid PET to aid in diagnosis of AD, possibly at early stages of disease. Finally, given the robust performance characteristics of this measure, these results support widespread use of tau/Aβ42 in clinical settings, including an ongoing phase III trial of a beta-site APP-cleaving enzyme (BACE) inhibitor in a prodromal AD population (clinicaltrials.gov https://clinicaltrials.gov/ct2/show/NCT01953601).
Research in context.
-
1.
Systematic review: The authors reviewed the literature using traditional (e.g., PubMed) sources. Cerebrospinal fluid (CSF) tau and Aβ42 analytes change with the development of Alzheimer's amyloid brain pathology. Few tau and Aβ42 assays are produced using good manufacturing practices, validated to clinical laboratory improvement amendments' standards, and have demonstrated good lot-to-lot and site-to-site analytical consistency.
-
2.
Interpretation: Using a novel assay meeting good manufacturing practices and Clinical Laboratory Improvement Amendments' standards, a CSF tau/Aβ42 cutoff ratio distinguished Alzheimer's disease (AD) from HC subjects and demonstrated good concordance with amyloid PET imaging. This suggests a tau/Aβ42 ratio is a useful alternative to amyloid PET for evaluating subjects at risk for AD.
-
3.
Future directions: These results support use of CSF tau/Aβ42 measures in clinical settings to evaluate subjects at risk for AD.
Acknowledgments
Mary E. Hanson, PhD, of Merck & Co., Inc., Kenilworth, NJ, USA, provided medical writing support; and Sheila Erespe, MS, of Merck & Co., Inc., Kenilworth, NJ, USA, provided editorial support. The authors would like to thank the study participants, clinicians, and researchers at the following sites: VU University Medical Center Amsterdam, The Netherlands; Duke University, Durham, North Carolina; Norwegian University of Science and Technology, Trondheim, Norway; AIBL (www.AIBL.csiro.au) including the Florey Institute of Neuroscience and Mental Health, the University of Melbourne, Parkville, Victoria Australia; Centre of Excellence for Alzheimer's Disease Research and Care, Edith Cowan University, Joondalup, Western Australia, Australia; and Commonwealth Scientific and Industrial Research Organization, Australia.
Author contributions: Y.M., J.S., K.W., C.S., M.S., A.S., P.S., C.E.T., C.W., M.M.B., G.D., J.L., and M.F.E. contributed to conception, design, or planning of the study. Y.M., J.S., K.W., O.L., M.S., A.S., C.E.T., J.B., S.L.M., G.B., S.B.S., L.R.W., C.W., A.C., M.M.B., G.D., D.D, S.R.R.-S., J.S., E.S., and M.T. contributed to acquisition of the data. Y.M., D.H., C.S., C.W., M.M.B., G.D., J.S., E.S., M.T., A.A., and M.F.E. contributed to analysis of the data. Y.M., D.H., C.S., O.L., M.S., P.S., C.E.T., S.L.M., C.W., M.M.B, G.D., D.D., J.L., and M.F.E. contributed to interpretation of the results. Y.M., D.H., M.S., C.W., and M.F.E. contributed to drafting of the manuscript. All authors performed critical review and revision of the manuscript.
All authors approved of submitted version and agree to be accountable for all aspects of the work and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Funding: Funding for this research was provided by Merck & Co., Inc., Kenilworth, NJ, USA, and was involved in the following: design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript.
Declaration of interest: Julie Stromswold, Kimberly Wilson, Daniel Holder, Cyrille Sur, Omar Laterza, Mary Savage, Arie Struyk, and Michael Egan are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA and may own stock or hold stock options in the Company. Yi Mo was an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, at the time the study was conducted and during the time the manuscript was written. He reports no other conflicts. Christy Weiss, Arturo Cowes, Michele Bush, Ganga DeSilva, Jackie Surls, Eileen Sagini, and Amy Altman are employees of Luminex Corporation, Austin, TX, USA and may own stock in the company. Charlotte E. Teunissen reports personal fees from advisory board of Fujirebio and Roche, nonfinancial support from research consumables from Euroimmun, IBL, Fujirebio, Invitrogen, and Mesoscale Discovery, performed contract research for IBL, Shire, Boehringer, Roche, and Probiodrug outside the submitted work. S. Lance Macauley reports research funding from Merck & Co., Inc. Philip Scheltens reports grants from GE Healthcare, Merck & Co., Inc., Piramal, Alzheimer Nederland, Stichting Dioraphte, and Stichting VUMC Fonds. He also reports research funding from AbbVie, Avraham Pharmaceuticals, and Jansen research Foundation; consulting fees from MD Start, Nutricia Research, Takeda Pharmaceuticals, and Probiodrug AG, EIP Pharma. All grants and consulting fees are paid to his institution. He receives no personal fees from anyone. Stephanie R. Rainey-Smith reports funds paid by Merck & Co., Inc., to Edith Cowan University, to cover the costs associated with biological sample and Supplementary Data collection. Johan Luthman reports that he was an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, at the time of the work presented. Merck & Co., Inc. was at that time period engaged in a research and development project together with Luminex, the developer of the assay used in the studies presented here. He is now an employee of Eisai Inc, a company that does not have any research and development agreements with Luminex. James Burke, Geir Bråthen, Sigrid Botne Sando, Linda R. White, and David G. Darby have nothing to declare.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.dadm.2017.02.004.
Supplementary data
References
- 1.Hampel H., Frank R., Broich K., Teipel S.J., Katz R.G., Hardy J. Biomarkers for Alzheimer's disease: academic, industry and regulatory perspectives. Nat Rev Drug Discov. 2010;9:560–574. doi: 10.1038/nrd3115. [DOI] [PubMed] [Google Scholar]
- 2.Jack C.R., Jr., Knopman D.S., Jagust W.J., Shaw L.M., Aisen P.S., Weiner M.W. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perrin R.J., Fagan A.M., Holtzman D.M. Multimodal techniques for diagnosis and prognosis of Alzheimer's disease. Nature. 2009;461:916–922. doi: 10.1038/nature08538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villemagne V.L., Burnham S., Bourgeat P., Brown B., Ellis K.A., Salvado O. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol. 2013;12:357–367. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- 5.Kang J.H., Korecka M., Toledo J.B., Trojanowski J.Q., Shaw L.M. Clinical utility and analytical challenges in measurement of cerebrospinal fluid amyloid-beta(1-42) and tau proteins as Alzheimer disease biomarkers. Clin Chem. 2013;59:903–916. doi: 10.1373/clinchem.2013.202937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lachno D.R., Romeo M.J., Siemers E.R., Vanderstichele H., Coart E., Konrad R.J. Validation of ELISA methods for quantification of total tau and phosporylated-tau181 in human cerebrospinal fluid with measurement in specimens from two Alzheimer's disease studies. J Alzheimers Dis. 2011;26:531–541. doi: 10.3233/JAD-2011-110296. [DOI] [PubMed] [Google Scholar]
- 7.Lachno D.R., Evert B.A., Vanderstichele H., Robertson M., Demattos R.B., Konrad R.J. Validation of assays for measurement of amyloid-beta peptides in cerebrospinal fluid and plasma specimens from patients with Alzheimer's disease treated with solanezumab. J Alzheimers Dis. 2013;34:897–910. doi: 10.3233/JAD-122317. [DOI] [PubMed] [Google Scholar]
- 8.Lewczuk P., Kornhuber J., Vanderstichele H., Vanmechelen E., Esselmann H., Bibl M. Multiplexed quantification of dementia biomarkers in the CSF of patients with early dementias and MCI: a multicenter study. Neurobiol Aging. 2008;29:812–818. doi: 10.1016/j.neurobiolaging.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Olsson A., Vanderstichele H., Andreasen N., De Meyer G., Wallin A., Holmberg B. Simultaneous measurement of beta-amyloid(1-42), total tau, and phosphorylated tau (Thr181) in cerebrospinal fluid by the xMAP technology. Clin Chem. 2005;51:336–345. doi: 10.1373/clinchem.2004.039347. [DOI] [PubMed] [Google Scholar]
- 10.Shaw L.M., Vanderstichele H., Knapik-Czajka M., Figurski M., Coart E., Blennow K. Qualification of the analytical and clinical performance of CSF biomarker analyses in ADNI. Acta Neuropathol. 2011;121:597–609. doi: 10.1007/s00401-011-0808-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jagust W.J., Landau S.M., Shaw L.M., Trojanowski J.Q., Koeppe R.A., Reiman E.M. Relationships between biomarkers in aging and dementia. Neurology. 2009;73:1193–1199. doi: 10.1212/WNL.0b013e3181bc010c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaw L.M., Vanderstichele H., Knapik-Czajka M., Clark C.M., Aisen P.S., Petersen R.C. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tolboom N., van der Flier W.M., Yaqub M., Boellaard R., Verwey N.A., Blankenstein M.A. Relationship of cerebrospinal fluid markers to 11C-PiB and 18F-FDDNP binding. J Nucl Med. 2009;50:1464–1470. doi: 10.2967/jnumed.109.064360. [DOI] [PubMed] [Google Scholar]
- 14.Palmqvist S., Zetterberg H., Mattsson N., Johansson P., Minthon L., Blennow K. Detailed comparison of amyloid PET and CSF biomarkers for identifying early Alzheimer disease. Neurology. 2015;85:1240–1249. doi: 10.1212/WNL.0000000000001991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trojanowski J.Q., Vandeerstichele H., Korecka M., Clark C.M., Aisen P.S., Petersen R.C. Update on the biomarker core of the Alzheimer's Disease Neuroimaging Initiative subjects. Alzheimers Dement. 2010;6:230–238. doi: 10.1016/j.jalz.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Brien J.T., Herholz K. Amyloid imaging for dementia in clinical practice. BMC Med. 2015;13:163. doi: 10.1186/s12916-015-0404-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berge G., Lauridsen C., Sando S.B., Holder D.J., Moller I., Aasly J.O. Effect of Tween-20 on core biomarkers measured in cerebrospinal fluid from patients with Alzheimer's Disease, mild cognitive impairment, or healthy control individuals. J Alzheimers Dis. 2015;49:493–502. doi: 10.3233/JAD-150234. [DOI] [PubMed] [Google Scholar]
- 18.Mattsson N., Andreasson U., Persson S., Carrillo M.C., Collins S., Chalbot S. CSF biomarker variability in the Alzheimer's Association quality control program. Alzheimers Dement. 2013;9:251–261. doi: 10.1016/j.jalz.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention DoLPSaS: Clinical Laboratory Improvement Amendments. Electronic Code of Federal Regulations.
- 20.Wilson E.B. Probable inference, the law of succession, and statistical inference. J Am Stat Soc. 1927;22:209–212. [Google Scholar]
- 21.Quintela-del-Rio A., Estévez-Pérez G. Nonparametric kernel distribution function estimation with kerdiest: an R package for bandwidth choice and applications. J Stat Softw. 2012;50:1–21. [Google Scholar]
- 22.Fagan A.M., Shaw L.M., Xiong C., Vanderstichele H., Mintun M.A., Trojanowski J.Q. Comparison of analytical platforms for cerebrospinal fluid measures of beta-amyloid 1-42, total tau, and p-tau181 for identifying Alzheimer disease amyloid plaque pathology. Arch Neurol. 2011;68:1137–1144. doi: 10.1001/archneurol.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sunderland T., Linker G., Mirza N., Putnam K.T., Friedman D.L., Kimmel L.H. Decreased beta-amyloid1-42 and increased tau levels in cerebrospinal fluid of patients with Alzheimer disease. JAMA. 2003;289:2094–2103. doi: 10.1001/jama.289.16.2094. [DOI] [PubMed] [Google Scholar]
- 24.GE Healthcare . General Electric Company; Arlington Heights, IL: 2014. Vizamyl (Flutemetamol F 18 Injection) Package Insert. [Google Scholar]
- 25.Lilly USA L . Eli Lilly and Company, USA; Indianapolis, IN, USA: 2013. Amyvid (Florbetapir F 18 Injection) Package Insert. [Google Scholar]
- 26.Piramal Imaging SA . Piramal Imaging; Matran, Switzerland: 2014. Neuraceq (Florbetaben F 18 Injection) Package Insert. [Google Scholar]
- 27.Coric V., Salloway S., van Dyck C.H., Dubois B., Andreasen N., Brody M. Targeting prodromal Alzheimer disease with avagacestat: a randomized clinical trial. JAMA Neurol. 2015;72:1324–1333. doi: 10.1001/jamaneurol.2015.0607. [DOI] [PubMed] [Google Scholar]
- 28.Janelidze S., Zetterberg H., Mattsson N., Palmqvist S., Vanderstichele H., Lindberg O. CSF Abeta42/Abeta40 and Abeta42/Abeta38 ratios: better diagnostic markers of Alzheimer disease. Ann Clin Transl Neurol. 2016;3:154–165. doi: 10.1002/acn3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zwan M., van Harten A., Ossenkoppele R., Bouwman F., Teunissen C., Adriaanse S. Concordance between cerebrospinal fluid biomarkers and [11C]PIB PET in a memory clinic cohort. J Alzheimers Dis. 2014;41:801–807. doi: 10.3233/JAD-132561. [DOI] [PubMed] [Google Scholar]
- 30.Landau S.M., Lu M., Joshi A.D., Pontecorvo M., Mintun M.A., Trojanowski J.Q. Comparing positron emission tomography imaging and cerebrospinal fluid measurements of beta-amyloid. Ann Neurol. 2013;74:826–836. doi: 10.1002/ana.23908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palmqvist S., Zetterberg H., Blennow K., Vestberg S., Andreasson U., Brooks D.J. Accuracy of brain amyloid detection in clinical practice using cerebrospinal fluid beta-amyloid 42: a cross-validation study against amyloid positron emission tomography. JAMA Neurol. 2014;71:1282–1289. doi: 10.1001/jamaneurol.2014.1358. [DOI] [PubMed] [Google Scholar]
- 32.Bloudek L.M., Spackman D.E., Blankenburg M., Sullivan S.D. Review and meta-analysis of biomarkers and diagnostic imaging in Alzheimer's disease. J Alzheimers Dis. 2011;26:627–645. doi: 10.3233/JAD-2011-110458. [DOI] [PubMed] [Google Scholar]
- 33.Formichi P., Battisti C., Radi E., Federico A. Cerebrospinal fluid tau, A beta, and phosphorylated tau protein for the diagnosis of Alzheimer's disease. J Cell Physiol. 2006;208:39–46. doi: 10.1002/jcp.20602. [DOI] [PubMed] [Google Scholar]
- 34.Committee for Medicinal Products for Human Use (CHMP): Qualification Opinion of Alzheimer's Disease Novel Methodologies/biomarkers for BMS-708163. EMA/CHMP/SAWP/102001/2011.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.