Abstract
Background and Objectives:
“Trifecta” in partial nephrectomy consists of negative surgical margins, minimal renal function decrease and absence of complications. In the present article, our single-center robot-assisted partial nephrectomy (RAPN) experience in T1b renal masses is reported in terms of strict Trifecta outcomes.
Methods:
This is a retrospective analysis of patients with a tumor diameter between 4 and 7 cm (stage T1b), who underwent RAPN by a single surgeon. Preoperative, intraoperative, and postoperative data were recorded and analyzed to evaluate short-term functional and oncologic outcomes. Patients with absence of grade ≥ 2 Clavien-Dindo complications, warm ischemia time (WIT) ≤25 minutes, ≤15% postoperative estimated glomerular filtration rate (eGFR) decrease and negative surgical margins were reported to achieve strict Trifecta outcomes. P < .05 was indicated statistically significant.
Results:
A total of 150 patients underwent RAPN, and 50 patients were identified with tumor size between 4 and 7 cm. Mean WIT was 20.8 ± 6.2 minutes and mean estimated blood loss (EBL) was 269 ± 191 mL. Surgical margins were negative in all patients. Eleven patients (22%) had a >15% eGFR decrease after surgery. Nine patients (18%) had WIT longer than 25 minutes. Four patients (8%) had grade ≥2 Clavien-Dindo complications. Twenty-nine (58%) patients had strict Trifecta outcomes. Mean follow-up was 44.2 ± 27.2 months. Tumor recurrence was not observed in any patient.
Conclusions:
Robot-assisted laparoscopic partial nephrectomy for T1b renal masses can be safely performed in experienced hands. Optimal strict Trifecta outcomes and recurrence rates can be achieved.
Keywords: Renal mass, Robot-assisted partial nephrectomy, T1b, Trifecta
INTRODUCTION
Nephron-sparing surgery remains the preferred surgical approach for the treatment of patients with T1b (4–7 cm) renal tumors, whenever technically feasible. Most urologists would agree that it should be favored over radical nephrectomy.1 Despite the fact that open partial nephrectomy has remained the gold standard technique for the management of these patients for many years, laparoscopic partial nephrectomy series have reported similar oncologic outcomes with significantly shorter hospital stay and lower analgesic requirement.2,3 Moreover, during the evolution of laparoscopy, introduction of the robotic platform enabled transfer of the experience from laparoscopic cases to this novel technology and thus robot-assisted partial nephrectomy (RAPN) has replaced standard laparoscopy in many high-volume centers.4 Gradually, larger and more complex tumors have been treated with robot assistance, yielding encouraging outcomes.5
“Trifecta” is a relatively new concept adopted from the radical prostatectomy literature to report the outcomes of partial nephrectomy. It was first reported in 2013 by Hung et al6 and consisted of providing negative surgical margins, minimal renal function decrease, and absence of complications in the same patient. Since renal function preservation correlates with warm ischemia time (WIT), some authors reported a WIT ≤ 25 minutes to be a constituent of “Trifecta” outcomes.7,8 Hence, after the first introduction, different definitions of Trifecta were reported. In some studies, it was defined as no complications, negative surgical margins, and WIT ≤25 minutes.7,8 Trifecta is not standardized, and there are sparse data reporting such outcomes in robot-assisted partial nephrectomy for T1b tumors in the literature. In this study, to evaluate renal function preservation more precisely, both WIT and postoperative day 30 eGFR decrease were considered. Patients with absence of grade ≥2 Clavien-Dindo complications, WIT ≤25 minutes, ≤15% postoperative eGFR decrease and negative surgical margins were reported to achieve strict Trifecta outcomes. To prove the wider feasibility and safety of RAPN in T1b renal masses, further standardized evidence is needed, because available data in the literature consist of small series, mostly reporting on small renal masses (≤4 cm) with nonstandardized evaluation of outcomes. To the best of our knowledge, the present article reports data from one of the largest single-center RAPN series, in terms of (strict) Trifecta outcomes for management of T1b renal masses.
METHODS
The study was a retrospective analysis of patients who underwent RAPN by a single surgeon between April 2008 and February 2016. From this cohort, patients with a tumor diameter between 4 and 7 cm were selected (stage T1b). Preoperative characteristics recorded included age, body mass index (BMI), American Society of Anesthesiologists (ASA) physical status score, comorbidities, hematocrit (Hct), serum creatinine (Cr) level, eGFR, size, side, and Padua score of the mass. Intraoperative and postoperative data noted operative time, WIT, surgical margin status, estimated blood loss (EBL), length of hospital stay (LOS), length of placement of drain and urethral catheter, intra- and postoperative complications, postoperative serum Cr level, Hct, eGFR, histological type and Fuhrman grade (when applicable) and follow-up period. eGFR was calculated with the modification of diet in renal disease equation9 1 month after surgery. The Padua score was used in efforts to classify patients according to individual anatomic details.10
Patients were evaluated with computed tomography (CT) or magnetic resonance imaging (MRI) before surgery. Renal vascular anatomy was investigated in detail on these images. RAPN was not favored for masses that were completely endophytic, or near the hilar vessels, or posteriorly located at the upper pole, or above 7 cm in diameter. Postoperative complications were documented based on the Clavien-Dindo grading system. Patient follow-up consisted of visits at months 1 and 3, and every 3 months thereafter for the first year and every 6 months for years 2–3. Physical examination and sonography imaging were performed at each visit and magnetic resonance imaging (MRI) at the 12-month follow-up.
Patients with an absence of grade ≥ 2 Clavien-Dindo complications, WIT ≤ 25 minutes ≤15% postoperative eGFR decrease and negative surgical margins were reported to achieve strict Trifecta outcomes.
For statistical analysis, Student's t test was used to compare the variables with the use of IBM SPSS v21. A p-value of < 0.05 indicated statistical significance.
Operative technique
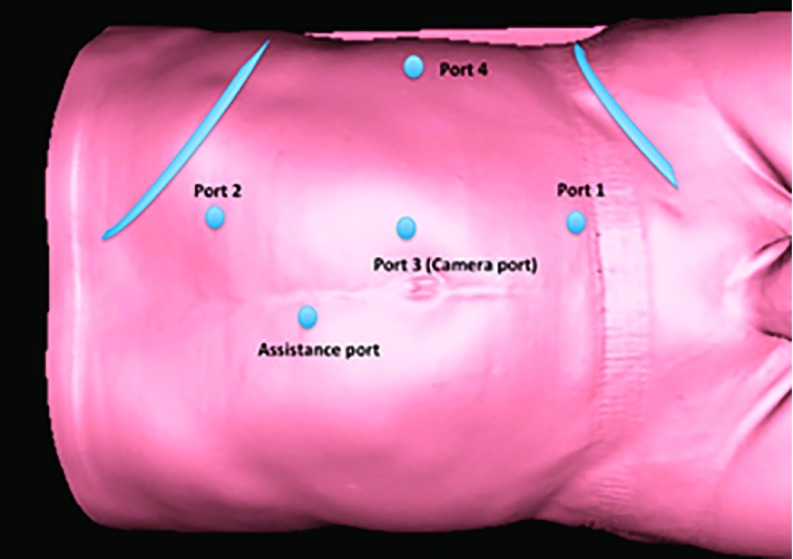
All procedures were performed by a single surgeon with extensive experience in robot-assisted surgery. Operations were performed using the 3 da Vinci surgical systems (S-HD, Si, or Xi; Intuitive Surgical, Sunnyvale, California, USA) over the years. First cases were performed with the S-HD system in 2008, and then the system was converted to the Si in 2009 and eventually to the Xi system in 2014. Since 2014, the Xi system had been used for the procedure. Patients were positioned in a modified flank position with the diseased side up and then flexed using the table break at the level of anterior superior iliac crest. Pneumoperitoneum was established using a Veress needle. A 5-port configuration was used as shown in Figure 1 (one more port is added for liver retraction on right side tumors).
Figure 1.
Port configuration (left side).
After dropping the bowel, the renal pedicle was dissected. The renal artery was visualized and prepared for clamping. The tumor margin and its proximity to the collecting system were delineated with the aid of the laparoscopic ultrasonography probe. Then, the circumference of the mass was scored with hot scissors. After mannitol infusion, the renal artery was clamped with either 1 or 2 Reliance bulldog clamps (Scanlan International, St. Paul, Minnesota, USA). The mass was subsequently excised using resection, enucleation, or a combination of both based on individual anatomy. The resection base was then sutured with a running 3/0 V-Loc suture (Medtronic, Minneapolis, Minnesota, USA) for hemostasis and collecting system repaired (if entered). Renorrhaphy was performed with No-0 polyglactin (Vicryl) sutures. Suture tension was controlled with sliding Hem-o-Loc clips (Teleflex, Morrisville, North Carolina, USA). Oxycellulose was placed on the renorrhaphy line in every case and additional hemostatic agent (human gelatin-thrombin matrix sealant), when necessary. The bulldog clamp was subsequently removed and the surgical field monitored for bleeding. A JJ urinary catheter was not inserted in any patient before or during the procedure.
RESULTS
A total of 150 patients underwent RAPN during the study period. Data presented focuses on 50 in whom the tumor size was between 4 and 7 cm. Mean age and BMI of these patients was 53.1 ± 12.7 y and 28.9 ± 6.2 kg/m2, respectively. Mean tumor size in the surgical specimen was 47.6 ± 6.9 mm. The tumor was on the left side in 28 and right side in 22. Among the 50 patients with pT1b tumor, 21 had associated comorbidities (8 hypertension, 11 diabetes mellitus, and 2 cardiac conditions) with an average ASA score of 1.7 ± 0.5. The mean Padua score was 9.8 ± 1.5 (range, 7–13).
Patient characteristics and operative data are depicted in Table 1. The mean duration of surgery was 145 ± 41 minutes, mean WIT was 20.8 ± 6.2 minutes and mean EBL was 269 ± 191 mL. Nine patients (18%) had WIT > 25 minutes. Pathological evaluation was consistent with clear-cell carcinoma in 35 (70%), papillary carcinoma type 1 in 6 (12%) and chromophobe carcinoma in 5 (10%). Two patients (4%) had benign renal cyst, 1 (2%) had angiomyolipoma and 1 (2%) had liposarcoma. Surgical margins were negative in all patients. Frozen section analysis was performed in 5 patients (10%) during surgery to confirm negative surgical margins.
Table 1.
Patient Characteristics and Operative Data
| Patient Characteristics | Operative Data | ||
|---|---|---|---|
| Age (years) | 53.1 ± 12.7 | Operative time (mins) | 145 ± 41 |
| BMI (kg/m2) | 28.9 ± 6.2 | WIT (mins) | 20.8 ± 6.2 |
| ASA score | 1.7 ± 0.5 | EBL (ml) | 269 ± 191 |
| Padua score | 9.8 ± 1.5 | Drain removal period (days) | 2.7 ± 0.7 |
| Tumor size (mm) | 47.6 ± 6.9 | Foley catheter removal period (days) | 1.5 ± .9 |
| Tumor side | 28 Left | Complications | 6/50 (12%) |
| 2 Clavien I | |||
| 22 Right | 1 Clavien II | ||
| 2 Clavien IIIB | |||
| 1 Clavien IV | |||
N = 50. Mean follow-up 40.1 ± 27.4 months.
Complications were observed in 6 patients (2 Clavien I, 1 Clavien II, 2 Clavien IIIB, and 1 Clavien IV). Two patients had postoperative bleeding that resolved with conservative management (Clavien I). One patient had postoperative bleeding necessitating transfusion (Clavien II) and another developed renal artery pseudoaneurysm 11 d after surgery and underwent a successful selective renal artery embolization (Clavien IIIB). Urinary extravasation was present in 1 patient and a JJ catheter was inserted (Clavien IIIB). Another patient developed necrotizing fasciitis in the perianal region, underwent surgical excision, and was followed up in the intensive care unit (Clavien IV). The mean drain and Foley catheter removal time points were 2.7 ± 0.7 and 1.5 ± 0.9 d, respectively. Mean length of hospital stay was 4.1 ± 0.8 d. Mean preoperative Cr level, eGFR value, and Hct levels were 0.87 ± 0.21 mg/dL, 92.3 ± 19.4 mL/min per 1.73 m2, and 41.9 ± 4.2%, respectively. Postoperative Cr and eGFR values (at month 1) were 0.95 ± 0.26 mg/dL and 86.74 ± 19.38 mL/min per 1.73 m2, respectively. Mean postoperative Hct was 36.05 ± 4.18%. Pre-and postoperative Cr, eGFR, and Hct differences were statistically significant in favor of the preoperative values with P = .001, .004, and .001 for Cr, eGFR, and Hct, respectively (Table 2). Eleven patients (22%) had a >15% decrease in eGFR after surgery. The mean follow-up was 44.2 ± 27.2 months.
Table 2.
Comparison of Pre- and Postoperative Renal Function and Hct
| Preoperative | Postoperative | P* | |
|---|---|---|---|
| Creatinine (mg/dL) | 0.87 ± 0.21 | 0.95 ± 0.26 | 0.001 |
| eGFR (mL/min per 1.73 m2) | 92.3 ± 19.4 | 86.74 ± 19.38 | 0.004 |
| Hct (%) | 41.9 ± 4.2 | 36.05 ± 4.18 | 0.001 |
N = 50. Hct, Hematocrit.
Student's t test.
Twenty-nine (58%) patients had strict Trifecta outcomes. Because all patients had negative surgical margins, Trifecta outcomes were based on WIT, postoperative eGFR decrease, and complications. Four patients (8%) had grade ≥ 2 Clavien-Dindo complications, 9 (18%) had WIT longer than 25 minutes, and 11 (22%) had >15% eGFR decrease, after surgery. Two patients with a >15% postoperative eGFR decrease had grade ≥ 2 Clavien-Dindo complications, and 1 patient with > 15% postoperative eGFR decrease had a WIT longer than 25 minutes (Table 3).
Table 3.
Strict Trifecta outcomes
| WIT | Preop–Postop eGFR Decrease | Complications (Clavien-Dindo) | Surgical Margins | ||||
|---|---|---|---|---|---|---|---|
| ≤25 min | 41 (82%) | ≤ 15% | 39 (78%) | < Gr 2 | 2 (4%) | (−) | 50 (100%) |
| >25 min | 9 (18%) | > 15% | 11 (22%) | ≥ Gr 2 | 4 (8%) | (+) | 0 |
Strict Trifecta was obtained in 29/50 (58%) of cases. Two patients had a >15% preop–postop eGFR decrease and grade ≥2 Clavien-Dindo complication, and 1 patient had >15% preop–postop eGFR decrease and a WIT >25 minutes.
DISCUSSION
Nephron sparing techniques with the use of the robotic platform are gaining widespread popularity among many centers across the world, and indications are continuously expanding. As patients harboring masses larger in diameter and complexity are considered candidates for RAPN, concerns for safety and oncological outcomes become evident. The use of the term Trifecta to describe the success and outcomes of RAPN is relatively new and may contribute standardized evaluation of outcomes.6 Trifecta criteria differ in the literature, but principally consist of absence of complications, negative surgical margins, and minimal renal function decrease. Because WIT of 25 minutes is reported to be significantly associated with new-onset stage IV chronic kidney disease,6 25 minutes was set as the cutoff for WIT in this series. Also, to ensure optimal renal function preservation evaluation, ≤15% postoperative eGFR decrease was established as a criterion of the Trifecta. Hence, 2 criteria for evaluation of renal function preservation was used. Absence of grade ≥2 Clavien-Dindo complications, WIT ≤25 minutes, ≤15% postoperative eGFR decrease, and negative surgical margins were determined as strict Trifecta outcomes. Fifty RAPN patients with T1b renal masses were evaluated with strict Trifecta outcomes.
Patel et al11 were the first to demonstrate the safety profile of RAPN in 15 patients with tumors >4 cm, comparing patients who underwent RAPN for T1a vs T1b tumors. Median console time was 275 minutes. Although WITs (25 minutes) were significantly longer and severe complication rates (19.8%) were significantly higher for T1b tumors, the feasibility of this operation was demonstrated.11 Likewise, Gupta et al12 reported a median WIT of 36 minutes, a longer console time (390 minutes) and a lower severe complication rate (6%) for T1b cancer. As robotic-assisted surgery experience increased, RAPN results improved. More recently, studies involving larger cohorts and multicentric experience have reported data showing very promising results.13–16 Nevertheless, major complication rate remains at 4.7%.17 In our series, 6 patients had complications (2 Clavien-Dindo I, 1 Clavien-Dindo II, 2 Clavien-Dindo IIIB, and 1 Clavien-Dindo IV). Four patients (8%) had ≥II Clavien-Dindo complications. Necrotizing fasciitis, a very rare complication (Clavien IV), would not be likely to be related directly to tumor size. Hence, practically, a ≥2 Clavien-Dindo complication rate was 6% in our series. Regarding strict Trifecta outcomes and excluding this Clavien IV complication, the complication rate was 6% (3 patients), and the strict Trifecta outcome was 60% in our series. From our point of view, a comparison of our experience in RAPN for T1a and T1b tumors is beyond the scope of this article. Nevertheless, it would be intriguing to mention that, in our cohort of patients with T1a tumors in whom we performed RAPN, the mean WIT was 16.64 minutes, the console time was 115.64 minutes, and the EBL was 180.19 mL, which, in combination, demonstrate a trend toward equivalent results between the T1a and T1b tumor groups. Of course, a more detailed statistical analysis and matching between the 2 groups is mandatory to draw safe conclusions.
The potential impact of positive surgical margins (PSMs) on recurrence rate and overall survival in renal cancer remains controversial. High-volume studies have not necessarily proved a clear correlation.18,19 Nevertheless, some have demonstrated that PSM influences tumor recurrence rate,20,21 but does not appear to have an effect on cancer-specific survival,21 suggesting that conclusions should not be drawn without long-term follow-up. The prevalence of PSM in nephron-sparing techniques has been reported to be in the range of 4.9–8.1% for the open and laparoscopic approaches.22 PSM range has been reported to be 0–8.7% for the robotic approach.10–16,22 In our series, we did not encounter any PSMs in the final pathology. Several intra-operative techniques can aid in accomplishing the above-mentioned goal of achieving negative surgical margins. In our view, using laparoscopic ultrasonography is critical. Its role in better visualization of the depth and margins of the tumor has been documented,23 and we used this device in every case. Clamping of the renal hilum definitely allows for higher quality vision of the tumor border in a relatively bloodless field (of course, at the expense of ischemia). Even though some studies in the literature failed to demonstrate a difference in PSMs between clamped and unclamped operations,24 we strongly believe that superior imaging allows for significantly more precise dissection and reconstruction. The hilum was not clamped in 2 cases in the series, both of whom had negative surgical margins. As with any other surgical procedure, surgeon experience is key for optimal operative and oncologic outcomes.25 The surgeon in this series has a background of a large experience with open, laparoscopic and eventually robotic partial nephrectomies after the introduction of robotic technology. Thus, surgeon expertise is probably another factor accounting for the favorable margin rate. Finally, some novel techniques, such as near-infrared fluorescence after intravenous injection of indocyanine green (ICG), may be valuable in obtaining intraoperative information about the tumor extent and vasculature and decrease the positive surgical margin rate, but its effectiveness is yet to be proven.26 We used this technology in sporadic cases in this series. Although ICG injection has eased tumor delineation, it is not wise to make a comment with very limited experience.
A vital outcome for all nephron-sparing procedures is the degree of preservation of renal function. Not surprisingly, most studies depicted a decline in eGFRs and an increase in serum Cr levels. eGFR is one of the most commonly used indicators of renal function and the reported deterioration rate remains between 5.6 and 14.7% before and after RAPN in the literature.11–16 In this study, a WIT of 25 minutes and ≤15% postoperative eGFR decrease were determined as strict Trifecta criteria to ensure optimal renal function preservation. Although pre- and postoperative Cr and eGFR differences were statistically significant in favor of preoperative values, our eGFR deterioration rate of 6% is in line with the above-mentioned outcomes in the literature supporting good functional outcomes of RAPN in T1b tumors at postoperative month 1.
Our results with 41 patients (82%) having WIT ≤ 25 minutes, an overall median WIT of 20 minutes, a console time of 144 minutes, an 8% ≥2 Clavien-Dindo complication rate, and no positive margins imply that RAPN is a potentially safe procedure for T1b tumors. Kim et al7 and Porpiglia et al8 reported Trifecta outcomes in 43and 69.5% of robot-assisted surgical T1b patients, respectively. In both of these studies WIT ≤ 25 minutes was used for evaluation of renal function preservation. If only WIT ≤ 25 minutes was used to evaluate renal function preservation in our series, the Trifecta rate would be 80%. Our strict Trifecta outcomes in 58% of patients prove the efficacy of RAPN for management of T1b renal masses.
The patients in this series had tumors with intermediate Padua score (>9) (7 being the minimum score and 13 the highest), suggesting relatively complex masses. Because the Padua system gives 2 points to size between 4 and 7 cm, most common, more complex anatomic features of the tumors were of endophytic location and nearness to the collecting system and hilus. Anatomic features of the tumor were evaluated before surgery, and RAPN was not favored for masses that were completely endophytic, or near the hilar vessels, or posteriorly located at the upper pole, or above 7 cm in diameter. Consequently, an important finding of the present study is that no recurrences were observed during follow-up.
We acknowledge certain limitations related to the study. It is retrospective, and to draw definite conclusions, prospective studies would be in order. It reflects experience on a small number of patients, even though, to the best of our knowledge, this study is among the largest in the literature in terms of RAPN for T1b tumors. In addition, evaluating the outcomes with strict Trifecta criteria may contribute to the standardized reporting and evaluation of the outcomes. Follow-up is limited to 44 months. Finally, the time of eGFR evaluation at follow-up (1 month) may be too early to determine final renal function outcome.
Robot-assisted laparoscopic partial nephrectomy for T1b renal masses may be safely performed in experienced hands. Optimal strict Trifecta outcomes and recurrence rates may be achieved. Strict Trifecta criteria may contribute to a standardized evaluation of outcomes.
Contributor Information
Ilter Tufek, Department of Urology, School of Medicine, Istanbul Acıbadem University, Istanbul, Turkey..
Panagiotis Mourmouris, Department of Urology, School of Medicine, Istanbul Acıbadem University, Istanbul, Turkey..
Tunkut Doganca, Department of Urology, Taksim Acıbadem Hospital, Istanbul, Turkey..
Can Obek, Department of Urology, Taksim Acıbadem Hospital, Istanbul, Turkey..
Omer Burak Argun, Department of Urology, School of Medicine, Istanbul Acıbadem University, Istanbul, Turkey..
Mustafa Bilal Tuna, Department of Urology, Acıbadem Maslak Hospital, Istanbul, Turkey..
Mehmet Selcuk Keskin, Department of Urology, School of Medicine, Istanbul Acıbadem University, Istanbul, Turkey.; Department of Urology, Acıbadem Maslak Hospital, Istanbul, Turkey.
Ali Rıza Kural, Department of Urology, School of Medicine, Istanbul Acıbadem University, Istanbul, Turkey..
References:
- 1. Ljungberg B, Bensalah K, Canfield S, et al. EAU Guidelines on Renal Cell Carcinoma: Update. Eur Urol. 2015;67:913–924. [DOI] [PubMed] [Google Scholar]
- 2. Marszalek M, Meixl H, Polajnar M, et al. Laparoscopic and open partial nephrectomy: a matched-pair comparison of 200 patients. Eur Urol. 2009;55:1171–1178. [DOI] [PubMed] [Google Scholar]
- 3. Gill IS, Kavoussi LR, Lane BR, et al. Comparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumors. J Urol. 2007;178:41–46. [DOI] [PubMed] [Google Scholar]
- 4. Aboumarzouk OM, Stein RJ, Eyraud R, et al. Robotic versus laparoscopic partial nephrectomy: a systematic review and meta-analysis. Eur Urol. 2012;62:1023–1033. [DOI] [PubMed] [Google Scholar]
- 5. Masson-Lecomte A, Yates DR, Bensalah K, et al. Robot-assisted laparoscopic nephron sparing surgery for tumors over 4 cm: operative results and preliminary oncologic outcomes from a multicenter French study. Eur J Surg Oncol. 2013;39:799–803. [DOI] [PubMed] [Google Scholar]
- 6. Hung AJ, Cai J, Simmons MN, Gill IS. “Trifecta” in partial nephrectomy. J Urol. 2013;189:36–42. [DOI] [PubMed] [Google Scholar]
- 7. Kim DK, Kim LHC, Raheem AA, et al. Comparison of trifecta and pentafecta outcomes between T1a and T1b renal masses following robot-assisted partial nephrectomy (RAPN) with minimum one year follow up: can RAPN for T1b renal masses be feasible? PLoS One. 2016;11:e0151738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Porpiglia F, Mari A, Bertolo R, et al. Partial nephrectomy in clinicalT1b renal tumors: multicenter comparative study of open, laparoscopic, and robot-assisted approach (the RECORd Project). Urology. 2016;89:45–51. [DOI] [PubMed] [Google Scholar]
- 9. Levey AS, Stevens LA, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ficarra V, Novara G, Secco S, et al. Preoperative aspects and dimensions used for an anatomical (PADUA) classification of renal tumours in patients who are candidates for nephron-sparing surgery. Eur Urol. 2009;56:786–793. [DOI] [PubMed] [Google Scholar]
- 11. Patel MN, Krane LS, Bhandari A, et al. Robot partial nephrectomy for renal tumors larger than 4 cm. Eur Urol. 2010;57:310–316. [DOI] [PubMed] [Google Scholar]
- 12. Gupta GN, Boris R, Chung P, et al. Robot-assisted laparoscopic partial nephrectomy for tumors greater than 4 cm and high nephrometry score: feasibility, renal functional, and oncological outcomes with minimum 1 year follow-up. Urol Oncol. 2013;31:51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Petros F, Sukumar S, Haber GP, et al. Multi-institutional analysis of robot assisted partial nephrectomy for renal tumors >4 cm versus ≤ 4 cm in 445 consecutive patients. J Endourol. 2012;26:642–646. [DOI] [PubMed] [Google Scholar]
- 14. Ficarra V, Bhayani S, Porter J, et al. Robot-assisted partial nephrectomy for renal tumors larger than 4 cm: results of a multicenter, international series. World J Urol. 2012;30:665–670. [DOI] [PubMed] [Google Scholar]
- 15. Janda G, Deal A, Yang H, et al. Single institution experience with robotic partial nephrectomy for renal masses greater than 4 cm. J Endourol. 2016;30:384–389. [DOI] [PubMed] [Google Scholar]
- 16. Masson-Lecomte A, Yates DR, Bensalah K, et al. Robot-assisted laparoscopic nephron sparing surgery for tumors over 4 cm: operative results and preliminary oncologic outcomes from a multicentre French study. Eur J Surg Oncol. 2013;39:799–803. [DOI] [PubMed] [Google Scholar]
- 17. Bi L, Zhang C, Li K, et al. Robotic partial nephrectomy for renal tumors larger than 4 cm: a systematic review and metaanalysis. PLoS One. 2013;8:e75050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yossepowitch O, Thompson RH, Leibovich BC, et al. Positive surgical margins at partial nephrectomy: predictors and oncological outcomes. J Urol. 2008;179:2158–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lopez-Costea MA, Fumadó L, Lorente D, et al. Positive margins after nephron-sparing surgery for renal cell carcinoma: long-term follow-up of patients on active surveillance. BJU Int. 2010;106:645–648. [DOI] [PubMed] [Google Scholar]
- 20. Bernhard JC, Pantuck AJ, Wallerand H, et al. Predictive factors for ipsilateral recurrence after nephron-sparing surgery in renal cell carcinoma. Eur Urol. 2010;57:1080–1086. [DOI] [PubMed] [Google Scholar]
- 21. Bensalah K, Pantuck AJ, Rioux-Leclercq N, et al. Positive surgical margin appears to have negligible impact on survival of renal cell carcinomas treated by nephron-sparing surgery. Eur Urol. 2010;57:466–471. [DOI] [PubMed] [Google Scholar]
- 22. Tabayoyong W, Abouassaly R, Kiechle JE, et al. Variation in surgical margin status by surgical approach among patients undergoing partial nephrectomy for small renal masses. J Urol. 2015;194:1548–1553. [DOI] [PubMed] [Google Scholar]
- 23. Marszalek M, Carini M, Chlosta P, et al. Positive surgical margins after nephron-sparing surgery. Eur Urol. 2012;61:757–763. [DOI] [PubMed] [Google Scholar]
- 24. Smith GL, Kenney PA, Lee Y, et al. Non-clamped partial nephrectomy: techniques and surgical outcomes. BJU Int. 2011;107:1054–1058. [DOI] [PubMed] [Google Scholar]
- 25. Mottrie A, De Naeyer G, Schatteman P, et al. Impact of the learning curve on perioperative outcomes in patients who underwent robotic partial nephrectomy for parenchymal renal tumours. Eur Urol. 3010;58:127–132. [DOI] [PubMed] [Google Scholar]
- 26. Tobis S, Knopf JK, Silvers CR, et al. Near infrared fluorescence imaging after intravenous indocyanine green: initial clinical experience with open partial nephrectomy for renal cortical tumors. Urology. 2012;79:958–964. [DOI] [PubMed] [Google Scholar]