Highlights
-
•
MTOR pathway genes are often mutated in ovarian clear cell carcinomas (OCCC).
-
•
11.2% of OCCC have targetable alterations only in the mTOR pathway.
-
•
MTOR pathway mutations in OCCC can underlie robust, lasting responses to everolimus.
Keywords: Ovarian clear cell carcinoma, PIK3CA, PTEN, Everolimus, Comprehensive genomic profiling
1. Introduction
Ovarian clear cell carcinoma (OCCC) accounts for 5–13% of ovarian carcinomas, and is associated with higher prevalence in women of Asian descent, poorer prognosis particularly at advanced stages, and relative platinum insensitivity compared to serous carcinomas. Loss of the SWI/SNF chromatin remodeling gene, ARID1A, and frequent PI3K pathway activation has been previously observed in OCCCs.
The mechanistic target of rapamycin (mTOR) is a serine/threonine kinase complex that regulates cellular growth, metabolism, and cell cycle progression. mTOR is directly involved in many cell signaling pathways such as the phosphatidylinositol-3-kinase (PI3k)/AKT pathway. PIK3CA mutation or amplification, AKT overexpression, and loss of PTEN (phosphatase and tensin homolog) lead to an increased mTOR activity (Husseinzadeh and Husseinzadeh, 2014). The PI3K/AKT/mTOR pathway is regarded as an attractive candidate for therapeutic interventions, and inhibitors targeting different components are under various stages of clinical development (Mabuchi et al., 2015).
There are four main categories of inhibitors targeting the mTOR pathway: mTOR inhibitors, PI3K inhibitors, dual mTOR/PI3K inhibitors, and AKT inhibitors. The rapamycin analog everolimus is an oral mTOR inhibitor currently approved for treatment-refractory advanced renal cell carcinoma, advanced pancreatic neuroendocrine tumors, and the tuberous sclerosis complex-associated tumors renal angiomyolipoma and subependymal giant-cell astrocytomas (Ray-Coquard et al., 2013). For both ovarian and endometrial cancers, everolimus is currently being evaluated in clinical trials either as monotherapy or in combination with cytotoxic and hormonal agents (Mabuchi et al., 2015, Ray-Coquard et al., 2013, Wheler et al., 2014, Myers, 2013).
Here we present a case study detailing a response to treatment with everolimus for a patient whose recurrent, metastatic OCCC harbored three alterations affecting PIK3CA and PTEN, detected by comprehensive genomic profiling (CGP) using next-generation sequencing (NGS) techniques. Additionally, CGP results of 125 advanced/recurrent stage OCCCs, including the case presented here, are included to highlight the frequency of clinically relevant GA (CRGA) that predict subsets of patients who may benefit from therapies targeting the PI3K/AKT/mTOR pathway.
2. Methods
Comprehensive genomic profiling (FoundationOne®) was performed on 125 consecutive clinically advanced/recurrent ovarian clear cell carcinomas (Table 1), as described previously (Frampton et al., 2013). In brief, DNA was extracted from 40 μm of formalin-fixed, paraffin-embedded sections, and CGP was performed on hybridization captured libraries of 236 (n = 73) or 315 (n = 52) genes, plus select introns frequently rearranged in cancer, were sequenced to high, uniform coverage (average 609 ×). Sequence data were analyzed for clinically relevant classes of genomic alterations, including base pair substitutions, insertions/deletions, copy number alterations, and rearrangements. Clinically relevant genomic alterations (CRGA) were defined as genomic alterations associated with response to therapies currently available or in target-driven clinical trials.
Table 1.
Characteristics of the ovarian clear cell carcinoma tumors analyzed by CGP.
| Total cases | 125 |
| Patient age (y) | |
| Average | 52.6 |
| Median | 52 |
| Range | 30–80 |
| Specimen sitesa | |
| Primary | 57 |
| Regional | 17 |
| Distant | 51 |
| Genomics | |
| Cases with reportable alterations | 123 |
| Cases without reportable alterations | 2 |
| Average GA/tumor | 5 |
Primary specimen site is the ovary; regional sites were the peritoneum, fallopian tube, pelvis, or uterus; distant sites were all others.
One sample analyzed was the liver metastastectomy sample of a 36-year-old woman with recurrent disease after progression following multiple lines of treatment received over the course of 5 years, including surgery, radiation therapy, hormonal therapy (anastrozole) and conventional chemotherapy (taxol/carboplatin, gemcitabine/carboplatin). After genomic analysis of her tumor metastasis, treatment with everolimus was initiated at 10 mg daily and continues with clinical benefit after 27 months.
Approval for this study, including a waiver of informed consent and a HIPAA waiver of authorization, was obtained from the Western Institutional Review Board (IRB# 20152817).
3. Results
A total of 125 OCCC, 45.6% from primary site tissue and 54.4% from metastatic sites (13.6% regional and 40.8% distant), were included in the analysis (Table 1). The patients were women aged 30–80 (median 52 y) with predominantly advanced stage OCCC. 112 cases (89.6%) had at least one CRGA (avg 2.2 CRGA/tumor). The most common CRGAs affected the following genes: PIK3CA (52.8%), ARID1A (51.2%), TP53 (21.6%), ZNF217 (17.6%), ERBB2 (12.8%), KRAS (8%), CCNE1 (7.2%), CRKL (4.8%). In the 52% of cases with ARID1A loss, PIK3CA activating GA co-occurred in more than half (56%) and TP53 LOF in 20%. In contrast to previously published studies, this cohort of OCCC had lower rates of focal MET amplification (1.6%), PTEN loss (5.6%), and MSI-H status (4%). KRAS alterations were found in 6/87 cases of OCCC with CRGA in the MTOR pathway; 4/38 OCCC harbored KRAS GA but lacked MTOR pathway CRGA.
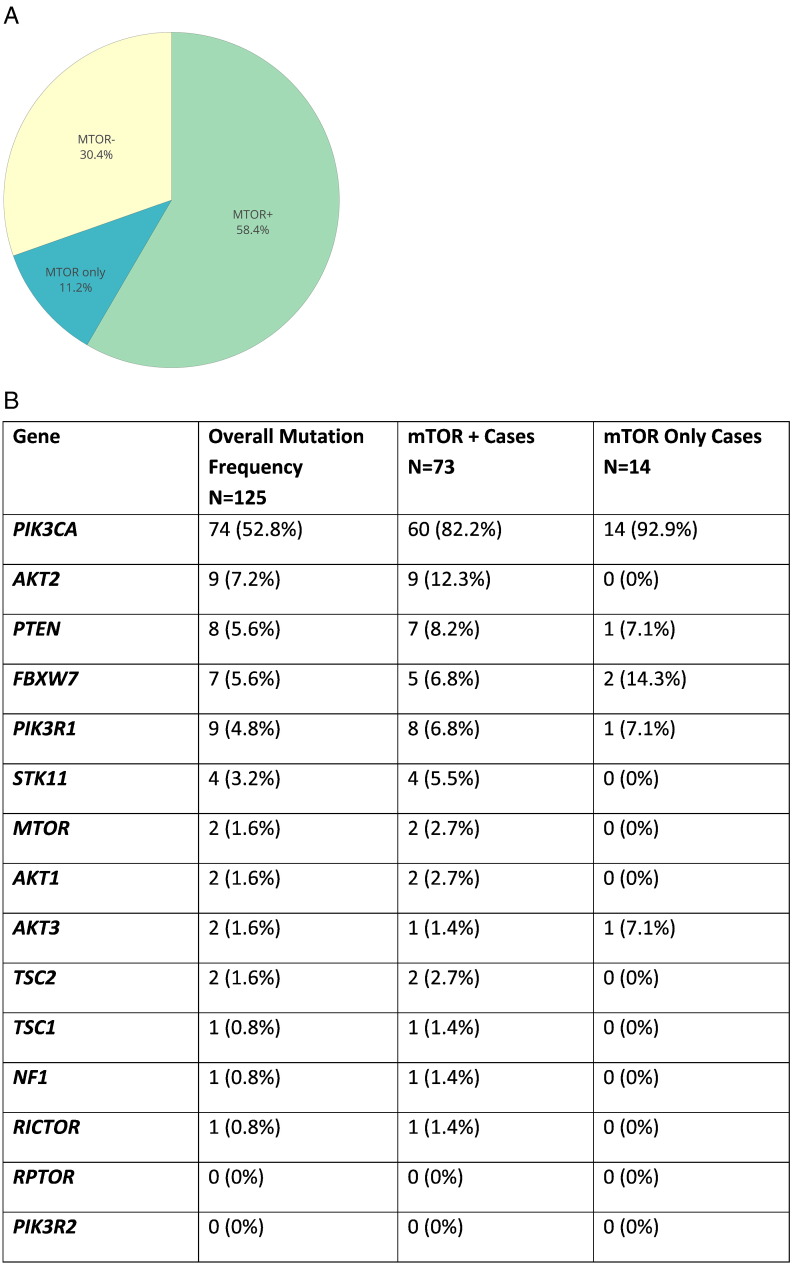
Of these 125 OCCC, 87 (69.6%) cases harbored mutations in at least one component of the mTOR pathway (Fig. 1a), with 14 (11.2%) cases harboring CRGA only in the mTOR pathway. One of these cases, harboring an activating mutation affecting PIK3CA (E545A) and two alterations that result in loss of PTEN activity (Q171*, R233*), is described in greater detail below. Mutations affecting PIK3CA were by far the most common PI3K/AKT/mTOR pathway alterations, and were found in 59.2% of all samples, 83.6% of samples with mutation of the mTOR pathway, and 92.9% of samples with CRGA in only the mTOR pathway.
Fig. 1.
Distribution of mutations affecting the PI3K/AKT/mTOR pathway in ovarian clear cell carcinoma. A. More than 69% of samples harbor at least one alteration in the MTOR pathway, and 11.2% of samples harbor CRGA only in the MTOR pathway. B. Distribution of mutations across genes that comprise the MTOR pathway. The number of alterations in each gene is listed. The percentage of cases affected is shown in parentheses. Some cases harbor multiple alterations in the same gene. See Supplemental Table 1 for more details on the types of alterations observed.
One patient whose tumor underwent CGP analysis is a 36-year-old woman who initially underwent emergency right salpingo-oophorectomy because of elevated WBC and severe pain in early 2010. Pathologic examination revealed clear cell carcinoma of the ovary, and within two weeks of her initial surgery, she underwent a laparoscopic hysterectomy, left salpingo-oophorectomy and staging. Final pathology confirmed stage IC moderately differentiated clear cell ovarian cancer (pT1c, pN0, pMx). In March and April 2010, the patient received 3 cycles of adjuvant chemotherapy with paclitaxel and carboplatin, and in July 2010 a PET-CT scan demonstrated no evidence of residual or metastatic ovarian carcinoma.
6 months later, an FDG avid 6.3 cm left lower pelvis recurrence, malignant ascites and left-sided hydronephrosis were detected on PET-CT and CT scans. The patient underwent 3 cycles of carboplatin and gemcitabine, with a partial radiologic response (tumor size decreased to 3.4 cm). The residual tumor was surgically resected via rectosigmoid resection with anastomosis and then subsequently treated by pelvic radiation therapy. No evidence of residual or recurrent disease was identified on CT scans in April and October 2011.
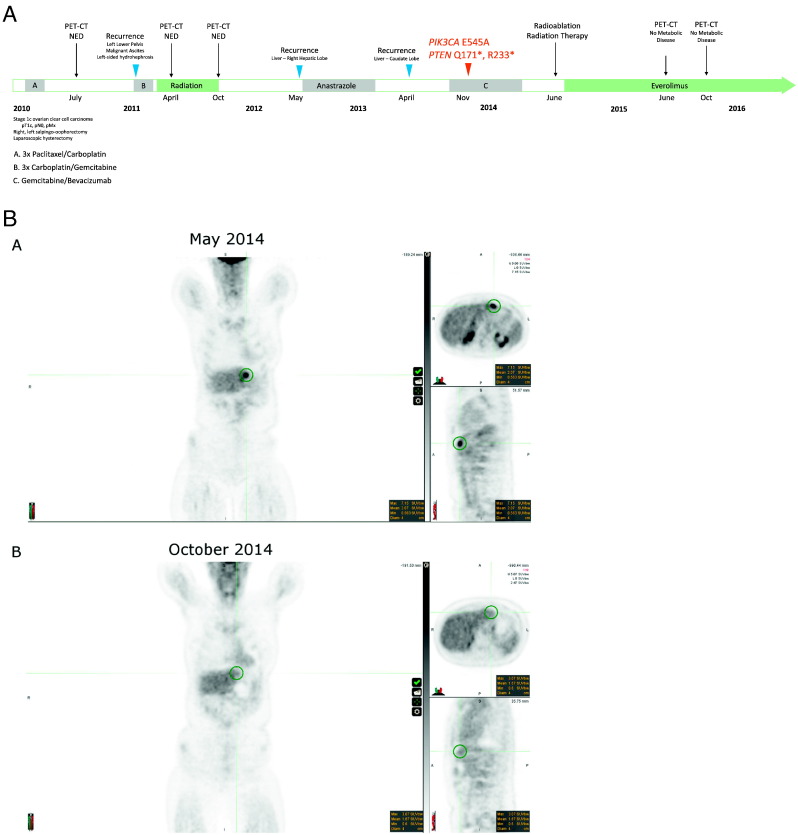
In May of 2012, 7 months after completing pelvic radiation, the patient had recurrent disease in the liver. A new 2.3 cm FDG avid focus in the right hepatic lobe was identified on a PET-CT scan. Patient then underwent surgical resection of a subcapsular lesion and segment 7 of the liver. Pathology confirmed a metastatic high-grade carcinoma consistent with ovarian primary. Subsequent additional foci suspicious for recurrence in adjacent to the distal left ureter and left posterior urinary bladder prompted a trial of anastrazole 1 mg daily as the tumor was ER positive. In April 2013, the patient experienced another recurrence in the liver. A 6 × 5.3 cm mass in the caudate lobe of the liver was resected and pathologically confirmed as metastatic high-grade ovarian carcinoma. This specimen was subsequently utilized for comprehensive genomic profiling. Disease progression continued with multiple new liver masses and marked left-sided hydronephrosis documented on imaging. The patient started salvage gemcitabine and bevacizumab in October 2013 with a positive treatment response consisting of an interval decrease in the extent of hypermetabolic malignancy in and adjacent to the liver on PET-CT in May 2014. The patient then underwent radiofrequency ablation and radiation therapy to the liver in June 2014 but was in need of additional options for systemic therapy, as the response was considered likely to be short-lived based on the previous disease course.
Genomic profiling of the 2013 liver metastatectomy specimen identified three CRGA affecting PIK3CA and PTEN, which are associated with sensitivity to mTOR inhibitors. CRGA in any of the other 315 genes included in the assay were not detected. Based on the CGP results, the patient began genomically-matched targeted treatment with everolimus 10 mg oral daily in July 2014. A follow-up PET-CT scan in October 2014 demonstrated resolution of previous liver lesions but a worsening of left hydronephrosis (Fig. 2). Everolimus therapy was continued despite a questionable new mild nonspecific elevation in the lateral right second rib on a February 2015 PET-CT scan. Follow-up scans in June 2015 and October 2015 were negative for metabolically active malignancy. As of September 2016, the patient has received 27 months of treatment with everolimus without significant toxicity (Fig. 2) and CA-125 levels remain low at 10.9. The plan is to continue everolimus as maintenance therapy until untoward side effects occur or disease progression.
Fig. 2.
Timeline of clinical history and response to radioablation followed by everolimus. A. Clinical history over time. B. Hypermetabolic malignancy in and adjacent to the liver, as observed by PET-CT in May 2014. The patient underwent radiofrequency ablation and radiation therapy to the liver in June 2014. Genomically-matched targeted treatment with everolimus (10 mg oral daily) begun in July 2014. Follow-up PET-CT scan in October 2014 demonstrated resolution of previous liver lesions but a worsening of left hydronephrosis.
4. Discussion
More than three quarters of OCCC harbor CRGA, most commonly affecting the PI3K/Akt/mTOR pathway, but also other targetable genes (Supplemental Table 1). A significant fraction of OCCC (11.2%) harbor CRGA only in the mTOR pathway, and we present a case of a robust and durable response to treatment with single agent everolimus for a patient with this genomic profile in late stage, heavily pretreated, multiply recurrent disease. It has been shown that patients with PIK3CA mutations are more responsive to PI3k/AKT/mTOR inhibitors than patients without these mutations (Janku et al., 2012, Janku et al., 2011). As a result, screening for these mutations early in the course of the disease could be crucial and can have important implications for selecting the appropriate targeted treatment consistent with the underlying genomic alteration.
The patient described in this study has experienced a durable response to single agent everolimus exceeding 27 months. The costs associated with both comprehensive genomic profiling and everolimus treatment were covered for the patient by their insurance provider. Coverage of the costs associated with everolimus treatment were approved after a peer review of the mechanistic rationale underlying treatment. The authors acknowledge that financials considerations are a component when considering the use of CGP and developing a viable treatment plan. The list prices of commercially available comprehensive genomic profiling assays are commonly several thousand dollars, although the actual cost to a patient or insurance provider may be less, depending on the individual circumstances. The cost of everolimus purchased through a pharmacy can range from $4000 to $13,000 for a 28-day supply. There are several processes that can be explored when CGP indicates treatment with a therapy that is not FDA approved or NCCN indicted, and which may not be immediately covered by most insurance providers. In addition to seeking insurance coverage, when the rationale for treatment is favorably considered, appeals for compassionate use can be made directly to the manufacturers. In our experience advocating on behalf of patients who have received comprehensive genomic profiling, access to therapy has been granted in approximately 60% of cases. Patient advocacy organizations are an additional resource for support and guidance in crafting these appeals. As evidence supporting the use of genomically matched targeted treatments continues to accumulate, an increasing number of clinical basket trials are underway and may be an appropriate venue for treatment. Our analysis indicates that nearly 70% of patients with OCCC may be positive for a biomarker indicating a potential benefit from treatment with mTOR inhibitors, while demonstrating a high level of co-occurrence of additional alterations that could impact the efficacy of single agent everolimus therapy. For example, 5% of OCC in this dataset show co-occurring RAS and MTOR pathway activation, and in a previous report a patient responded to combination therapy targeting the PI3K and RAS pathways (Castro et al., 2015). This report supports the use of comprehensive genomic profiling to identify those patients who are positive for an oncogenic event in any one of 15 components of the PI3K/AKT/mTOR pathway (Fig. 1b), and to assess for the presence or absence of additional drivers that may attenuate a response or underlie resistance to treatment with therapies such as everolimus. In this study, a robust response was observed for a patient whose tumor harbored only alterations in the mTOR pathway; for the additional 60% of patients whose OCCC have alterations in the mTOR pathway, combinations of therapies targeting multiple pathways may be required to achieve similar clinical benefit. Clinical trials of rare ovarian carcinoma subtypes, such as clear cell carcinoma, including broad molecular profiling to investigate the efficacy of biomarker matched targeted therapy, are needed.
Conflict disclosure
JAE and LMG are employees of and own equity in Foundation Medicine, Inc. JC and MM have no conflicts to disclose.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.gore.2017.02.007.
Appendix A. Supplementary data
Supplementary Table 1
References
- Husseinzadeh N., Husseinzadeh H.D. mTOR inhibitors and their clinical application in cervical, endometrial and ovarian cancers: a critical review. Gynecol. Oncol. 2014;133:375–381. doi: 10.1016/j.ygyno.2014.02.017. [DOI] [PubMed] [Google Scholar]
- Mabuchi S., Kuroda H., Takahashi R., Sasano T. The PI3K/AKT/mTOR pathway as a therapeutic target in ovarian cancer. Gynecol. Oncol. 2015;137:173–179. doi: 10.1016/j.ygyno.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Ray-Coquard I., Favier L., Weber B., Roemer-Becuwe C., Bougnoux P., Fabbro M., Floquet A., Joly F., Plantade A., Paraiso D., Pujade-Lauraine E. Everolimus as second- or third-line treatment of advanced endometrial cancer: ENDORAD, a phase II trial of GINECO. Br. J. Cancer. 2013;108:1771–1777. doi: 10.1038/bjc.2013.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheler J.J., Moulder S.L., Naing A., Janku F., Piha-Paul S.A., Falchook G.S., Zinner R., Tsimberidou A.M., Fu S., Hong D.S., Atkins J.T., Yelensky R., Stephens P.J., Kurzrock R. Anastrozole and everolimus in advanced gynecologic and breast malignancies: activity and molecular alterations in the PI3K/AKT/mTOR pathway. Oncotarget. 2014;5:3029–3038. doi: 10.18632/oncotarget.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers A.P. New strategies in endometrial cancer: targeting the PI3K/mTOR pathway–the devil is in the details. Clin. Cancer Res. 2013;19:5264–5274. doi: 10.1158/1078-0432.CCR-13-0615. [DOI] [PubMed] [Google Scholar]
- Frampton G.M., Fichtenholtz A., Otto G.A., Wang K., Downing S.R., He J., Schnall-Levin M., White J., Sanford E.M., An P., Sun J., Juhn F., Brennan K., Iwanik K., Maillet A., Buell J., White E., Zhao M., Balasubramanian S., Terzic S., Richards T., Banning V., Garcia L., Mahoney K., Zwirko Z., Donahue A., Beltran H., Mosquera J.M., Rubin M.A., Dogan S., Hedvat C.V., Berger M.F., Pusztai L., Lechner M., Boshoff C., Jarosz M., Vietz C., Parker A., Miller V.A., Ross J.S., Curran J., Cronin M.T., Stephens P.J., Lipson D., Yelensky R. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat. Biotechnol. 2013;31:1023–1031. doi: 10.1038/nbt.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janku F., Wheler J.J., Westin S.N., Moulder S.L., Naing A., Tsimberidou A.M., Fu S., Falchook G.S., Hong D.S., Garrido-Laguna I., Luthra R., Lee J.J., Lu K.H., Kurzrock R. PI3K/AKT/mTOR inhibitors in patients with breast and gynecologic malignancies harboring PIK3CA mutations. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012;30:777–782. doi: 10.1200/JCO.2011.36.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janku F., Tsimberidou A.M., Garrido-Laguna I., Wang X., Luthra R., Hong D.S., Naing A., Falchook G.S., Moroney J.W., Piha-Paul S.A., Wheler J.J., Moulder S.L., Fu S., Kurzrock R. PIK3CA mutations in patients with advanced cancers treated with PI3K/AKT/mTOR axis inhibitors. Mol. Cancer Ther. 2011;10:558–565. doi: 10.1158/1535-7163.MCT-10-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro M.P., Whitcomb B.P., Zajchowski D.A., Coleman R.L. Successful use of next generation genomic sequencing (NGS)-directed therapy of clear cell carcinoma of the ovary (CCCO) with trametinib and metformin in a patient with chemotherapy-refractory disease. Gynecol. Oncol. Res. Pract. 2015;2 doi: 10.1186/s40661-015-0013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1