Abstract
Certain gouty subjects with excessive de novo purine synthesis are deficient in hypoxanthineguanine phosphoribosyltransferase (HG-PRTase [EC 2.4.2.8]). The mechanism of accelerated uric acid formation in these patients was explored by measuring the incorporation of glycine-14C into various urinary purine bases of normal and enzyme-deficient subjects during treatment with the xanthine oxidase inhibitor, allopurinol.
In the presence of normal HG-PRTase activity, allopurinol reduced purine biosynthesis as demonstrated by diminished excretion of total urinary purine or by reduction of glycine-14C incorporation into hypoxanthine, xanthine, and uric acid to less than one-half of control values. A boy with the Lesch-Nyhan syndrome was resistant to this effect of allopurinol while a patient with 12.5% of normal enzyme activity had an equivocal response. Three patients with normal HG-PRTase activity had a mean molar ratio of hypoxanthine to xanthine in the urine of 0.28, whereas two subjects who were deficient in HG-PRTase had reversal of this ratio (1.01 and 1.04). The patterns of 14C-labeling observed in HG-PRTase deficiency reflected the role of hypoxanthine as precursor of xanthine. The data indicate that excessive uric acid in HG-PRTase deficiency is derived from hypoxanthine which is insufficiently reutilized and, as a consequence thereof, catabolized inordinately to uric acid. The data provide evidence for cyclic interconversion of adenine and hypoxanthine derivatives. Cleavage of inosinic acid to hypoxanthine via inosine does not contribute significantly to the formation of uric acid in either normal man or in patients with HG-PRTase deficiency.
HG-PRTase was not completely absent in red blood cells from a boy with the Lesch-Nyhan syndrome; with hypoxanthine as substrate, the activity in erythrocyte hemolysates was 0.64% of normal values.
Full text
PDF
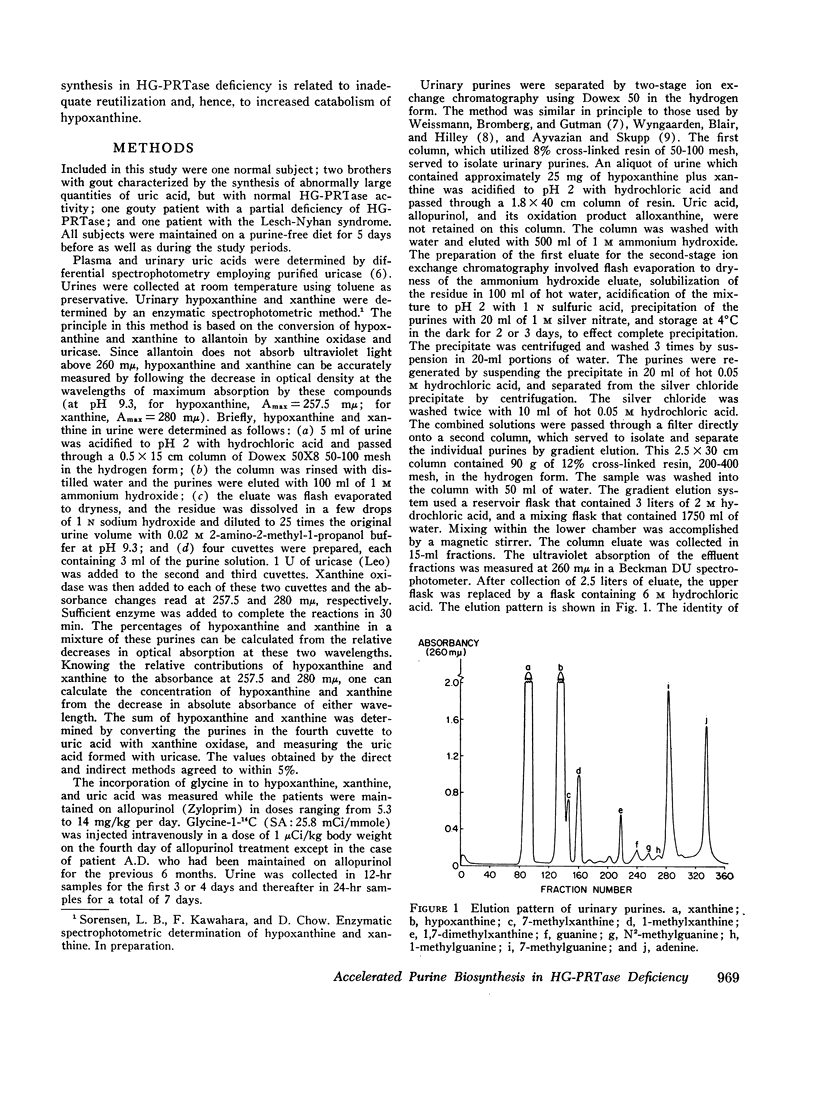

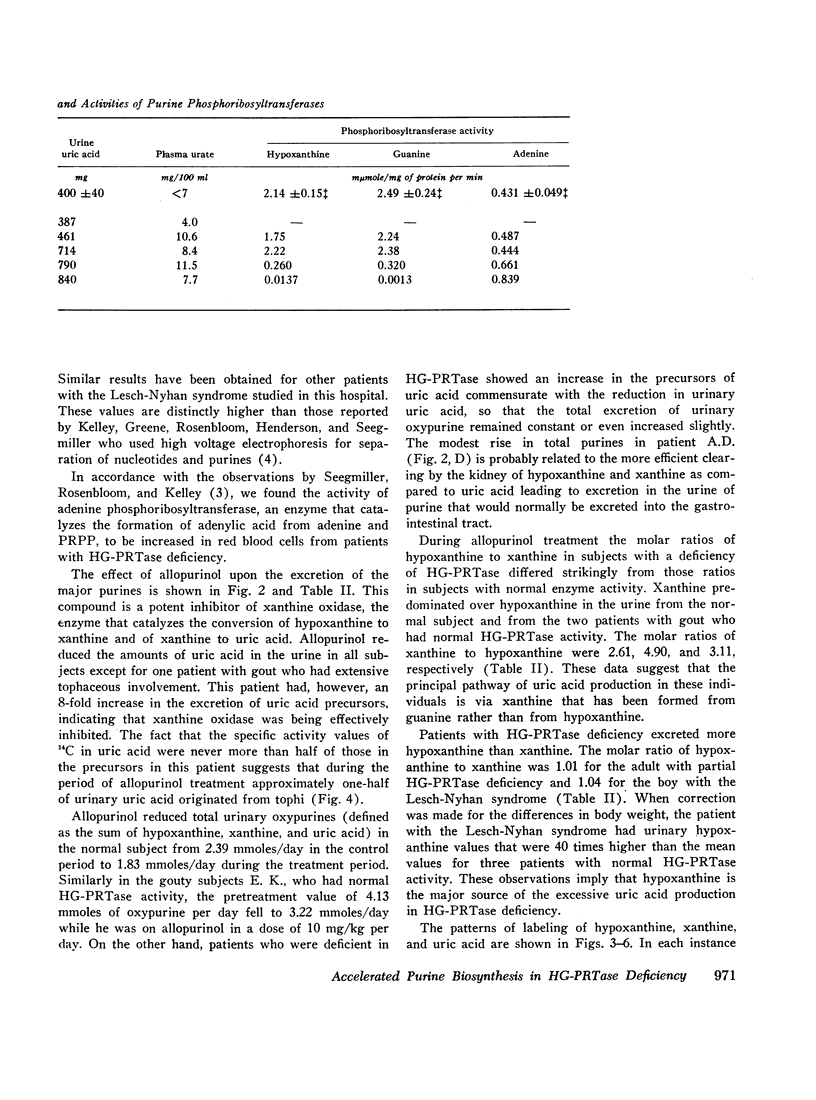
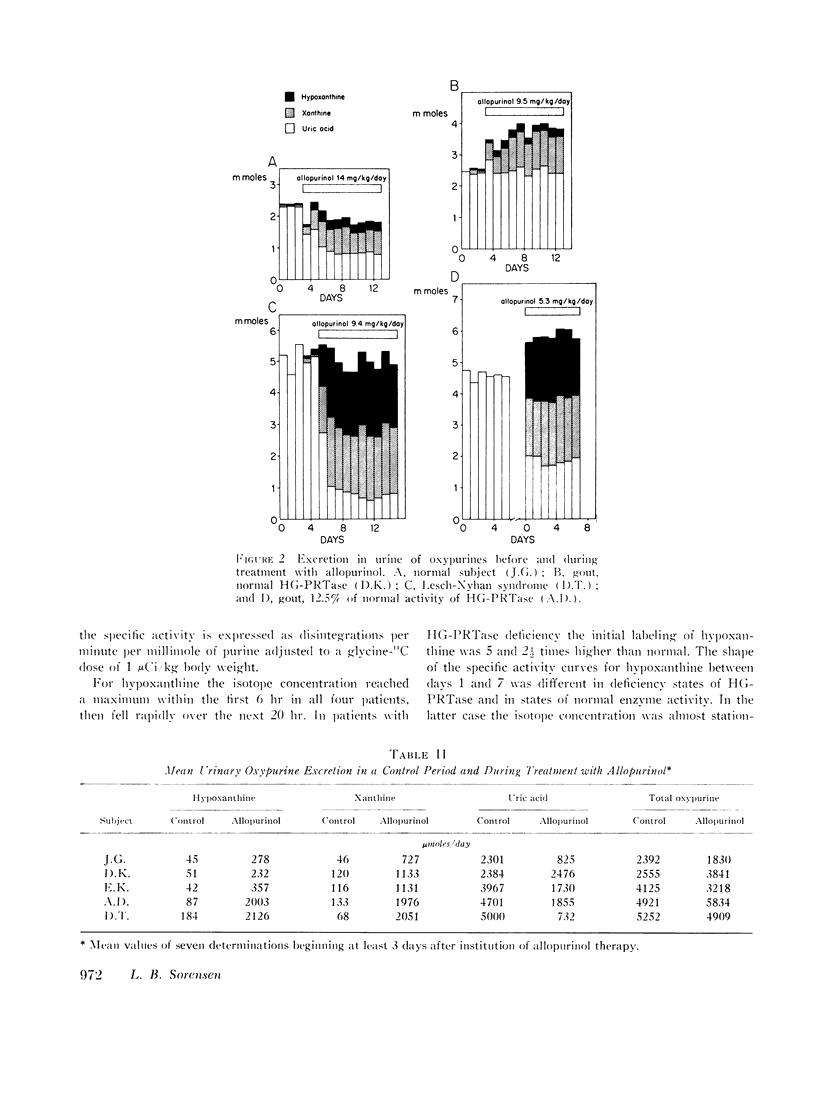
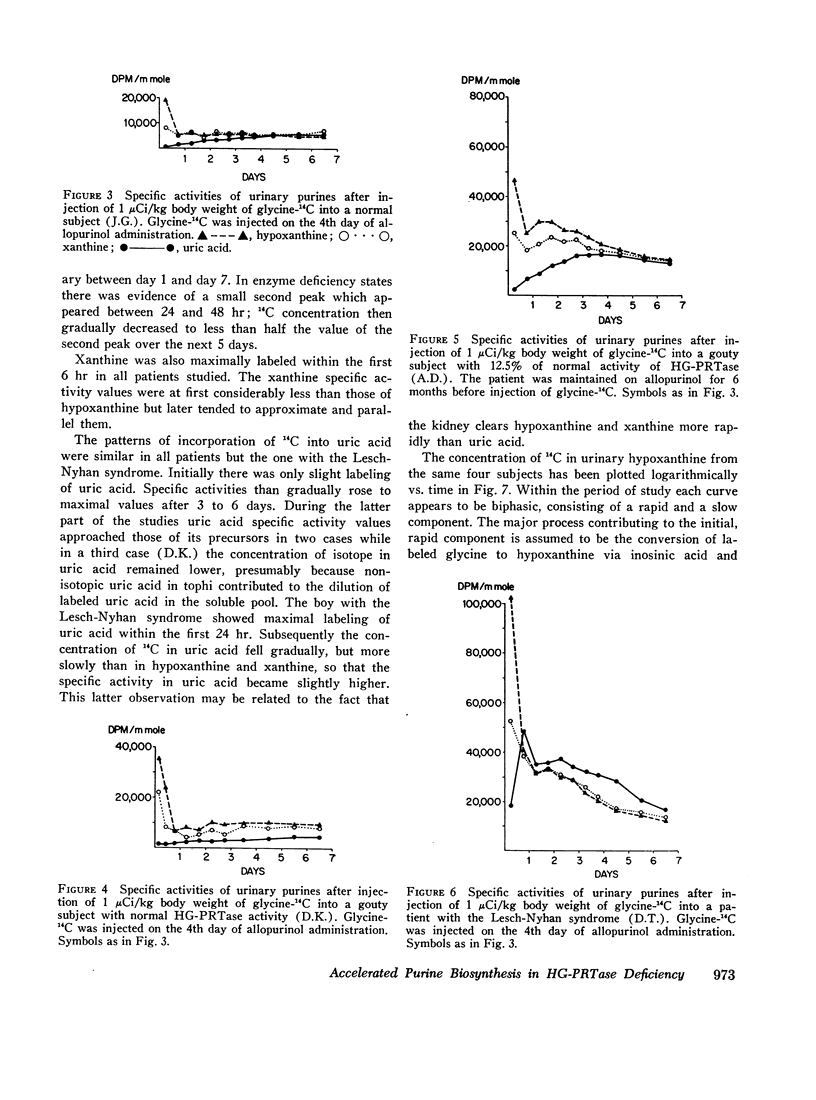
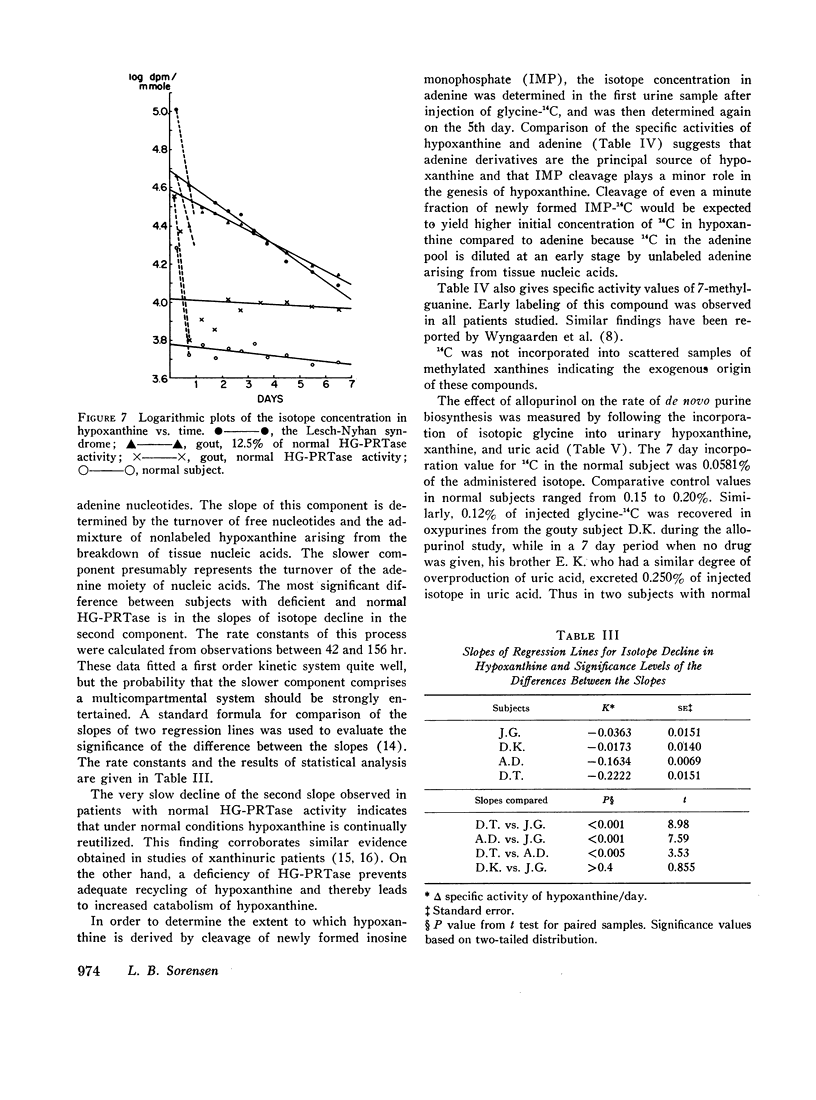


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AYVAZIAN J. H., SKUPP S. THE STUDY OF PURINE UTILIZATION AND EXCRETION IN A XANTHINURIC MAN. J Clin Invest. 1965 Jul;44:1248–1260. doi: 10.1172/JCI105231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayvazian J. H., Skupp S. Study of the utilization and excretion of dietary purines in a xanthinuric man. J Clin Invest. 1966 Dec;45(12):1859–1864. doi: 10.1172/JCI105490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROMBERG P. A., GUTMAN A. B., WEISSMANN B. The purine bases of human urine. I. Separation and identification. J Biol Chem. 1957 Jan;224(1):407–422. [PubMed] [Google Scholar]
- Balis M. E. Seminars on Lesch-Nyhan syndrome. Aspects of purine metabolism. Fed Proc. 1968 Jul-Aug;27(4):1067–1074. [PubMed] [Google Scholar]
- Bradford M. J., Krakoff I. H., Leeper R., Balis M. E. Study of purine metabolism in a xanthinuric female. J Clin Invest. 1968 Jun;47(6):1325–1332. doi: 10.1172/JCI105824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carcassi A., Marcolongo R., Jr, Marinello E., Riario-Sforza G., Boggiano C. Liver xanthine oxidase in gouty patients. Arthritis Rheum. 1969 Feb;12(1):17–20. doi: 10.1002/art.1780120104. [DOI] [PubMed] [Google Scholar]
- Kelley W. N., Greene M. L., Rosenbloom F. M., Henderson J. F., Seegmiller J. E. Hypoxanthine-guanine phosphoribosyltransferase deficiency in gout. Ann Intern Med. 1969 Jan;70(1):155–206. doi: 10.7326/0003-4819-70-1-155. [DOI] [PubMed] [Google Scholar]
- Kelley W. N., Rosenbloom F. M., Miller J., Seegmiller J. E. An enzymatic basis for variation in response to allopurinol. Hypoxanthine-guanine phosphoribosyltransferase deficiency. N Engl J Med. 1968 Feb 8;278(6):287–293. doi: 10.1056/NEJM196802082780601. [DOI] [PubMed] [Google Scholar]
- Kelley W. N., Rosenbloom F. M., Seegmiller J. E. The effects of azathioprine (imuran) on purine synthesis in clinical disorders of purine metabolism. J Clin Invest. 1967 Sep;46(9):1518–1529. doi: 10.1172/JCI105643. [DOI] [PMC free article] [PubMed] [Google Scholar]
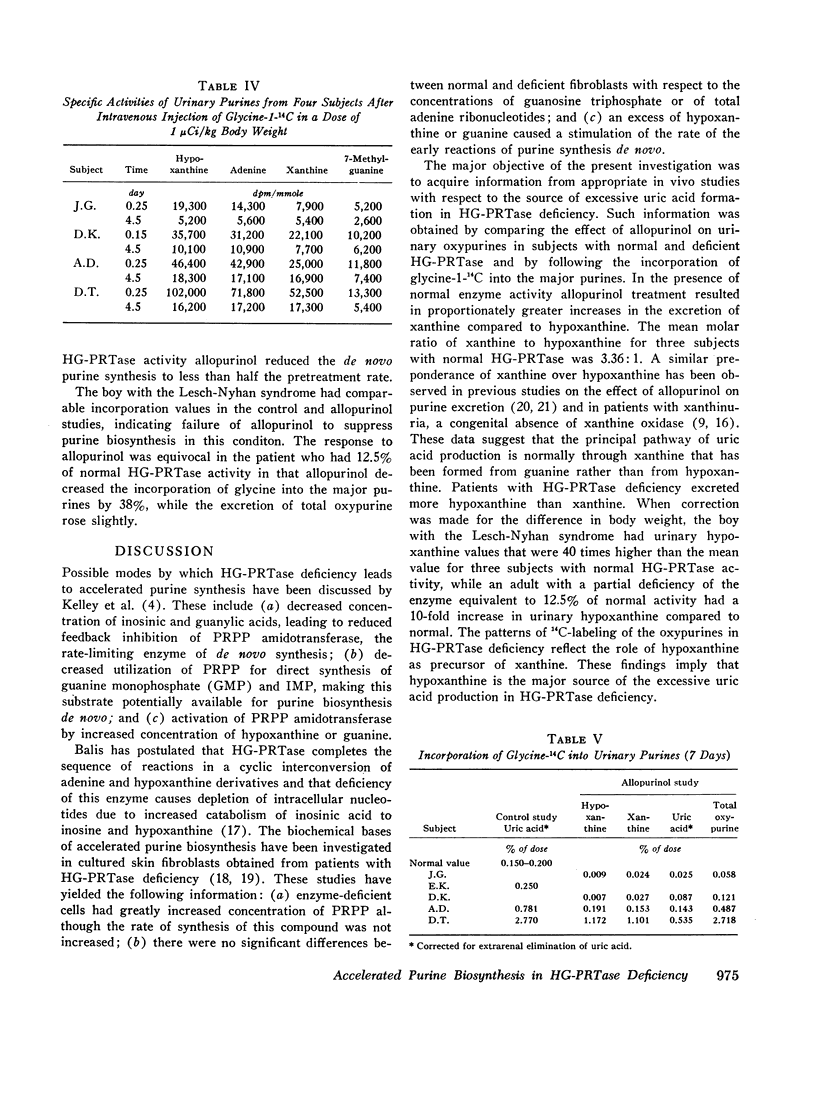
- LESCH M., NYHAN W. L. A FAMILIAL DISORDER OF URIC ACID METABOLISM AND CENTRAL NERVOUS SYSTEM FUNCTION. Am J Med. 1964 Apr;36:561–570. doi: 10.1016/0002-9343(64)90104-4. [DOI] [PubMed] [Google Scholar]
- MCCOLLISTER R. J., GILBERT W. R., Jr, ASHTON D. M., WYNGAARDEN J. B. PSEUDOFEEDBACK INHIBITION OF PURINE SYNTHESIS BY 6-MERCAPTOPURINE RIBONUCLEOTIDE AND OTHER PURINE ANALOGUES. J Biol Chem. 1964 May;239:1560–1563. [PubMed] [Google Scholar]
- POMALES R., BIEBER S., FRIEDMAN R., HITCHINGS G. H. Augmentation of the incorporation of hypoxanthine into nucleic acids by the administration of an inhibitor of xanthine oxidase. Biochim Biophys Acta. 1963 May 28;72:119–120. [PubMed] [Google Scholar]
- Rosenbloom F. M., Henderson J. F., Caldwell I. C., Kelley W. N., Seegmiller J. E. Biochemical bases of accelerated purine biosynthesis de novo in human fibroblasts lacking hypoxanthine-guanine phosphoribosyltransferase. J Biol Chem. 1968 Mar 25;243(6):1166–1173. [PubMed] [Google Scholar]
- Rosenbloom F. M., Henderson J. F., Kelly W. N., Seegmiller J. E. Accelerated purine biosynthesis de novo in skin fibroblasts deficient in hypoxanthine--guanine phosphoribosyltransferase activity. Biochim Biophys Acta. 1968 Aug 23;166(1):258–260. doi: 10.1016/0005-2787(68)90512-1. [DOI] [PubMed] [Google Scholar]
- SEEGMILLER J. E., GRAYZEL A. I., LASTER L., LIDDLE L. Uric acid production in gout. J Clin Invest. 1961 Jul;40:1304–1314. doi: 10.1172/JCI104360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SORENSEN L. B. The pathogenesis of gout. Arch Intern Med. 1962 Apr;109:379–390. doi: 10.1001/archinte.1962.03620160005002. [DOI] [PubMed] [Google Scholar]
- STIRPE F., DELLACORTE E. REGULATION OF XANTHINE DEHYDROGENASE IN CHICK LIVER. EFFECT OF STARVATION AND OF ADMINISTRATION OF PURINES AND PURINE NUCLEOSIDES. Biochem J. 1965 Feb;94:309–313. doi: 10.1042/bj0940309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegmiller J. E., Klinenberg J. R., Miller J., Watts R. W. Suppression of glycine-15N incorporation into urinary uric acid by adenine-8-13C in normal and gouty subjects. J Clin Invest. 1968 May;47(5):1193–1203. doi: 10.1172/JCI105808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen L. B. Suppression of the shunt pathway in primary gout by azathioprine. Proc Natl Acad Sci U S A. 1966 Mar;55(3):571–575. doi: 10.1073/pnas.55.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetman L., Nyhan W. L. Excretion of hypoxanthine and xanthine in a genetic disease of purine metabolism. Nature. 1967 Aug 19;215(5103):859–860. doi: 10.1038/215859a0. [DOI] [PubMed] [Google Scholar]
- WYNGAARDEN J. B., BLAIR A. E., HILLEY L. On the mechanism of overproduction of uric acid in patients with primary gout. J Clin Invest. 1958 Apr;37(4):579–590. doi: 10.1172/JCI103641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YUE T. F., GUTMAN A. B. EFFECT OF ALLOPURINOL (4-HYDROXYPYRAZOLO-(3,4-D)PYRIMIDINE) ON SERUM AND URINARY URIC ACID IN PRIMARY AND SECONDARY GOUT. Am J Med. 1964 Dec;37:885–898. doi: 10.1016/0002-9343(64)90131-7. [DOI] [PubMed] [Google Scholar]


