Abstract
Purpose
Frontotemporal lobar degeneration (FTLD) is a common cause of early onset dementia. Behavioral variant frontotemporal dementia (bvFTD), its most common subtype, is characterized by deep alterations in behavior and personality. In 2011, new diagnostic criteria were suggested that incorporate imaging criteria into diagnostic algorithms. The study aimed at validating the potential of imaging criteria to individually predict diagnosis with machine learning algorithms.
Materials & methods
Brain atrophy was measured with structural magnetic resonance imaging (MRI) at 3 Tesla in a multi-centric cohort of 52 bvFTD patients and 52 healthy control subjects from the German FTLD Consortium's Study. Beside group comparisons, diagnosis bvFTD vs. controls was individually predicted in each subject with support vector machine classification in MRI data across the whole brain or in frontotemporal, insular regions, and basal ganglia known to be mainly affected based on recent meta-analyses. Multi-center effects were controlled for with a new method, “leave one center out” conjunction analyses, i.e. repeatedly excluding subjects from each center from the analysis.
Results
Group comparisons revealed atrophy in, most consistently, the frontal lobe in bvFTD beside alterations in the insula, basal ganglia and temporal lobe. Most remarkably, support vector machine classification enabled predicting diagnosis in single patients with a high accuracy of up to 84.6%, where accuracy was highest in a region-of-interest approach focusing on frontotemporal, insular regions, and basal ganglia in comparison with the whole brain approach.
Conclusion
Our study demonstrates that MRI, a widespread imaging technology, can individually identify bvFTD with high accuracy in multi-center imaging data, paving the road to personalized diagnostic approaches in the future.
Abbreviations: bvFTD, behavioral variant frontotemporal dementia; FTLD, frontotemporal lobar degeneration; FEW, family wise error; GMD, gray matter density; MNI, Montreal Neurological Institute; MPRAGE, magnetization-prepared rapid gradient echo; MRI, magnetic resonance imaging; SVM, support vector machine; VBM, voxel based morphometry
Keywords: Atrophy, Behavioral variant frontotemporal dementia, Diagnostic criteria, Frontotemporal lobar degeneration, MRI, Pattern classification
Highlights
-
•
Diagnostic criteria for behavioral variant frontotemporal dementia include imaging.
-
•
Study validates MRI's potential to predict diagnosis with machine learning algorithms.
-
•
Support vector machine classification enabled high classification accuracy.
-
•
Accuracy was higher in disease-specific than whole-brain approaches.
-
•
Structural MRI can individually identify behavioral variant frontotemporal dementia.
1. Introduction
Frontotemporal lobar degeneration (FTLD) is a common cause of early onset dementia (Rabinovici and Miller, 2010). Its prevalence and incidence is similar to Alzheimer's disease in individuals under the age of 65 years. Behavioral variant frontotemporal dementia (bvFTD), its most common subtype, is characterized by deep alterations in behavior and personality (Neary et al., 1998). In 2011, new diagnostic criteria were suggested that divide bvFTD into three different diagnostic categories: “possible bvFTD”, a strictly clinical diagnosis, “probable bvFTD”, if clinical criteria are complemented with fitting imaging data (frontal and/or anterior temporal atrophy, hypometabolism or hypoperfusion), and bvFTD with “definite FTLD pathology”, when either a known pathogenic mutation or histopathological evidence is present (Rascovsky et al., 2011). Recent meta-analyses on the neural correlates of bvFTD validated these imaging criteria and showed a decreased gray matter density (GMD) mainly in the frontal cortex, suggesting that bvFTD is mainly a frontal brain disease in contrast to other neurodegenerative diseases (Pan et al., 2012, Schroeter, 2012, Schroeter and Neumann, 2011, Schroeter et al., 2007, Schroeter et al., 2008, Schroeter et al., 2009, Schroeter et al., 2014).
Incorporating imaging criteria into diagnostic algorithms for bvFTD is a decisive step in improving these criteria. The aim of our study was to further validate imaging criteria for bvFTD by measuring brain atrophy with magnetic resonance imaging (MRI) in a multi-centric cohort, and by using this data to predict diagnosis in each single patient. Note that usage of multi-center data is a precondition for future application in clinical routine. Accordingly, we calculated structural brain differences between bvFTD patients and healthy controls with voxel based morphometry (VBM) (Ashburner and Friston, 2000) using the multi-center cohort of the German FTLD Consortium. Recently, machine learning techniques have been applied to neuroimaging data in order to distinguish between patients and control subjects or different diseases on an individual level (Dukart et al., 2011, Dukart et al., 2013a, Dukart et al., 2013b, Klöppel et al., 2008). In this study we used support vector machine (SVM) algorithms to differentiate between bvFTD patients and healthy controls on an individual level. We hypothesized that bvFTD is characterized mainly by frontotemporal lobe atrophy and that SVM algorithms identify bvFTD patient and control subjects with a high accuracy that might even be increased by focusing on disease-specific brain areas, namely fronto(temporal) regions, the insula and basal ganglia in comparison with whole brain approaches, based on recent meta-analyses (Pan et al., 2012, Schroeter, 2012, Schroeter et al., 2014).
2. Methods
2.1. Patients and control subjects
Fifty-two patients with bvFTD were included in the study. Diagnosis was based on the newest diagnostic criteria by Rascovsky et al. (2011). Of those fifty-two bvFTD patients twenty met the criteria for possible bvFTD, twenty-eight the criteria for probable bvFTD, and four met the criteria for genetically confirmed bvFTD. Twenty-four were female, and duration since initial symptoms averaged 3.7 ± 3.7 years (results are generally reported as mean ± standard deviation). Disease severity was 5.5 ± 3.5 or 7.7 ± 4.2 as measured with the Clinical Dementia Rating scale (CDR) and the FTLD-modified Clinical Dementia Rating scale (FTLD-CDR). The data was retrieved from the German FTLD Consortium, a prospective multicenter study on FTLD (http://www.ftld.de; Otto et al., 2011). Fifty-two (twenty-four female) control subjects were recruited overall, thirty subjects from the different centers in the German FTLD Consortium's study and, additionally, twenty-two from the Max-Planck-Institute Leipzig database.
All subjects were thoroughly examined by taking a case history, neurological examination, neuropsychological testing and MRI (see below). Overall the bvFTD and the healthy control subject group were exactly gender matched and didn't differ in mean age (bvFTD 61.5 ± 10.0 years; healthy control subjects 63.1 ± 9.8 years; Student's t-test p = 0.76). The study was approved by the ethics committees of the University of Ulm, and of the other universities involved in the FTLD Consortium, and was in accordance with the latest version of the Declaration of Helsinki. Each patient provided a written informed consent.
2.2. Acquisition and analysis of imaging data
High resolution Magnetization-Prepared Rapid Gradient Echo (MPRAGE) T1-images were acquired by seven different centers throughout Germany. MRIs were performed on five different 3 Tesla Siemens scanners (Allegra Ulm, Biograph Munich, TrioTim Erlangen & Goettingen, Verio Leipzig & Rostock, Skyra Bonn) with three different sequences (Erlangen/Bonn/Goettingen/Leipzig/Munich: TR = 2300 ms, TI = 900 ms, TE = 2.98 ms; Rostock: TR = 2500 ms, TI = 1100 ms, TE = 2.98 ms; Ulm: TR = 2200 ms, TI = 1200 ms, TE = 4.38 ms).
In total, the 52 bvFTD patients were compared to 52 healthy control subjects. Generally, two different analyses were conducted. The first one consisted of 19, exactly center-matched, patients and control subjects, i.e. patients were compared with control subjects from exactly the same center, to enable a maximally controlled pilot analysis. The second analysis included all available subjects, and could therefore not be exactly matched according to center. Both groups had no significant differences in age and gender distribution (see above). Possible center effects were controlled for with an additional analysis (see below).
2.3. Group comparison patients vs. control subjects – VBM analyses
GMD differences between bvFTD patients and the control cohort were analyzed with VBM by applying the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm/vbm8-for-spm8/), implemented in SPM8 (Wellcome Trust Centre for Neuroimaging, London, UK), running on Matlab 7.1. Data were pre-processed according to the VBM8 toolbox. Firstly, high resolution images were normalized to the same stereotactic space by registering each individual image to the same template image, the Montreal Neurological Institute (MNI) 152 standard template, using non-linear transformations. The images were segmented into gray matter, white matter, and cerebrospinal fluid volumes. The gray matter volumes were then smoothed by convolving an isotropic Gaussian kernel (12 × 12 × 12 mm). The result of the preprocessing steps is referred to as GMD, which was compared in the next step. The statistical analysis was performed voxel-wise by using the general linear model and implementing a two-sample t-test. Results were controlled for potentially confounding factors, namely age, gender, total intracranial volume, and the center where the MRI was conducted. A significance threshold of p < 0.05, family wise error (FWE) corrected on the cluster level, was used for the 19 vs. 19 pilot analysis. For the final 52 vs. 52 analysis a threshold of p < 0.05 FWE corrected on the voxel level was used due to higher statistical power by the larger number of participants. Note that center effects were controlled for in both group comparisons (see below).
2.4. Group comparison patients vs. control subjects – conjunction analyses
These analyses were performed to control for potential center effects in the aforementioned group comparisons. To investigate the influence of inter-center variability, group comparisons (patients vs. control subjects) were conducted repeatedly with the “leave one center out” approach, i.e. excluding systematically subjects from one center from the analysis. After these processing steps, a conjunction analysis was performed by overlaying the results of the six, respectively seven different VBM analyses, in order to identify those brain networks that were affected by bvFTD in all analyses, and accordingly, independent from center. The threshold was set to p < 0.05 FWE on the cluster level here.
2.5. Group comparison patients vs. control subjects – SVM classification analyses
The final analysis investigated the possibility to classify patients individually solely from MRI data. The classification was obtained by SVM, using the libSVM software package (Software available at http://www.csie.ntu.edu.tw/~cjlin/libsvm) (Chang and Lin, 2011). SVMs are supervised learning models, used to analyze data, build classifiers and regression analyses. They work in two phases. In the training phase, a subset of the available data points, in this study all the voxels of the brain or all the voxels of the region of interest are used to find a linear hyperplane to separate the two classes (e.g. patients vs. controls) optimally. In the testing phase, new, previously unseen data is classified depending on their relative position to the hyperplane.
For classification we used the “leave one (subject) out” approach. In this approach one subject of each group (e.g. patients and control) is used for testing and the remaining ones are used for training the classifier, until every subject has been left out. The masks were created using the Wake Forest University School of Medicine (WFU) PickAtlas (Maldjian et al., 2003). Classifications were run for both of the above mentioned analyses, and with different regions of interest for each analysis (see Table 1).
Table 1.
Results of support vector machine classification to separate patients and control subjects
| Region of interest | Analysis (N) | Accuracy %) | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) |
|---|---|---|---|---|---|---|
| Whole Brain | ||||||
| 19 vs. 19 | 73.6 | 63.2 | 84.2 | 80.0 | 69.6 | |
| 52 vs. 52 | 81.7 | 78.9 | 84.6 | 83.7 | 80.0 | |
| Frontal Lobe | ||||||
| 19 vs. 19 | 78.9 | 68.4 | 89.5 | 86.7 | 73.9 | |
| 52 vs. 52 | 80.7 | 76.9 | 84.6 | 83.3 | 78.6 | |
| Frontal Lobe, Insula, Basal Ganglia | ||||||
| 19 vs 19 | 78.9 | 68.4 | 89.5 | 86.7 | 73.9 | |
| 52 vs 52 | 82.7 | 80.7 | 84.6 | 84.0 | 81.5 | |
| Temporal Lobe | ||||||
| 19 vs. 19 | 71.1 | 73.7 | 68.4 | 70.0 | 72.2 | |
| 52 vs. 52 | 78.8 | 76.9 | 80.8 | 80.0 | 77.8 | |
| Frontal & Temporal Lobe | ||||||
| 19 vs. 19 | 76.3 | 68.4 | 84.2 | 81.3 | 72.7 | |
| 52 vs. 52 | 84.6 | 80.7 | 88.5 | 87.5 | 82.1 | |
| Frontal & Temporal Lobe, Insula, Basal Ganglia | ||||||
| 19 vs. 19 | 73.6 | 63.2 | 84.2 | 80.0 | 69.6 | |
| 52 vs. 52 | 84.6 | 80.7 | 88.5 | 87.5 | 82.1 | |
3. Results
3.1. Group comparison patients vs. control subjects – VBM analyses
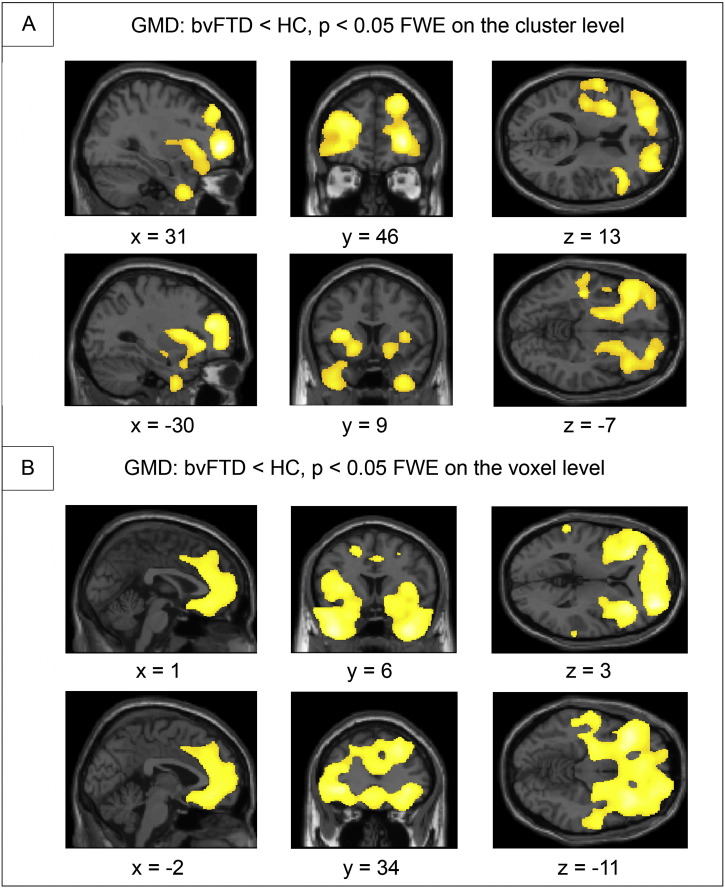
As illustrated in Fig. 1, the VBM-analysis revealed reductions in GMD mainly in the frontal and temporal lobe, insulae and basal ganglia in bvFTD. For the first center-matched analysis with 19 bvFTD patients vs. 19 control subjects, brain regions included bilaterally the globus pallidus, frontal pole, insular cortex, temporal pole, left precentral gyrus, postcentral gyrus, amygdala, Heschl's gyrus, and central opercular gyrus. The second analysis including 52 bvFTD patients vs. 52 control subjects revealed changes bilaterally in the anterior cingulate, frontomedian, paracingulate cortices, superior, middle and inferior frontal gyri, frontal pole, orbital gyrus, insular cortex, temporal pole, middle temporal gyrus, amygdala, putamen, nucleus accumbens, and right pre- and postcentral gyrus. Note that neither disease duration nor disease severity as measured with the FTLD-CDR correlated with GMD in our study, preventing a potential impact of these two clinical factors on our results.
Fig. 1.
Group comparison between behavioral variant frontotemporal dementia (bvFTD) vs. healthy control cohort (HC) for gray matter density (GMD). A: 19 patients with bvFTD vs. 19 center-matched control subjects. B: 52 patients with bvFTD vs. 52 control subjects. Family wise error (FWE) correction. Coordinates in Montreal Neurological Institute (MNI) space. Left side of the brain is shown on the left.
3.2. Group comparison patients vs. control subjects – conjunction analyses
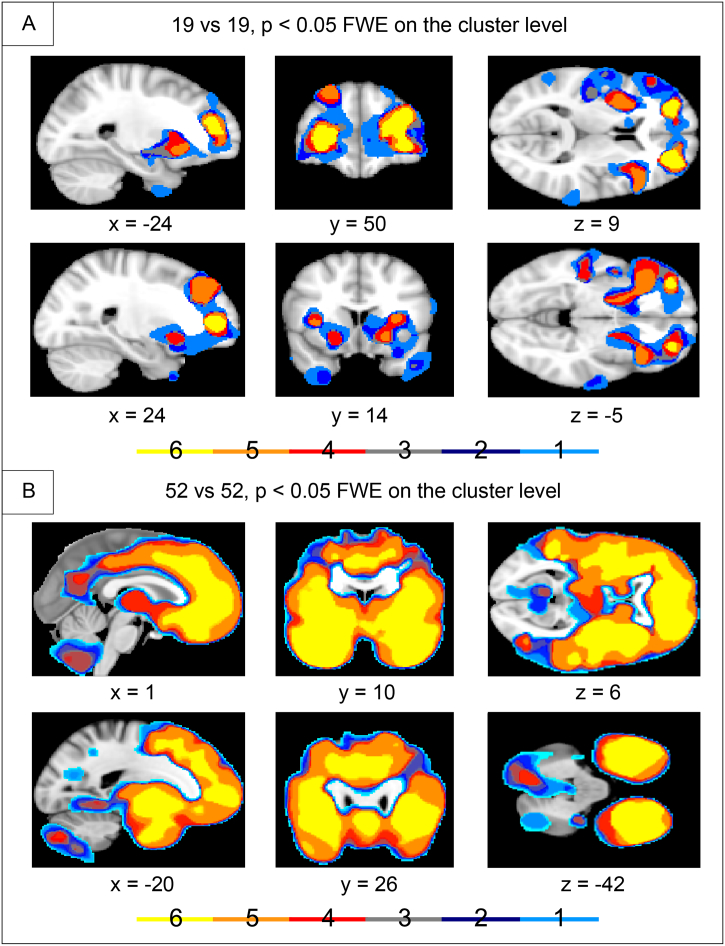
The conjunction analysis revealed different degrees of concordance in the two analyses (Fig. 2). In the 19 bvFTD vs. 19 control subjects analysis, the highest concordance was found in bilateral frontal pole. These were the only areas showing GMD loss in all of the six analyses. As opposed to this, the 52 bvFTD vs. 52 control subjects analysis showed a high concordance in both, the frontal and temporal lobes, insula and the basal ganglia. More specifically it identified the superior frontal gyrus, frontal pole, frontoorbital and frontomedian cortex, paracingulate and anterior cingulate/midcingulate cortex, middle and superior temporal gyrus, planum polare, insular cortex, putamen, nucleus accumbens, left angular gyrus, and right supramarginal gyrus (see Fig. 2).
Fig. 2.
Conjunction analyses across group comparison between behavioral variant frontotemporal dementia (bvFTD) vs. control cohort, leaving for each analysis one center, i.e. respective patients and control subjects, out. Differences in gray matter density (GMD). The scale illustrates the number of overlapping centers. A: 19 patients with bvFTD vs. 19 center-matched control subjects. B: 52 patients with bvFTD vs. 52 control subjects. Family wise error (FWE) correction. Coordinates in Montreal Neurological Institute (MNI) space. Left side of the brain is shown on the left.
3.3. Group comparison patients vs. controls subjects – SVM classification analyses
Results of the final analyses, investigating the possibility to individually classify patients solely from MRI data, are illustrated in detail (accuracy, sensitivity, specificity, positive and negative predictive value) in Table 1. Classification accuracy ranged from 71.1% to 78.9% in the 19 bvFTD patients vs. 19 control subjects analyses, and from 78.8% to 84.6% in the 52 bvFTD patients vs. 52 control subjects analyses. Obviously, classification accuracy was generally strong with higher values in the larger cohort, presumably due to a higher statistical power. Accordingly, description of results will be focused on the larger cohort including 52 patients and 52 control subjects in the following. The frontal lobe mask revealed better results for accuracy than the temporal lobe mask (80.7% vs. 78.8%). Both masks reached slightly lower accuracy than using all the voxels of the brain (81.7%). Adding the insula and basal ganglia to the frontal mask increased accuracy to 82.7%. The highest accuracy with 84.6% could be obtained by using a mask including the frontal and temporal lobe alone, or combined with the insula and basal ganglia. More detailed masks did not lead to better classification. Overall a high specificity was achieved.
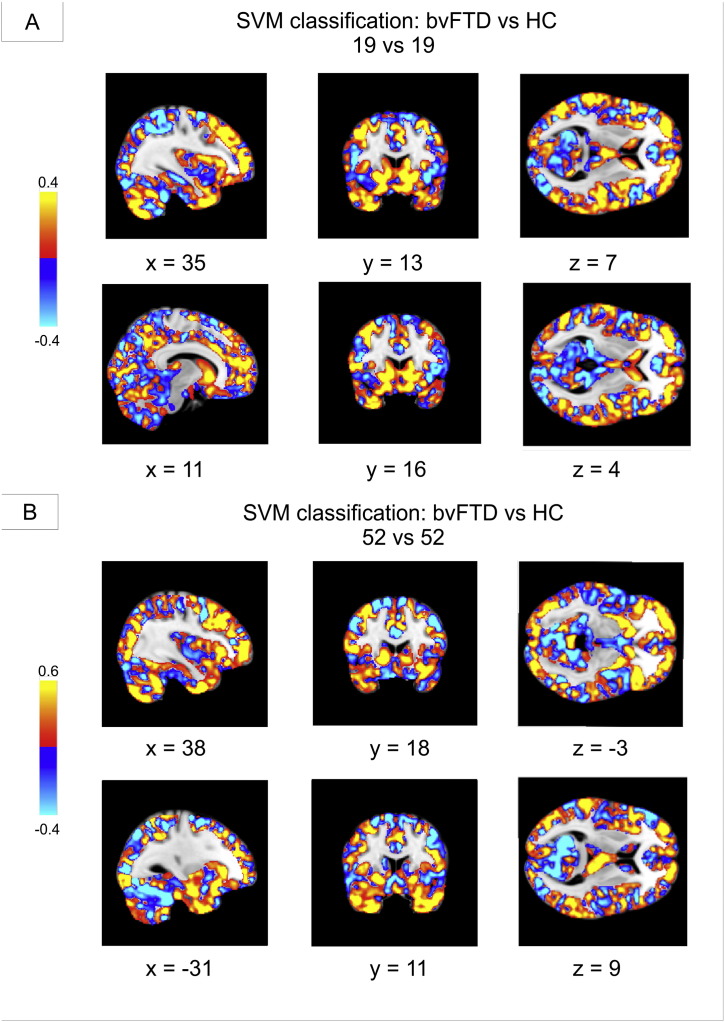
Fig. 3 illustrates weights of voxels most relevant for SVM classification between bvFTD patients and healthy controls across the whole brain. Relevant voxels for bvFTD classification were located in the frontal and temporal cortex, the insula, basal ganglia, and the cerebellum.
Fig. 3.
Weights of voxels most relevant for support vector machine (SVM) classification between patients with behavioral variant frontotemporal dementia (bvFTD) and healthy controls (HC). The most relevant voxels for classification as bvFTD are shown in red-yellow, for HC in blue. SVM classification was performed on all voxels within the gray matter mask (tissue probability > 0.4). A: 19 patients with bvFTD vs. 19 center-matched control subjects. B: 52 patients with bvFTD vs. 52 control subjects. Coordinates in Montreal Neurological Institute (MNI) space. Left side of the brain is shown on the right.
4. Discussion
This study aimed at further validating imaging criteria for bvFTD by measuring brain atrophy with MRI in a multi-centric cohort, and by predicting diagnosis in each patient individually based on pattern recognition algorithms (SVM classification).
In line with our hypothesis the group comparison between bvFTD and control subjects revealed most pronounced and most consistent GMD loss in frontal brain regions in both of the conducted group comparison analyses. Remarkably, the conjunction analysis of the smaller of the two cohorts revealed the greatest overlap between the centers in the bilateral frontal pole. This is of particular note since this cohort was characterized by a lower average of duration since initial symptoms occurred (2.4 ± 2.6 vs. 3.7 ± 3.7 years), and, accordingly, lower disease severity (CDR 5.0 ± 3.5 vs. 5.5 ± 3.5; FTLD-CDR 5.8 ± 4.1 vs. 7.7 ± 4.2; Student's t-test p = 0.12, 0.62, 0.52) as compared to the larger cohort, suggesting that the frontal lobe may be the first affected brain region in bvFTD patients, as has been postulated before (Schroeter et al., 2014). The results support the assumption that bvFTD might be regarded as a mainly frontal disease as suggested by powerful meta-analyses across imaging studies, histopathological studies, and conceptual theories for this neurodegenerative disease (Kim et al., 2012, Pan et al., 2012, Schroeter, 2012, Schroeter et al., 2014, Seeley et al., 2006). Furthermore, we found GMD differences in the insulae and basal ganglia in agreement with the aforementioned meta-analytic and histopathological findings, and, additionally, in the temporal lobe. Such regional alterations have been previously described for different subtypes of bvFTD (Whitwell et al., 2009) and in autopsy proven FTLD patients (Rabinovici et al., 2007).
In line with the major aim of this study, the diagnosis bvFTD could be predicted in individual patients by applying pattern recognition algorithms to MRI data as shown for the first time to our knowledge. The SVM analyses supplied very reasonable results, varying in accuracy between 71.1% and 78.9% in the 19 vs. 19 comparison and between 78.8% and 84.6% in the 52 vs. 52 comparison, depending on the selected region of interest. Using all voxels of the frontal lobe led to better classification accuracy than using all voxels of the temporal lobe. This again supports the assumption that the primarily affected or core network lies within the frontal lobe.
The highest accuracy was reached by using all the voxels of the frontal and temporal lobe alone or together with the insula and basal ganglia in order to distinguish between patients and control subjects (Table 1). These results underline the importance of the insula and basal ganglia in bvFTD (Kim et al., 2012, Schroeter et al., 2014). The relevance of the four aforementioned brain regions for pattern classification was confirmed by the analysis investigating the importance of the several brain regions contributing to the correct classification in the whole brain analysis (see Fig. 3). Of note, the cerebellum contributed also to correct classification here.
One might conclude that SVM classification enables individual diagnosis of bvFTD with structural MRI – a finding supporting the inclusion of imaging criteria into diagnostic algorithms. For clinical applications, we suggest conducting analyses across the frontal, temporal lobes, insula and basal ganglia in order to distinguish between patients and healthy control subjects with highest reliability. Incorporating additional biomarkers into the classifier, such as from cerebrospinal fluid, or other imaging modalities, such as positron emission tomography, shall increase diagnostic accuracy in the future (Dukart et al., 2011, Dukart et al., 2013a, Steinacker et al., 2017, Tahmasian et al., 2016).
For clinical purposes in the framework of personalized medicine future studies shall focus on detecting neurodegenerative diseases with such approaches as early as possible, i.e. in prestages such as mild cognitive or mild behavioral impairment or mild neurocognitive disorder, where treatment might be easier to conduct, and allow differential diagnosis with other neurodegenerative disease subtypes. Especially a comparison with the most frequent dementia type – Alzheimer's disease – would be of interest. Here, previous studies have shown that bvFTD/FTLD can be differentiated with high accuracy from Alzheimer's disease with the same approaches (Dukart et al., 2011, Klöppel et al., 2008, Möller et al., 2016). Note that regions-of-interest and analysis techniques might differ in diagnostic vs. differential diagnostic procedures as shown for imaging approaches recently (Dukart et al., 2010, Dukart et al., 2011, Dukart et al., 2013a, Dukart et al., 2013b).
Inter-center variability, as revealed in the conjunction analysis of the group comparisons, was more prominent in the smaller cohort (Fig. 2). The reason for inter-center variability could be manifold. Firstly, the disease stages might vary between centers, and secondly, the centers used different MRI scanners or even scanning parameters. The overlap between detected GMD differences across the different centers in the larger cohort shows a greater similarity between the centers in the expected areas of the frontal and temporal lobe, and the basal ganglia, presumably due to higher numbers of subjects included and, consequently, higher statistical power.
In sum, the conjunction analyses prove that disease effects are greater than the effect of inter-center variability. We suggest applying such conjunction analyses with the “leave one center out” approach, i.e. excluding repeatedly subjects from each center from the analysis, also to other multi-centric data to control for inter-center differences/bias.
One might discuss as a limitation of this study the different scanning conditions between the centers, varying from different types of scanners to different scanning sequences. More subtle effects of the disease might therefore be overlooked or some over-interpreted because of inter-center variability. We tried to approach this problem with the center conjunction analyses (see above), which pointed out the main areas showing GMD loss across all centers. Note that this approach can be transferred to other multi-center studies to validate their findings. Here, further studies shall add also other scanner types beyond Siemens scanners. On the other hand the multi-center design might be discussed as an important advantage of the study. By conducting analyses across multi-centric data we guaranteed a real-life scenario ensuring that our approach can be transferred to and applied in clinical settings. Another shortcoming of this study is the fact that most of the patients do not have a definite diagnosis yet, i.e. no detected pathogenic mutation or histopathological evidence. In order to be as precise as possible only patients diagnosed according to the newest diagnostic criteria were included in the study (Rascovsky et al., 2011). Future studies shall approve our automatic classification approach in genetically and histopathologically proven cohorts.
5. Conclusion
Recently, new diagnostic criteria were suggested that incorporate imaging criteria into diagnostic algorithms for behavioral variant frontotemporal dementia (bvFTD). The study aimed at validating these imaging criteria for individual diagnosis by predicting diagnosis from imaging data with machine learning algorithms. Brain atrophy was measured with structural magnetic resonance imaging (MRI) at 3 Tesla in a multi-centric cohort of 52 bvFTD patients and 52 healthy control subjects from the German FTLD Consortium's Study. Note that usage of multi-center data is a precondition for future application in clinical routine. Support vector machine classification predicted diagnosis in single patients with a high accuracy of up to 84.6%, where accuracy was higher in a region-of-interest approach focusing on a disease-specific network including frontotemporal, insular regions and basal ganglia in comparison with the whole brain approach. Our study demonstrates that MRI, a widespread imaging technology, can individually identify bvFTD with high accuracy, paving the road to personalized diagnostic approaches in the future.
Conflict of interest
The authors have no conflict of interest to declare.
Acknowledgments
This work was supported by the German Federal Ministry of Education and Research (BMBF) by a grant given to German FTLD Consortium [grant number FKZ O1GI1007A], by LIFE - Leipzig Research Center for Civilization Diseases at the University of Leipzig - funded by the European Union, European Regional Development Fund and by the Free State of Saxony within the framework of the excellence initiative (KS & MLS), by the Parkinson's Disease Foundation [grant number PDF-IRG-1307; KS, SB, KM & MLS], by the Michael Fox Foundation [grant number 11362; KM & MLS], by the MaxNetAging Research School of the Max Planck Society (SB).
Contributor Information
Sebastian Meyer, Email: smeyer@cbs.mpg.de.
Karsten Mueller, Email: karstenm@cbs.mpg.de.
Katharina Stuke, Email: stuke@cbs.mpg.de.
Sandrine Bisenius, Email: bisenius@cbs.mpg.de.
Janine Diehl-Schmid, Email: janine.diehl-schmid@tum.de.
Frank Jessen, Email: frank.jessen@uk-koeln.de.
Jan Kassubek, Email: jan.kassubek@uni-ulm.de.
Johannes Kornhuber, Email: johannes.kornhuber@uk-erlangen.de.
Albert C. Ludolph, Email: albert.ludolph@rku.de.
Johannes Prudlo, Email: johannes.prudlo@med.uni-rostock.de.
Anja Schneider, Email: anja.schneider@dzne.de.
Katharina Schuemberg, Email: schuemberg@cbs.mpg.de.
Igor Yakushev, Email: igor.yakushev@tum.de.
Markus Otto, Email: markus.otto@uni-ulm.de.
Matthias L. Schroeter, Email: schroet@cbs.mpg.de.
References
- Ashburner J., Friston K.J. Voxel-based morphometry—the methods. NeuroImage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Chang C.C., Lin C.J. LIBSVM: a library for support vector machines. ACM Trans. Intell. Syst. Technol. 2011;2 [Google Scholar]
- Dukart J., Mueller K., Horstmann A., Vogt B., Frisch S., Barthel H., Becker G., Möller H.E., Villringer A., Sabri O., Schroeter M.L. Differential effects of global and cerebellar normalization on detection and differentiation of dementia in FDG-PET studies. NeuroImage. 2010;49:1490–1495. doi: 10.1016/j.neuroimage.2009.09.017. [DOI] [PubMed] [Google Scholar]
- Dukart J., Mueller K., Horstmann A., Barthel H., Moller H.E., Villringer A., Sabri O., Schroeter M.L. Combined evaluation of FDG-PET and MRI improves detection and differentiation of dementia. PLoS One. 2011;6 doi: 10.1371/journal.pone.0018111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukart J., Mueller K., Barthel H., Villringer A., Sabri O., Schroeter M.L., Alzheimer's Disease Neuroimaging I. Meta-analysis based SVM classification enables accurate detection of Alzheimer's disease across different clinical centers using FDG-PET and MRI. Psychiatry Res. 2013;212:230–236. doi: 10.1016/j.pscychresns.2012.04.007. [DOI] [PubMed] [Google Scholar]
- Dukart J., Perneczky R., Forster S., Barthel H., Diehl-Schmid J., Draganski B., Obrig H., Santarnecchi E., Drzezga A., Fellgiebel A., Frackowiak R., Kurz A., Muller K., Sabri O., Schroeter M.L., Yakushev I. Reference cluster normalization improves detection of frontotemporal lobar degeneration by means of FDG-PET. PLoS One. 2013;8 doi: 10.1371/journal.pone.0055415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E.J., Sidhu M., Gaus S.E., Huang E.J., Hof P.R., Miller B.L., DeArmond S.J., Seeley W.W. Selective frontoinsular von Economo neuron and fork cell loss in early behavioral variant frontotemporal dementia. Cereb. Cortex. 2012;22:251–259. doi: 10.1093/cercor/bhr004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöppel S., Stonnington C.M., Chu C., Draganski B., Scahill R.I., Rohrer J.D., Fox N.C., Jack C.R., Jr., Ashburner J., Frackowiak R.S. Automatic classification of MR scans in Alzheimer's disease. Brain. 2008;131:681–689. doi: 10.1093/brain/awm319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Möller C., Pijnenburg Y.A., van der Flier W.M., Versteeg A., Tijms B., de Munck J.C., Hafkemeijer A., Rombouts S.A., van der Grond J., van Swieten J., Dopper E., Scheltens P., Barkhof F., Vrenken H., Wink A.M. Alzheimer disease and behavioral variant frontotemporal dementia: automatic classification based on cortical atrophy for single-subject diagnosis. Radiology. 2016;279:838–848. doi: 10.1148/radiol.2015150220. [DOI] [PubMed] [Google Scholar]
- Neary D., Snowden J.S., Gustafson L., Passant U., Stuss D., Black S., Freedman M., Kertesz A., Robert P.H., Albert M., Boone K., Miller B.L., Cummings J., Benson D.F. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Otto M., Ludolph A.C., Landwehrmeyer B., Forstl H., Diehl-Schmid J., Neumann M., Kretzschmar H.A., Schroeter M.L., Kornhuber J., Danek A. German consortium for frontotemporal lobar degeneration. Nervenarzt. 2011;82:1002–1005. doi: 10.1007/s00115-011-3261-3. [DOI] [PubMed] [Google Scholar]
- Pan P.L., Song W., Yang J., Huang R., Chen K., Gong Q.Y., Zhong J.G., Shi H.C., Shang H.F. Gray matter atrophy in behavioral variant frontotemporal dementia: a meta-analysis of voxel-based morphometry studies. Dement. Geriatr. Cogn. Disord. 2012;33:141–148. doi: 10.1159/000338176. [DOI] [PubMed] [Google Scholar]
- Rabinovici G.D., Miller B.L. Frontotemporal lobar degeneration: epidemiology, pathophysiology, diagnosis and management. CNS Drugs. 2010;24:375–398. doi: 10.2165/11533100-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovici G.D., Seeley W.W., Kim E.J., Gorno-Tempini M.L., Rascovsky K., Pagliaro T.A., Allison S.C., Halabi C., Kramer J.H., Johnson J.K., Weiner M.W., Forman M.S., Trojanowski J.Q., Dearmond S.J., Miller B.L., Rosen H.J. Distinct MRI atrophy patterns in autopsy-proven Alzheimer's disease and frontotemporal lobar degeneration. Am. J. Alzheimers Dis. Other Demen. 2007;22:474–488. doi: 10.1177/1533317507308779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K., Hodges J.R., Knopman D., Mendez M.F., Kramer J.H., Neuhaus J., van Swieten J.C., Seelaar H., Dopper E.G., Onyike C.U., Hillis A.E., Josephs K.A., Boeve B.F., Kertesz A., Seeley W.W., Rankin K.P., Johnson J.K., Gorno-Tempini M.L., Rosen H., Prioleau-Latham C.E., Lee A., Kipps C.M., Lillo P., Piguet O., Rohrer J.D., Rossor M.N., Warren J.D., Fox N.C., Galasko D., Salmon D.P., Black S.E., Mesulam M., Weintraub S., Dickerson B.C., Diehl-Schmid J., Pasquier F., Deramecourt V., Lebert F., Pijnenburg Y., Chow T.W., Manes F., Grafman J., Cappa S.F., Freedman M., Grossman M., Miller B.L. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter M.L. Considering the frontomedian cortex in revised criteria for behavioural variant frontotemporal dementia. Brain. 2012;135 doi: 10.1093/brain/aws030. author reply e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter M.L., Neumann J. Combined imaging markers dissociate Alzheimer's disease and frontotemporal lobar degeneration — an ALE meta-analysis. Front. Aging Neurosci. 2011;3:10. doi: 10.3389/fnagi.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter M.L., Raczka K., Neumann J., Yves von Cramon D. Towards a nosology for frontotemporal lobar degenerations — a meta-analysis involving 267 subjects. NeuroImage. 2007;36:497–510. doi: 10.1016/j.neuroimage.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Schroeter M.L., Raczka K., Neumann J., von Cramon D.Y. Neural networks in frontotemporal dementia — a meta-analysis. Neurobiol. Aging. 2008;29:418–426. doi: 10.1016/j.neurobiolaging.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Schroeter M.L., Stein T., Maslowski N., Neumann J. Neural correlates of Alzheimer's disease and mild cognitive impairment: a systematic and quantitative meta-analysis involving 1351 patients. NeuroImage. 2009;47:1196–1206. doi: 10.1016/j.neuroimage.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter M.L., Laird A.R., Chwiesko C., Deuschl C., Schneider E., Bzdok D., Eickhoff S.B., Neumann J. Conceptualizing neuropsychiatric diseases with multimodal data-driven meta-analyses — the case of behavioral variant frontotemporal dementia. Cortex. 2014;57:22–37. doi: 10.1016/j.cortex.2014.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Carlin D.A., Allman J.M., Macedo M.N., Bush C., Miller B.L., Dearmond S.J. Early frontotemporal dementia targets neurons unique to apes and humans. Ann. Neurol. 2006;60:660–667. doi: 10.1002/ana.21055. [DOI] [PubMed] [Google Scholar]
- Steinacker P., Semler E., Anderl-Straub S., Diehl-Schmid J., Schroeter M.L., Uttner I., Foerstl H., Landwehrmeyer B., von Arnim C.A.F., Kassubek J., Oeckl P., Huppertz H.J., Fassbender F., Fliessbach K., Prudlo J., Roßmeier C., Kornhuber J., Schneider A., Volk A.E., Lauer M., Danek A., Ludolph A.C., Otto M. Neurofilament as a blood marker for diagnosis and monitoring of primary progressive aphasias. Neurology. 2017 doi: 10.1212/WNL.0000000000003688. published online. [DOI] [PubMed] [Google Scholar]
- Tahmasian M., Shao J., Meng C., Grimmer T., Diehl-Schmid J., Yousefi B.H., Forster S., Riedl V., Drzezga A., Sorg C. Based on the network degeneration hypothesis: separating individual patients with different neurodegenerative syndromes in a preliminary hybrid PET/MR study. J. Nucl. Med. 2016;57:410–415. doi: 10.2967/jnumed.115.165464. [DOI] [PubMed] [Google Scholar]
- Whitwell J.L., Przybelski S.A., Weigand S.D., Ivnik R.J., Vemuri P., Gunter J.L., Senjem M.L., Shiung M.M., Boeve B.F., Knopman D.S., Parisi J.E., Dickson D.W., Petersen R.C., Jack C.R., Jr., Josephs K.A. Distinct anatomical subtypes of the behavioural variant of frontotemporal dementia: a cluster analysis study. Brain. 2009;132:2932–2946. doi: 10.1093/brain/awp232. [DOI] [PMC free article] [PubMed] [Google Scholar]