Abstract
Prospective longitudinal evaluation of adolescents at ultra-high-risk (UHR) for the development of psychosis enables an enriched neurodevelopmental perspective of disease progression in the absence of many of the factors that typically confound research with formally psychotic patients (antipsychotic medications, drug/alcohol dependence). The cerebellum has been linked to cognitive dysfunction and symptom severity in schizophrenia and recent work from our team suggests that it is a promising target for investigation in UHR individuals as well. However, the cerebellum and cerebello-thalamo-cortical networks have not been investigated developmentally or with respect to disease progression in this critical population. Further, to date, the types of longitudinal multimodal connectivity studies that would substantially inform our understanding of this area have not yet been conducted. In the present investigation 26 UHR and 24 healthy control adolescents were administered structured clinical interviews and scanned at baseline and then again at 12-month time points to investigate both functional and structural connectivity development of cerebello-thalamo-cortical networks in conjunction with symptom progression. Our results provide evidence of abnormal functional and structural cerebellar network development in the UHR group. Crucially, we also found that cerebello-thalamo-cortical network development and connectivity at baseline are associated with positive symptom course, suggesting that cerebellar networks may be a biomarker of disease progression. Together, these findings provide support for neurodevelopmental models of psychotic disorders and suggest that the cerebellum and respective networks with the cortex may be especially important for elucidating the pathophysiology of psychosis and highlighting novel treatment targets.
Keywords: Cerebellum, Diffusion tensor imaging, Functional connectivity, Psychosis risk, Longitudinal, Neuroimaging
Highlights
-
•
The cerebellum is an important target of research across the psychosis spectrum.
-
•
Cerebellar networks were investigated over 12 months in youth at-risk for psychosis.
-
•
Network development differs from controls in the at-risk group.
-
•
Cerebello-cortical network connectivity predicts worsening positive symptoms.
-
•
Supports neurodevelopmental models of psychosis and implicates the cerebellum.
1. Introduction
Psychosis includes a debilitating series of mental illnesses and improving etiological understanding is especially important for designing targeted interventions that may be implemented prior to onset. In recent years, focus of investigation in this area has shifted to also include individuals at ultra-high-risk (UHR) for psychosis, a population where between 15 and 35% of individuals go on to develop a psychotic disorder such as schizophrenia within 2 years (Cannon et al., 2008, Mittal et al., 2010, Yung et al., 2007). At the present time, neurodevelopmental and disconnectivity hypotheses have been instrumental in providing a framework for understanding this vital risk period (Friston and Frith, 1995, Insel, 2010). Within this context, it is important to consider that while there has been very important progress in this area, a sorely needed developmentally-informed perspective remains hampered by the lack of longitudinal studies that included brain data at multiple time points, and consider both structural and functional connectivity.
The cerebellum and cerebello-thalamo-cortical (CTC) networks have been implicated in psychosis, typically as part of a cognitive dysmetria framework (Andreasen et al., 1998, Andreasen et al., 1996, Andreasen and Pierson, 2008, Picard et al., 2008). Recently, we suggested that cerebellar functional deficits may contribute to symptomatology due to dysfunctional internal models of behavior and altered interactions with the cortex (Bernard et al., 2015). Furthermore, resting state functional connectivity MRI (fcMRI) in UHR individuals demonstrates that cerebello-thalamo-cortical connectivity strength is related to positive and negative symptom severity (Bernard et al., 2014). It may be the case that developmental differences in CTC networks are associated with symptom progression in UHR individuals, and can serve as a possible biomarker of disease progression. However, while there is an emerging literature suggesting cerebellar functional deficits and neural differences in UHR populations (Bernard et al., 2014, Borgwardt et al., 2007, Dean et al., 2015, Dean et al., 2013, Pantelis et al., 2003), development within CTC networks has not been investigated in UHR populations longitudinally with respect to disease course. Such an investigation would provide key new insights into the role of CTC networks in disease progression and the etiology of schizophrenia and psychosis more broadly.
In this study, we investigated a sample of UHR and healthy control adolescents to investigate two questions. First, does cerebello-thalamo-cortical network development (functional and structural) differ between healthy adolescents and UHR individuals? Second, does CTC network activity relate to disease progression in this population? We focused on cerebellar Lobule V and Crus I and II; in patients with schizophrenia, motor abnormalities including (e.g., deficits in postural control and motor learning; Marvel et al., 2007, Marvel et al., 2004), particularly as they relate to the cerebellum, are well-defined (reviewed in Bernard and Mittal, 2014, Bernard and Mittal, 2015), and cognitive dysfunction represents a core deficit of the disease (Elvevag and Goldberg, 2000) making these networks particularly interesting targets of investigation. In our recent work investigating structural and functional network development during adolescence in healthy controls we found that functional connectivity between Crus I and II with lateral and medial prefrontal cortex is decreased over 12-months. This stands in contrast with Lobule V, a key motor network, where there are no further changes. These findings suggest that development of cerebello-frontal networks is protracted relative to motor development (Bernard et al., 2016). Thus, we hypothesized that functional connectivity development in the UHR group would differ with respect to controls. With respect to Crus I and Crus II connectivity we predicted that this would be manifest in one of two ways: as increases over time, or no changes. In normally developing youth, there were no changes in Lobule V connectivity in healthy controls (Bernard et al., 2016); therefore, we expected to see either increases or decreases in the UHR group indicative of abnormal developmental patterns. Regarding white matter, consistent with our prior work investigating white matter development in UHR populations (Bernard et al., 2015, Mittal et al., 2014), we predicted that there would be a significant interaction such that FA would decrease in the UHR group, but increase in controls. We expected a smaller degree of change in the Lobule V white matter networks given the normative developmental patterns of motor regions (Bernard et al., 2016). Finally, we hypothesized that both functional and structural measures of cerebello-thalamo-cortical connectivity would be associated with symptom progression in the UHR group.
2. Methods
2.1. Participants
Adolescents between 12 and 21 years old were recruited at the Adolescent Development and Preventive Treatment (ADAPT) program and the Institutional Review Board approved all procedures. Exclusion criteria for both groups included history of head injury, the presence of a neurological disorder, life-time substance dependence as assessed by the Structured Clinical Interview for Axis-I DSM-IV Disorders (First et al., 1995), and the presence of any contraindications for the magnetic resonance imaging environment. For UHR participants, we also excluded those with an Axis I psychotic disorder. In control subjects, we excluded all those with an Axis I disorder, or the presence of a psychotic disorder in first-degree relatives.
In total, 68 UHR and 78 control participants have been enrolled in the study. At the time of this investigation, 36 UHR and 42 control individuals were eligible for 12-month follow-up. 8 UHR individuals were lost to attrition, and 2 were excluded from our analysis due to issues with brain imaging data quality. Of the controls, 3 declined to complete the baseline scan, and 5 declined to complete the scan at 12-month follow-up. 7 control participants were lost to attrition. This level of attrition is largely comparable to other longitudinal studies following this population (Cannon et al., 2008). Finally, 3 additional participants were excluded due to issues with data quality and collection at the scanner. Our final sample for analysis included 26 UHR (18.65 ± 1.74 years old, 8 female, 4 left-handed) and 24 control (17.83 ± 2.50 years old, 13 female, 1 left-handed) participants who were assessed with a clinical interview and completed a neuroimaging session at both baseline and 12-month follow-up. Given that left-handedness is more common in patients with schizophrenia (Dragovic and Hammond, 2005), the inclusion of left-handed individuals results in a more representative high-risk sample. Analyses with these individuals removed are presented in the Supplemental material. Demographic variables are presented in Table 1.
Table 1.
Demographic variables and symptom severity in UHR and control participants. Mean and standard deviation are presented. Statistical significance, in cases where there is a trend or significant group difference is provided. Positive and negative symptoms differed significantly at both baseline and follow-up. Specific p-values and statistics are provided in text.
| UHR | Control | ||
|---|---|---|---|
| N | 26 (8 female) |
24 (13 female) |
|
| Baseline Age (years) | 18.65 (1.74) |
17.83 (2.50) |
|
| Parent education (years) | 16.63 (1.82) |
16.29 (2.40) |
|
| Subject education (years) | 12.77 (1.73) |
11.95 (2.42) |
|
| Alcohol frequency⁎ | 1.96 (1.82) |
0.96 (1.08) |
|
| Positive symptoms⁎⁎⁎ | Baseline | 11.96 (3.69) |
0.58 (1.21) |
| Follow-up | 11.04 (6.77) |
0.08 (0.28) |
|
| Negative symptoms⁎⁎⁎ | Baseline | 10.85 (6.95) |
0.42 (0.77) |
| Follow-up | 7.27 (6.43) |
0.25 (0.53) |
|
| Scanner motion (mm) | Baseline | 0.26 (0.34) |
0.21 (0.47) |
| Follow-up | 0.30 (0.34) |
0.21 (0.10) |
|
| Scanner outliers (number) | Baseline# | 5.27 (4.63) |
8.08 (6.30) |
| Follow-up | 8.07 (10.52) |
4.67 (5.52) |
|
p < 0.1.
p < 0.05.
p < 0.001.
Longitudinal data from the healthy control participants included have been analyzed previously as part of an investigation on cerebellar development (Bernard et al., 2016). Here, the control subjects are identical to those used in (Bernard et al., 2016), and serve as a point of comparison for our UHR group. All analyses presented here are entirely new, and focus on group differences, or within group analyses of the UHR sample, which has not been studied in this context. Subsets of the UHR data have been investigated longitudinally (Bernard et al., 2015, Dean et al., 2015, Mittal et al., 2014), but this work has not focused on fcMRI with respect to symptom progression. Indeed, to our knowledge this represents the first longitudinal multimodal imaging study of UHR individuals with respect to symptom progression.
2.2. Symptom assessment
The Structured Interview for Prodromal Syndromes (SIPS) measures distinct categories of prodromal symptom domains including positive and negative dimensions and is scored from 0 to 6 for each symptom. Inclusion in the UHR group was determined by moderate levels of positive symptoms (a SIPS score of 3–5 in one or more of the 5 positive symptom categories; Table 1), and/or a decline in global functioning in association with the presence of schizotypal personality disorder, and/or a family history of schizophrenia (Miller et al., 1999). A total of 3 UHR participants (12.5%) had a family history of schizophrenia, and two of these individuals showed attenuated positive symptoms as well. All interviewers had inter-rater reliabilities that exceeded Kappa ≥ 80. The SIPS was administered at baseline to confirm a prodromal syndrome and include individuals in the UHR group, and at 12-month follow-up to assess symptom change. We quantified total positive and negative symptoms for our subsequent analyses. The frequency of alcohol consumption was measured based on self-report on a scale from 0 to 5 where 0 indicates “never uses” and 5 indicates “almost daily use” (Drake et al., 1996).
2.3. Comorbidities
Consistent with other reports (Fusar-Poli et al., 2014, Rosen et al., 2006), the UHR group comorbidities included major depressive disorder (n = 5, 20.1%), dysthymic disorder (n = 1, 4%), bipolar disorder (n = 3, 12.5%), obsessive compulsive disorder (n = 1, 4%), post-traumatic stress disorder (n = 1, 4%), anxiety disorders (n = 7, 29.2%).
2.4. Neuroimaging procedure, data preprocessing and analysis
Participants completed a brain imaging session that included structural, fcMRI, and DTI scans, acquired using a 3-Tesla Siemens Tim Trio MRI scanner (Siemens AG, Munich, Germany) using a standard 12-channel head coil. All fcMRI data were pre-processed using FSL (v.5.0.7; http://fsl.fmrib.ox.ac.uk/fsl), and analysis was performed in the CONN toolbox version 15.a (Whitfield-Gabrieli and Nieto Castañón, 2012) implemented with SPM 12 (Wellcome Trust Centre for Neuroimaging). Cerebello-thalamo-cortical white matter tracts were mapped for Lobule V as well as Crus I. These tracts have been previously well delineated in healthy adults (Salmi et al., 2010), thus we restricted our tractography to these regions, and used methods identical to those in our work looking at healthy development (Bernard et al., 2016). Diffusion weighted images were processed using FSL's FDT toolbox. The parameters used for data collection and preprocessing match closely those used in our previously published brain imaging work (e.g., Bernard et al., 2016, Bernard and Mittal, 2014, Bernard and Mittal, 2015, Bernard et al., 2015). Specific details are provided in the Supplemental material.
First, we conducted analyses to investigate developmental differences in the Lobule V, Crus I and Crus II networks in UHR and control individuals using group x time repeated measures ANOVAs in the CONN toolbox. This was followed up with within-subjects paired t-tests of the UHR group to better understand the within-group patterns of cerebello-cortical resting state development. Finally, we conducted correlation analyses within the CONN toolbox to determine whether baseline connectivity patterns are correlated with symptom change at the whole-brain level, and to investigate the relationships between structural and functional connectivity. All resulting statistical maps were first thresholded at the voxel-level at puncorr < 0.001 and corrected at the cluster-level to a false-discovery rate (FDR) of p < 0.05 (Chumbley and Friston, 2009). Because this is an important clinical population, we also reported trend-level findings (pFDR < 0.08), as these trends may be of clinical importance.
Mean FA was then extracted for each person from the tracts connecting Lobule V to the motor cortex, and Crus I to the prefrontal cortex (both via the thalamus) by masking the FA maps with masks of the respective tracts. We have also previously investigated these tracts in healthy controls only (Bernard et al., 2016), and found there is good correspondence with those mapped by Salmi and colleagues (Salmi et al., 2010). We refer readers to our prior work, which shows in detail these tracts as they were mapped here in the healthy control participants (Bernard et al., 2016).
2.5. Additional statistical analyses
Demographic, symptom, and measures of FA from our DTI scans were analyzed IBM SPSS 22 (IBM Corporation Armonk, NY, 2012). Group differences in demographic variables were analyzed using independent samples t-tests (analysis of demographic variables, symptomatology, psychiatric comorbidities and scanner motion are presented in the Supplemental material). Changes in symptoms over time, motion, and FA in the individual tracts of interest were all analyzed using 2 × 2 group by time repeated measures ANOVA. Analyses of the relationships between FA and symptom severity were conducted using Pearson correlation and linear modeling. We were interested in understanding how changes in white matter relate to symptom progression, and as such we used a difference score (time 2–time 1). To understand how functional and structural connectivity at baseline together act as a marker of disease severity at time 2 (quantified as symptom severity), we used stepwise regression models, that were again initially limited to cerebello-thalamo-motor connectivity. Stepwise regression models were subsequently computed using connectivity between Lobule V and M1, in conjunction with baseline FA to predict symptom severity at 12-month follow-up. In all cases, unless otherwise noted in the results section, statistics were evaluated using p < 0.05.
3. Results
3.1. Resting state connectivity development in UHR individuals
We found a significant interaction with Crus I, wherein connectivity between Crus I and the occipital cortex was different over time in the two groups (Table 2), supporting a differential pattern of development in UHR individuals. To further unpack these results we conducted within group analyses of connectivity change in the UHR group, comparing Time 2 to Time 1. Consistent with predictions, we found a different pattern of development in the cerebello-prefrontal resting state networks associated with the Crus I and Crus II seed regions. In the UHR group, we observed no differences in functional connectivity in Crus I and Crus II when conducting a paired t-test comparing the two time points, unlike our results in healthy controls (Bernard et al., 2016). However, consistent with our findings in healthy controls, there were no differences over time in Lobule V connectivity in the UHR group. Supplementary Fig. 2 presents the within-group patterns of cerebello-cortical connectivity at follow-up, while Supplementary Table 3 presents group differences.
Table 2.
Group by Time interaction Crus I.
| Seed region | Region | BA | Cluster size | MNI coordinates |
T-value | P(FDR) | ||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| Crus I | Occipital pole | 18 | 216 | 8 | − 92 | 12 | 4.16 | 0.008 |
| Supracalcarine cortex | 18 | 4 | − 86 | 8 | 3.62 | |||
3.2. Resting state connectivity and symptom progression in UHR individuals
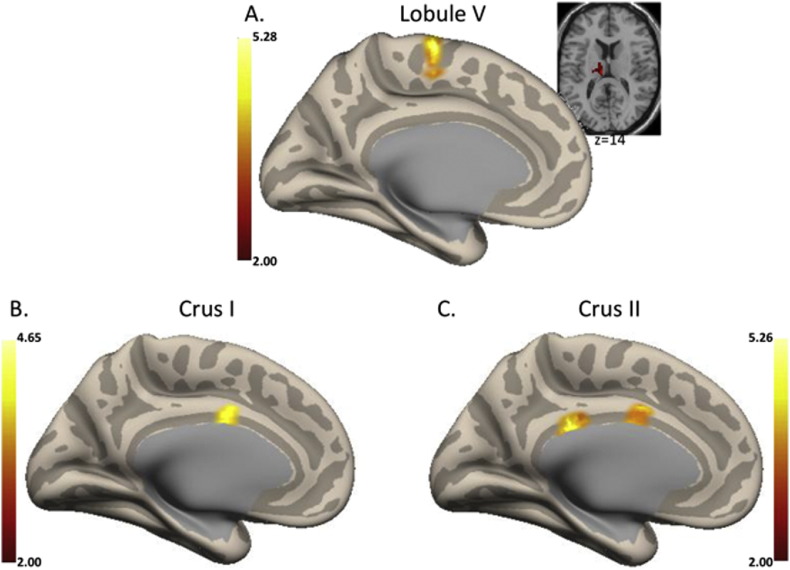
To evaluate the utility of baseline connectivity as a marker of symptom progression in UHR populations, we investigated correlations between baseline connectivity and both positive and negative symptom change. With respect to positive symptoms, we found that functional connectivity between the cerebellum and cortex using all cerebellar seeds was positively associated with worsening positive symptoms. In Lobule V, higher connectivity in the medial motor regions, supplementary motor area, and the thalamus at baseline were associated with worsening symptoms over 12-months, though the thalamus finding was trend-level. In both Crus I and II, connectivity with the cingulate cortex was associated with worsening symptoms (see Fig. 1 and Supplementary Table 4). There were no significant associations with respect to baseline connectivity and negative symptom change.
Fig. 1.
Correlations between baseline cerebello-cortical connectivity and positive symptom change. A. Lobule V (left medial), B. Crus I (left medial), and C. Crus II (left medial) networks. In all cases, higher connectivity at baseline was associated with worsening positive symptoms. For Lobule V, the medial motor cortical regions are presented on the inflated medial surface, while thalamic connectivity is presented on an axial slice. Coordinates of the significant regions and the associated statistics are presented in Table 3. L: left hemisphere; R: right hemisphere. Figure depicts the z-scores of connectivity from the r-to-z transform computed in CONN.
3.3. White matter development and symptom progression in UHR individuals
Investigations using paired t-tests in the UHR group alone found that there were no significant differences in any of the tracts over the course of one year (Lobule V-thalamic: t(25) = 1.12, p = 0.27; Crus I-thalamic: t(25) = 0.94, p = 0.35; thalamo-motor: t(25) = 0.14, p = 0.89; thalamo-prefrontal: t(25) = 1.35, p = 0.19). Using 2 × 2 (group × time) ANOVAs to look at each of the four tract segments, we found trend-level interactions after correcting for multiple comparisons (Bonferroni, p < 0.0125) in FA in the Lobule V-thalamic tract segment (F(1,48) = 5.02, p = 0.03), the thalamo-prefrontal tract segment (F(1,48) = 4.42, p = 0.041), and the Crus I-thalamic tract segment (F(1,48) = 3.59, p = 0.064). There was no significant group by time interaction in the thalamo-motor tract (F(1,48) = 1.73, p = 0.195). Thus, there is a trend that suggests an abnormal pattern of development in cerebello-thalamo-cortical white matter in UHR individuals (Supplementary Fig. 3).
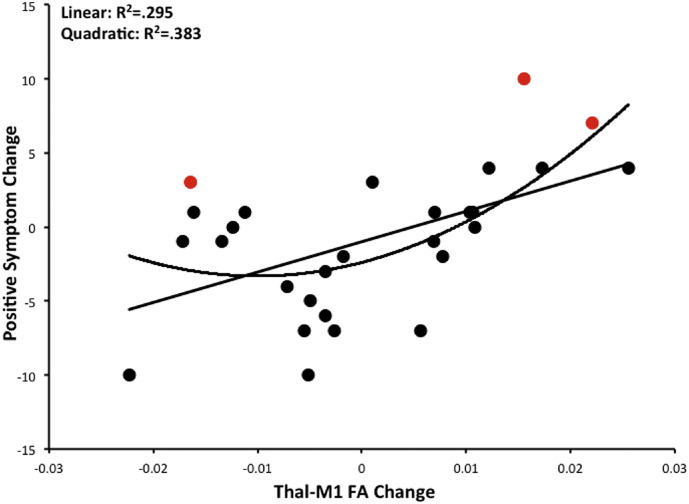
Pairwise correlations between FA change in the four tracts of interest and both positive and negative symptom change were completed, and analyses were Bonferroni corrected to p < 0.006. There was a positive correlation between FA change in the thalamo-motor tract and positive symptom change (r(26) = 0.54, p = 0.004; Fig. 2), after correction for multiple comparisons. None of the other correlations were significant (in all cases, p > 0.27) with respect to positive symptoms, and there were no significant correlations with negative symptoms (in all cases p > 0.31). In conjunction with the resting state connectivity data, this provides converging evidence suggesting that cerebello-thalamo-motor tracts are related to positive symptom progression in UHR individuals. In the UHR sample, those with FA increases were those that were worsening over time with respect to symptoms, while those with FA decreases showed improving symptoms.
Fig. 2.
FA change and positive symptom progression. FA change in the thalamo-motor tract was significantly positively associated with worsening positive symptoms using a linear model. Those with continued increases in FA had more positive symptoms at 12-months, indicative of a poor disease course. However, a quadratic model fits the data equally well, such that those UHR individuals with the greatest degree of change (increase or decrease) in FA are those that show the largest increases in positive symptom progression. Points shown in red indicate the three individuals who received a conversion diagnosis at 12-month follow-up.
Furthermore, visual inspection of the scatterplot of this relationship indicated that a quadratic relationship may also fit the data. We therefore computed an exploratory analysis of a quadratic model. The quadratic model was significant (R2 = 0.383, F(2,23) = 7.15, p = 0.004), such that those that show either decreases or increases in FA in this tract are those with the greatest changes in positive symptom severity. The greatest deviations in white matter (from no change) were associated with worsening positive symptoms. Finally, 3 UHR participants received a conversion diagnosis of a psychotic disorder during the 12-month follow-up period. They are highlighted in Fig. 2. Those that converted showed the some of the greatest changes in white matter FA (either increases or decreases). However, there were individuals with comparable degrees of white matter change that did not convert. Additional follow-up on these individuals is ongoing.
3.4. Resting state connectivity, white matter, and symptom progression
Using step-wise multiple regression we investigated both baseline Lobule V-motor resting state connectivity strength and thalamo-motor FA, with positive symptoms at 12-months as the dependent variable. In the first block of our analysis we entered baseline positive symptoms; we then entered baseline fcMRI strength and FA in blocks 2 and 3, respectively. The model was significant (F(3,25) = 9.36, p < 0.001) and accounted for approximately 56% of the variance in time 2 positive symptoms. The inclusion of baseline Lobule V-motor connectivity strength accounted for 9% of the variance in symptoms at follow-up. The inclusion of Lobule V-motor connectivity represents a statistically significant improvement in the model, as revealed by a significant ΔR2. Thalamo-motor FA did not significantly improve the model. Specific statistics regarding this model are provided in Table 3. Notably, the order in which the resting state and FA variables were entered does not change the model or the betas. Results including Crus I and II in the model are presented in the Supplemental material. Future work with larger samples is warranted to follow-up on the impact and utility of these cerebello-frontal networks in predicting positive symptoms over time. Our results here however suggest that baseline cerebello-motor connectivity is a potentially important predictor of time 2 positive symptoms.
Table 3.
Baseline connectivity predicts time 12-month symptoms. Results of the step-wise regression investigating time two positive symptoms. Baseline positive symptoms were entered in the first block, followed by Lobule V-motor cortex connectivity, and thalamo-motor FA. Significant changes in R2 are indicated, as are significant β-values. *p < 0.05, ***p < 0.001 Thalamo-Motor FA.
| Baseline positive symptoms |
Lobule V-motor connectivity |
Thalamo-motor FA |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | df | F | P | ΔR2 | df | F | P | ΔR2 | df | F | P | |
| T2 positive symptoms | 0.462 | 1,24 | 20.61 | 0.000⁎⁎⁎ | 0.090 | 1,23 | 4.61 | 0.04* | 0.009 | 1,22 | 0.446 | 0.51 |
| β | 0.680⁎⁎⁎ | 0.30⁎ | − 0.095 | |||||||||
4. Discussion
In taking a longitudinal approach and combining functional and structural neuroimaging measures, the present study found that UHR individuals show abnormal patterns of cerebello-thalamo-cortical network development. Furthermore, in the UHR group alone, both functional and structural measures of cerebello-thalamo-cortical connectivity were associated with positive symptom progression over the course of one year. To our knowledge, this is the first study of a UHR sample to investigate longitudinal symptom progression with respect to changes in fcMRI and DTI. Notably, abnormal FA development in the UHR group was associated with disease progression, and baseline connectivity measures are associated with symptom change. Interestingly, these associations were present for the cerebello-thalamo-motor networks in both domains, providing converging evidence for the importance of this circuit in the pathophysiology of psychosis. Most importantly, when baseline Lobule V-motor functional connectivity was included in regression models that account for baseline positive symptoms, there were significant improvements in the amount of variance explained. That is, higher connectivity at baseline was associated with worse positive symptoms 12-months later.
Broadly, these findings indicate CTC circuit developmental abnormalities in UHR individuals. Though speculative, this may be due to delayed neuronal maturity, similar to what was proposed by Insel (Insel, 2010). In younger adults, functional networks between the cerebellum and prefrontal cortex show continued development in later adolescence (Bernard et al., 2016). Here, in the UHR group, we did not see any significant development in these networks over 12-months. Furthermore, patterns of FA changes over time differ in the UHR and control groups at the trend level (after conservative multiple-comparisons corrections). These patterns are consistent with abnormal cerebello-cortical connectivity in schizophrenia (Shinn et al., 2015). Schizophrenia patients show decreased prefrontal-cerebellar connectivity with Crus I and Crus II (Repovs et al., 2011, Shinn et al., 2015), where we see abnormal developmental patterns. Abnormal development may contribute to the aberrant patterns present later in the disease.
Cerebellar networks, particularly those between Lobule V and the motor cortex were shown here to be associated with positive symptom change over 12-months in UHR individuals. In the case of Lobule V- motor connectivity, this may be indicative of abnormal sensorimotor integration. Such deficits may contribute to unusual thoughts and hallucinations as the individual is unable to effectively integrate this information or attribute its source (Bernard et al., 2015). Increased cerebellar connectivity with these regions may be indicative of abnormal sensorimotor integration processes at baseline that contribute to later disease progression. Shergill and colleagues have suggested that abnormal sensory prediction error may be related to the positive symptoms of schizophrenia, and linked hallucinations with sensorimotor cortical activation (Shergill et al., 2005). Indeed, recent work has demonstrated that there is hyper-connectivity between the cerebellum and motor networks (Shinn et al., 2015), and heightened thalamo-motor connectivity is seen in UHR individuals that convert to psychosis (Anticevic et al., 2015), further highlighting the importance of this connectivity pattern. Thus, while one might expect to see frontal regions associated with positive symptom progression, a growing literature implicates sensorimotor cortex, and here, motor regions of the cerebellum. We suggest that forward modeling and sensory prediction may be especially important for positive symptom progression.
Somewhat surprisingly, we did not see any associations between cerebello-thalamo-cortical connectivity and negative symptom progression. Our own work, and that from others has linked the cerebellum and cerebellar-mediated behavior to negative symptoms (Bernard et al., 2014, Dean et al., 2015, Demirtas-Tatlidede et al., 2010). However, a recent review of negative symptoms has largely implicated cortico-striatal, frontal, and temporal circuits (Millan et al., 2014). While the authors acknowledge that the cerebellum and thalamus may contribute to negative symptom severity (Millan et al., 2014), our findings here suggest that cerebello-thalamo-cortical networks may not contribute to progression. Finally, it is notable that on average we see an improvement in negative symptom severity in the UHR group and this may have impacted our ability to detect any predictive value in the negative symptom domain.
Though we found interesting associations between FA change and symptom change, it is notable that FA was initially higher in the UHR group, and the decreases over time may be a normalizing of sorts. With that in mind, it appears to be the case that in this group, any additional change over the 12-month period is associated with worsening positive symptoms. In UHR individuals where there are increases in FA, while this pattern is consistent with that seen in normative development (e.g Insel, 2010), it may be making matters worse. Those that show decreases in FA may be normalizing, or this may also be the start of a downward trend such that FA continues to decrease as they progress further through the course of the disease. These findings are certainly somewhat surprising and counterintuitive, and the best way to resolve them is with further longitudinal follow-up. Furthermore, as the literature on white matter in psychosis risk grows, it seems to be the case that the results themselves are highly mixed (Bloemen et al., 2010, Carletti et al., 2012, Karlsgodt et al., 2009). It does not seem to be the case that simply high or low FA values are consistently found; rather, there are widespread anomalies in FA that speak to broader aberrant developmental.
While our results provide key new findings with respect to cerebello-thalamo-cortical network development in psychosis risk and their contributions to positive symptom progression, several limitations need to be considered. First, while the present sample size is comparable to or larger than other longitudinal DTI studies in this population (17–37 people) (Bloemen et al., 2010, Peters et al., 2010, Walterfang et al., 2008), consortiums combining data from a number of different recruitment centers show significant promise for investigating brain changes in UHR populations (Cannon et al., 2015), and are important for replication. Second, though consistent with recent conversion rates in UHR groups (Yung et al., 2007), only 3 participants received conversion diagnoses during the 12-month follow-up period. As such, we were unable to look at differences in conversion, and had to focus solely on symptom progression. Furthermore, we refer to conversion broadly, and this is not specific to schizophrenia, consistent with the standard in the prodromal field (Cannon et al., 2008). Relatedly, when we examine the UHR group as a whole, on average there is no significant increase in positive symptoms. With that said, it is important to keep in mind that UHR populations are very heterogeneous and a large majority do not convert (Addington et al., 2011). Identifying factors that identify the smaller but clinically signficant group that will experience an escalating course of illness is therefore critical. Our DTI findings largely point to thalamic-connectivity. We may have picked up on a thalamic effect. While the resting state findings implicate the cerebello-thalamo-cortical circuit more broadly, future work teasing apart the relative contribution of the cerebellum and thalamus is needed, particularly given recent findings focused on the thalamus (Anticevic et al., 2015). Finally, the association between thalamo-motor FA change and time two positive symptoms was in a tract that shows no changes in FA over time. Thus, interpretation of this finding is somewhat difficult. However, we suggest that this is perhaps due to the heterogeneity of the UHR sample with respect to FA change (as seen in Fig. 2). This is certainly a complex finding, and follow-up work is necessary to better understand the implications.
Together, this investigation provides further support for complementary neurodevelopmental and disconnectivity models, and implicates the cerebellum and its networks with the cortex in the progression of symptoms. While this work is only a first step, it suggests that the cerebellum and its networks may be an especially useful target of research and remediation.
Financial disclosures
The authors have no financial conflicts of interest.
Acknowledgments
This work was supported by National Institutes of Health Grants R01MH094650 and R21/R33MH103231 to V.A.M., F32MH102898 to J.A.B., and F32DA034412 to J.M.O. J.A.B. is supported in part by a Brain & Behavior Research Foundation NARSAD Young Investigator Award as the Donald & Janet Boardman Family Investigator.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2017.03.001.
Appendix A. Supplementary data
Supplementary material
References
- Addington J., Ph D., Cornblatt B.A., Ph D., Cadenhead K.S., Cannon T.D., Ph D., Mcglashan T.H., Perkins D.O., Seidman L.J., Ph D., Walker E.F., Ph D., Woods S.W., Heinssen R., Ph D. At clinical high risk for psychosis: outcome for Nonconverters. Am. J. Psychiatry. 2011;168:800–805. doi: 10.1176/appi.ajp.2011.10081191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen N.C., Pierson R. The role of the cerebellum in schizophrenia. Biol. Psychiatry. 2008;64:81–88. doi: 10.1016/j.biopsych.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen N.C., O'Leary D.S., Cizadlo T., Arndt S., Rezai K., Ponto L.L., Watkins G.L., Hichwa R.D. Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proc. Natl. Acad. Sci. U. S. A. 1996;93:9985–9990. doi: 10.1073/pnas.93.18.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen N.C., Paradiso S., O'Leary D.S. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr. Bull. 1998;24:203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- Anticevic A., Haut K., Murray J.D., Repovs G., Yang G.J., Diehl C., McEwen S.C., Bearden C.E., Addington J., Goodyear B., Cadenhead K.S., Mirzakhanian H., Cornblatt B.a., Olvet D., Mathalon D.H., McGlashan T.H., Perkins D.O., Belger A., Seidman L.J., Tsuang M.T., van Erp T.G.M., Walker E.F., Hamann S., Woods S.W., Qiu M., Cannon T.D. Association of thalamic dysconnectivity and conversion to psychosis in youth and young adults at elevated clinical risk. JAMA Psychiat. 2015;6519:1–10. doi: 10.1001/jamapsychiatry.2015.0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard J.A., Mittal V. Cerebellar motor dysfunction in schizophrenia and psychosis risk : the importance of regional cerebellar analysis approaches. Front. Psych. 2014;5:160. doi: 10.3389/fpsyt.2014.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard J.A., Mittal V.A. Dysfunctional activation of the cerebellum in schizophrenia: a functional neuroimaging meta-analysis. Clin. Psychol. Sci. 2015;3:545–566. doi: 10.1177/2167702614542463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard J.A., Dean D.J., Kent J.S., Orr J.M., Pelletier-Baldelli A., Lunsford-Avery J., Gupta T., Mittal V.A. Cerebellar networks in individuals at ultra high-risk of psychosis: impact on postural sway and symptom severity. Hum. Brain Mapp. 2014;35:4064–4078. doi: 10.1002/hbm.22458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard J.A., Orr J.M., Mittal V.A. Abnormal hippocampal–thalamic white matter tract development and positive symptom course in individuals at ultra-high risk for psychosis. NPJ Schizophr. 2015;1:15009. doi: 10.1038/npjschz.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard J.A., Orr J.M., Mittal V.A. Differential motor and prefrontal cerebello-cortical network development: evidence from multimodal neuroimaging. NeuroImage. 2016;124:291–601. doi: 10.1016/j.neuroimage.2015.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloemen O.J.N., de Koning M.B., Schmitz N., Nieman D.H., Becker H.E., de Haan L., Dingemans P., Linszen D.H., van Amelsvoort T. a M.J. White-matter markers for psychosis in a prospective ultra-high-risk cohort. Psychol. Med. 2010;40:1297–1304. doi: 10.1017/S0033291709991711. [DOI] [PubMed] [Google Scholar]
- Borgwardt S.J., Riecher-Rössler A., Dazzan P., Chitnis X., Aston J., Drewe M., Gschwandtner U., Haller S., Pflüger M., Rechsteiner E., D'Souza M., Stieglitz R.-D., Radü E.-W., McGuire P.K. Regional gray matter volume abnormalities in the at risk mental state. Biol. Psychiatry. 2007;61:1148–1156. doi: 10.1016/j.biopsych.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Cannon T.D., Cadenhead K., Cornblatt B., Woods S.W., Addington J., Walker E., Seidman L.J., Perkins D., Tsuang M., McGlashan T., Heinnsen R. Prediction of psychosis in youth at high clinical risk. Arch. Gen. Psychiatry. 2008;65:28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon T.D., Chung Y., He G., Sun D., Jacobson A., van Erp T.G.M., McEwen S., Addington J., Bearden C.E., Cadenhead K., Cornblatt B., Mathalon D.H., McGlashan T., Perkins D., Jeffries C., Seidman L.J., Tsuang M., Walker E., Woods S.W., Heinssen R. Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol. Psychiatry. 2015;77:147–157. doi: 10.1016/j.biopsych.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carletti F., Woolley J.B., Bhattacharyya S., Perez-Iglesias R., Fusar Poli P., Valmaggia L., Broome M.R., Bramon E., Johns L., Giampietro V., Williams S.C.R., Barker G.J., McGuire P.K. Alterations in white matter evident before the onset of psychosis. Schizophr. Bull. 2012;38:1170–1179. doi: 10.1093/schbul/sbs053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumbley J.R., Friston K.J. False discovery rate revisited: FDR and topological inference using Gaussian random fields. NeuroImage. 2009;44:62–70. doi: 10.1016/j.neuroimage.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Dean D.J., Bernard J.A., Orr J.M., Pelletier-Baldelli a., Gupta T., Carol E.E., Mittal V.a. Cerebellar morphology and procedural learning impairment in neuroleptic-naive youth at ultrahigh risk of psychosis. Clin. Psychol. Sci. 2013;2:152–164. doi: 10.1177/2167702613500039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D.J., Kent J.S., Bernard J.A., Orr J.M., Gupta T., Pelletier-Baldelli A., Carol E.E., Mittal V.a. Increased postural sway predicts negative symptom progression in youth at ultrahigh risk for psychosis. Schizophr. Res. 2015;10–13 doi: 10.1016/j.schres.2014.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirtas-Tatlidede A., Freitas C., Cromer J.R., Safar L., Ongur D., Stone W.S., Seidman L.J., Schmahmann J.D., Pascual-Leone A. Safety and proof of principle study of cerebellar vermal theta burst stimulation in refractory schizophrenia. Schizophr. Res. 2010;124:91–100. doi: 10.1016/j.schres.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragovic M., Hammond G. Handedness in schizophrenia: a quantitative review of evidence. Acta Psychiatr. Scand. 2005;111:410–419. doi: 10.1111/j.1600-0447.2005.00519.x. [DOI] [PubMed] [Google Scholar]
- Drake R., Mueser K.T., McHuge G.J. Clinical rating scales: alcohol use scale (AUS), drug use scale (DUS), and substance abuse treatment scale (SATS) In: Dickey B., Sederer L.I., editors. Outcomes Assessment in Clinical Practice. Williams and Wilkins; Baltimore: 1996. pp. 113–116. [Google Scholar]
- Elvevag B., Goldberg T.E. Cognitive impairment in schizophrenia is the core of the disorder. Crit. Rev. Neurobiol. 2000;1 [PubMed] [Google Scholar]
- First M., Spitzer R., Gibbon M., Williams J. Patient ed. American Psychiatric Press; Washington DC: 1995. Structured Clinical Interview for the DSM-IV Axis I Disorders (SCID-I) [Google Scholar]
- Friston K.J., Frith C.D. Schizophrenia: a disconnection syndrome? Clin. Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- Fusar-Poli P., Nelson B., Valmaggia L., Yung A.R., McGuire P.K. Comorbid depressive and anxiety disorders in 509 individuals with an at-risk mental state: impact on psychopathology and transition to psychosis. Schizophr. Bull. 2014;40:120–131. doi: 10.1093/schbul/sbs136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T.R. Rethinking schizophrenia. Nature. 2010;468:187–193. doi: 10.1038/nature09552. [DOI] [PubMed] [Google Scholar]
- Karlsgodt K.H., Niendam T.A., Bearden C.E., Cannon T.D. White matter integrity and prediction of social and role functioning in subjects at ultra-high risk for psychosis. Biol. Psychiatry. 2009;66:562–569. doi: 10.1016/j.biopsych.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvel C.L., Schwartz B.L., Rosse R.B. A quantitative measure of postural sway deficits in schizophrenia. Schizophr. Res. 2004;68:363–372. doi: 10.1016/j.schres.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Marvel C.L., Turner B.M., O'Leary D.S., Johnson H.J., Pierson R.K., Ponto L.L.B., Andreasen N.C. The neural correlates of implicit sequence learning in schizophrenia. Neuropsychology. 2007;21:761–777. doi: 10.1037/0894-4105.21.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan M.J., Fone K., Steckler T., Horan W.P. Negative symptoms of schizophrenia: clinical characteristics, pathophysiological substrates, experimental models and prospects for improved treatment. Eur. Neuropsychopharmacol. 2014;24:645–692. doi: 10.1016/j.euroneuro.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Miller T.J., Mcglashan T.H., Woods S.W., Stein K., Driesen N., Corcoran C.M., Hoffman R., Davidson L. Symptom assessment in schizophrenic prodromal states. Psychiatry Q. 1999;70:273–287. doi: 10.1023/a:1022034115078. [DOI] [PubMed] [Google Scholar]
- Mittal V. a, Walker E.F., Bearden C.E., Walder D., Trottman H., Daley M., Simone A., Cannon T.D. Markers of basal ganglia dysfunction and conversion to psychosis: neurocognitive deficits and dyskinesias in the prodromal period. Biol. Psychiatry. 2010;68:93–99. doi: 10.1016/j.biopsych.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal V.A., Dean D.J., Bernard J.A., Orr J.M., Pelletier-Baldelli A., Carol E.E., Gupta T., Turner J., Leopold D.R., Robustelli B.L., Millman Z.B. Neurological soft signs predict abnormal cerebellar-thalamic tract development and negative symptoms in adolescents at high risk for psychosis: a longitudinal perspective. Schizophr. Bull. 2014;50:1204–1215. doi: 10.1093/schbul/sbt199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantelis C., Velakoulis D., McGorry P.D., Wood S.J., Suckling J., Phillips L.J., Yung A.R., Bullmore E.T., Brewer W., Soulsby B., Desmond P., McGuire P.K. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- Peters B.D., Dingemans P.M., Dekker N., Blaas J., Akkerman E., van Amelsvoort T.a., Majoie C.B., den Heeten G.J., Linszen D.H., de Haan L. White matter connectivity and psychosis in ultra-high-risk subjects: a diffusion tensor fiber tracking study. Psychiatry Res. Neuroimaging. 2010;181:44–50. doi: 10.1016/j.pscychresns.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Picard H., Amado I., Mouchet-Mages S., Olié J.-P., Krebs M.-O. The role of the cerebellum in schizophrenia: an update of clinical, cognitive, and functional evidences. Schizophr. Bull. 2008;34:155–172. doi: 10.1093/schbul/sbm049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repovs G., Csernansky J.G., Barch D.M. Brain network connectivity in individuals with schizophrenia and their siblings. Biol. Psychiatry. 2011;69:967–973. doi: 10.1016/j.biopsych.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen J.L., Miller T.J., D'Andrea J.T., McGlashan T.H., Woods S.W. Comorbid diagnoses in patients meeting criteria for the schizophrenia prodrome. Schizophr. Res. 2006;85:124–131. doi: 10.1016/j.schres.2006.03.034. [DOI] [PubMed] [Google Scholar]
- Salmi J., Pallesen K., Neuvonen T. Cognitive and motor loops of the human cerebro-cerebellar system. J. Cogn. Neurosci. 2010:2663–2676. doi: 10.1162/jocn.2009.21382. [DOI] [PubMed] [Google Scholar]
- Shergill S.S., Samson G., Bays P.M., Frith C.D., Wolpert D.M. Evidence for sensory prediction deficits in schizophrenia. Am. J. Psychiatry. 2005;162:2384–2386. doi: 10.1176/appi.ajp.162.12.2384. [DOI] [PubMed] [Google Scholar]
- Shinn A.K., Baker J.T., Lewandowski K.E., Öngür D., Cohen B.M. Aberrant cerebellar connectivity in motor and association networks in schizophrenia. Front. Hum. Neurosci. 2015;9:1–16. doi: 10.3389/fnhum.2015.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walterfang M., McGuire P.K., Yung A.R., Phillips L.J., Velakoulis D., Wood S.J., Suckling J., Bullmore E.T., Brewer W., Soulsby B., Desmond P., McGorry P.D., Pantelis C. White matter volume changes in people who develop psychosis. Br. J. Psychiatry. 2008;193:210–215. doi: 10.1192/bjp.bp.107.043463. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto Castañón A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Yung A.R., Yuen H.P., Berger G., Francey S., Hung T.-C., Nelson B., Phillips L., McGorry P. Declining transition rate in ultra high risk (prodromal) services: dilution or reduction of risk? Schizophr. Bull. 2007;33:673–681. doi: 10.1093/schbul/sbm015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material