Abstract
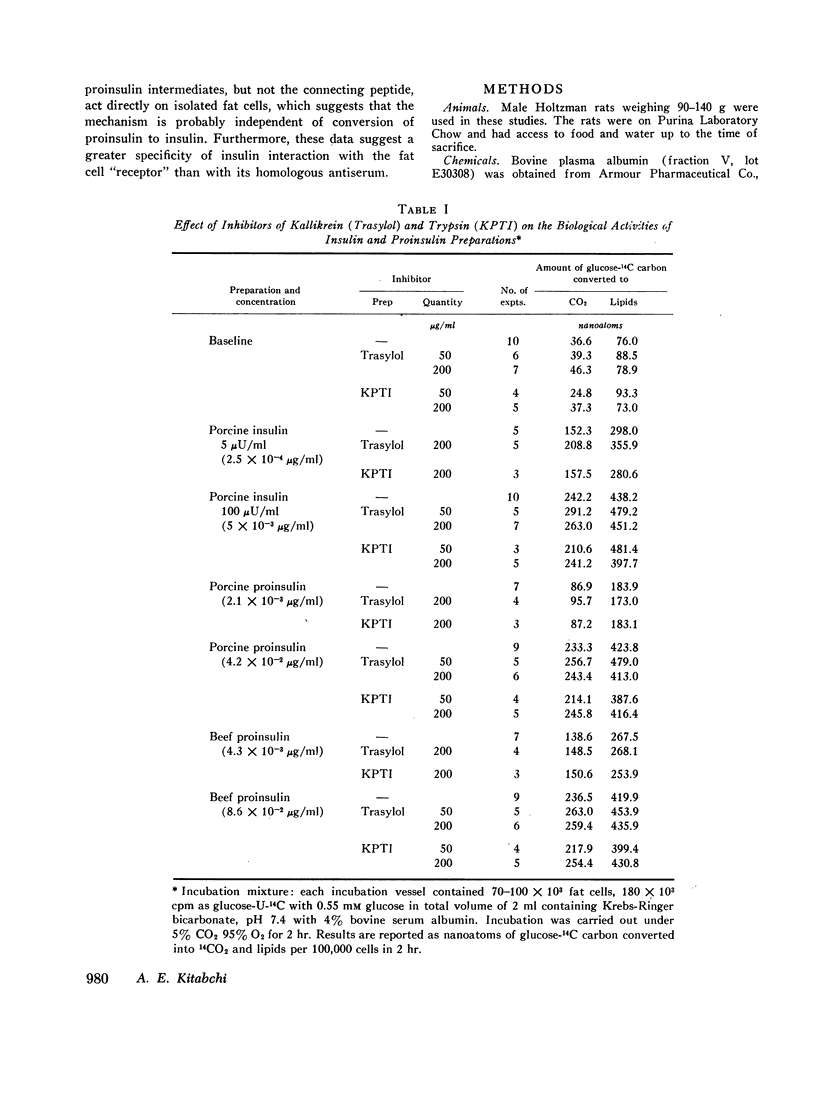
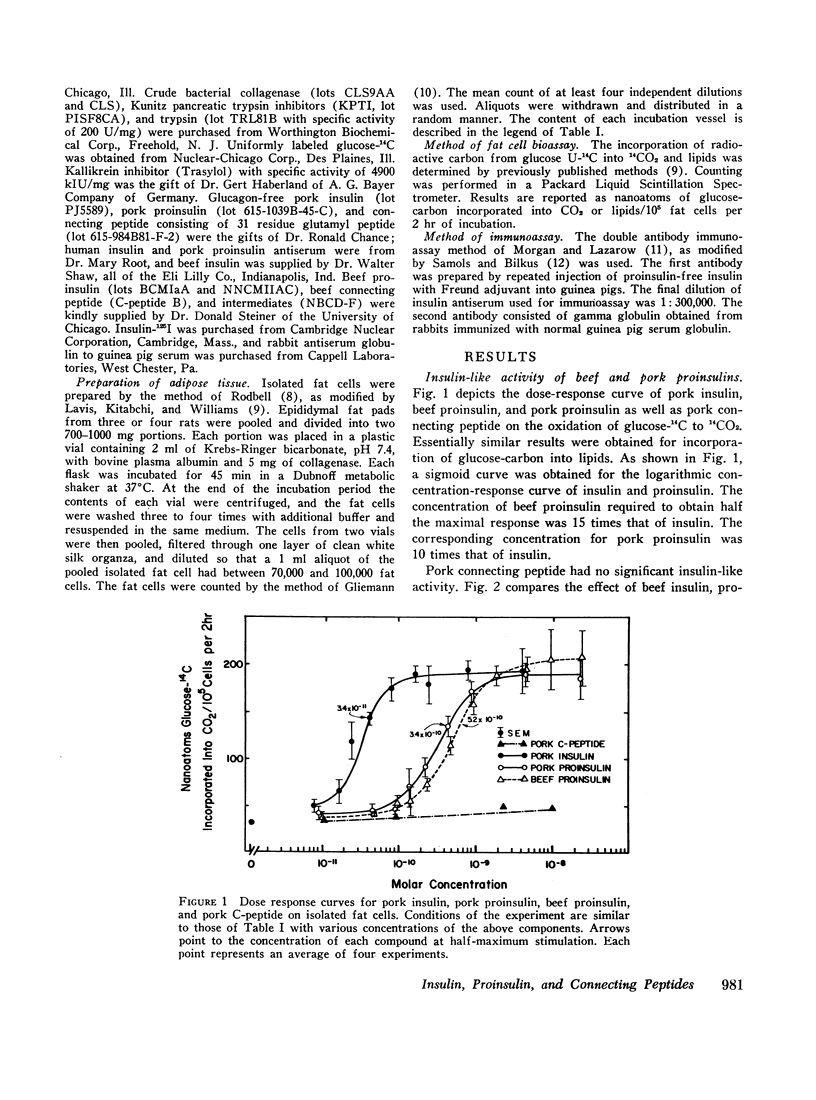
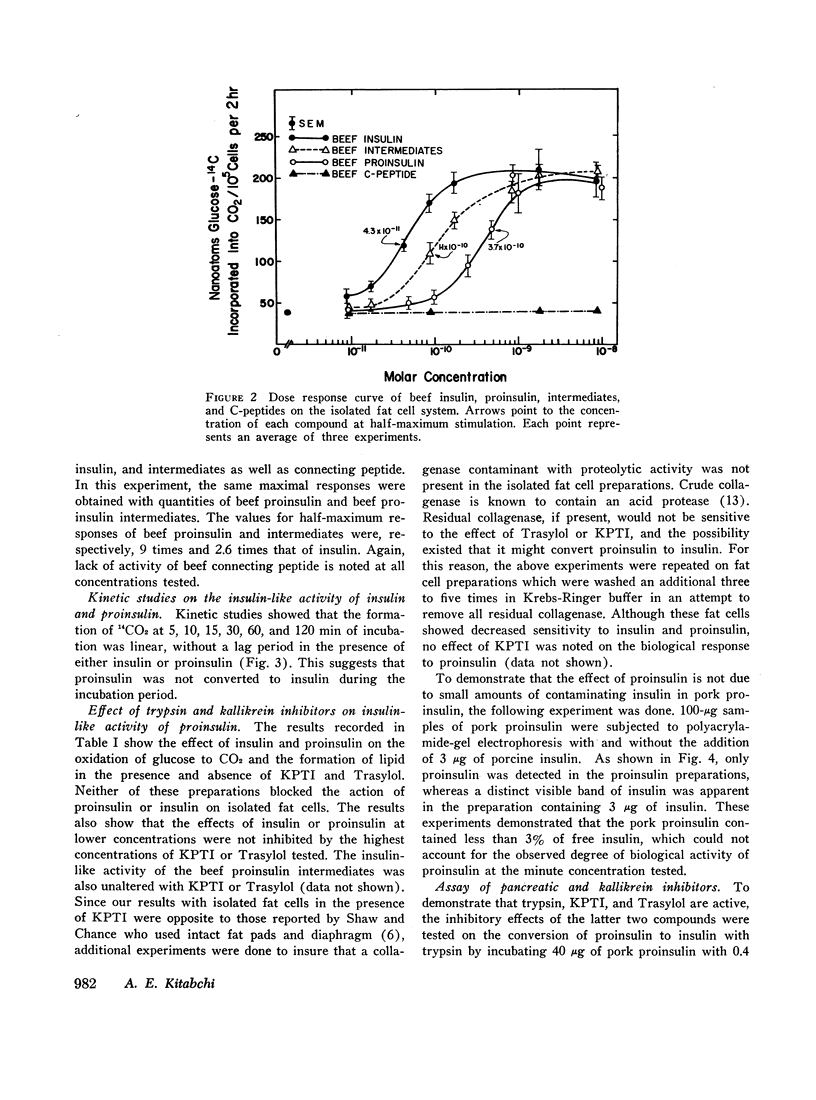
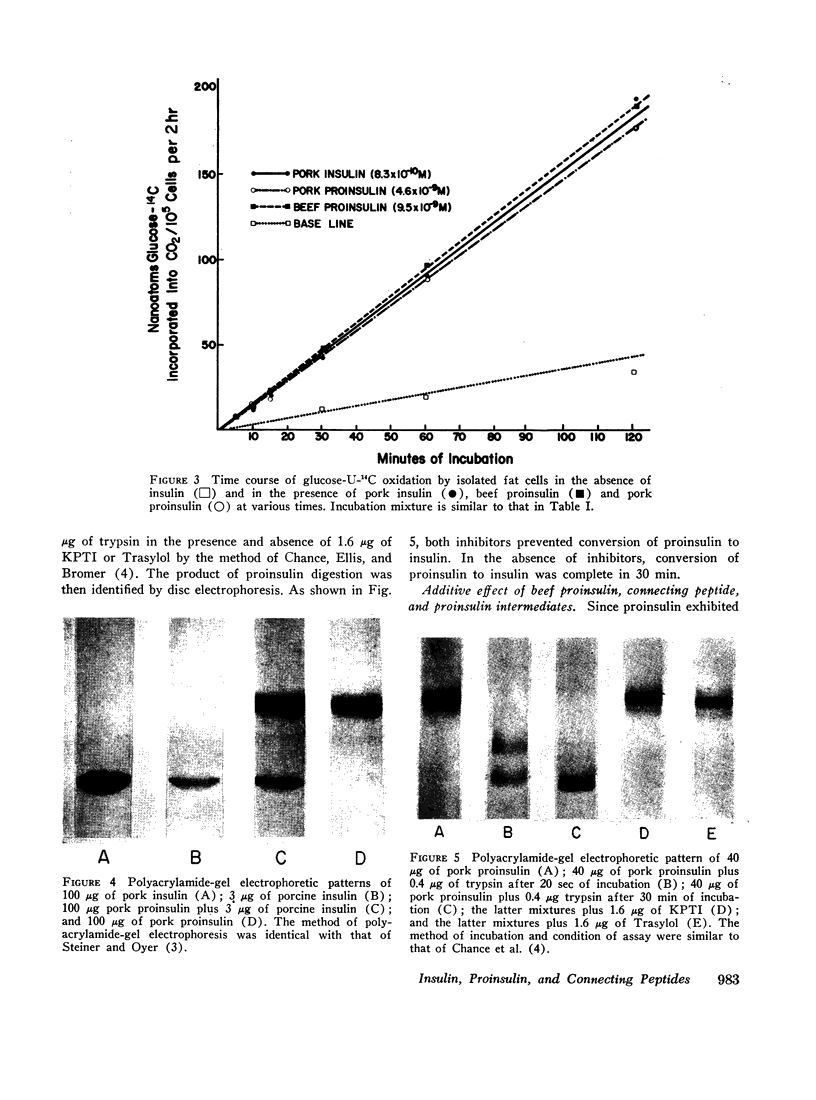
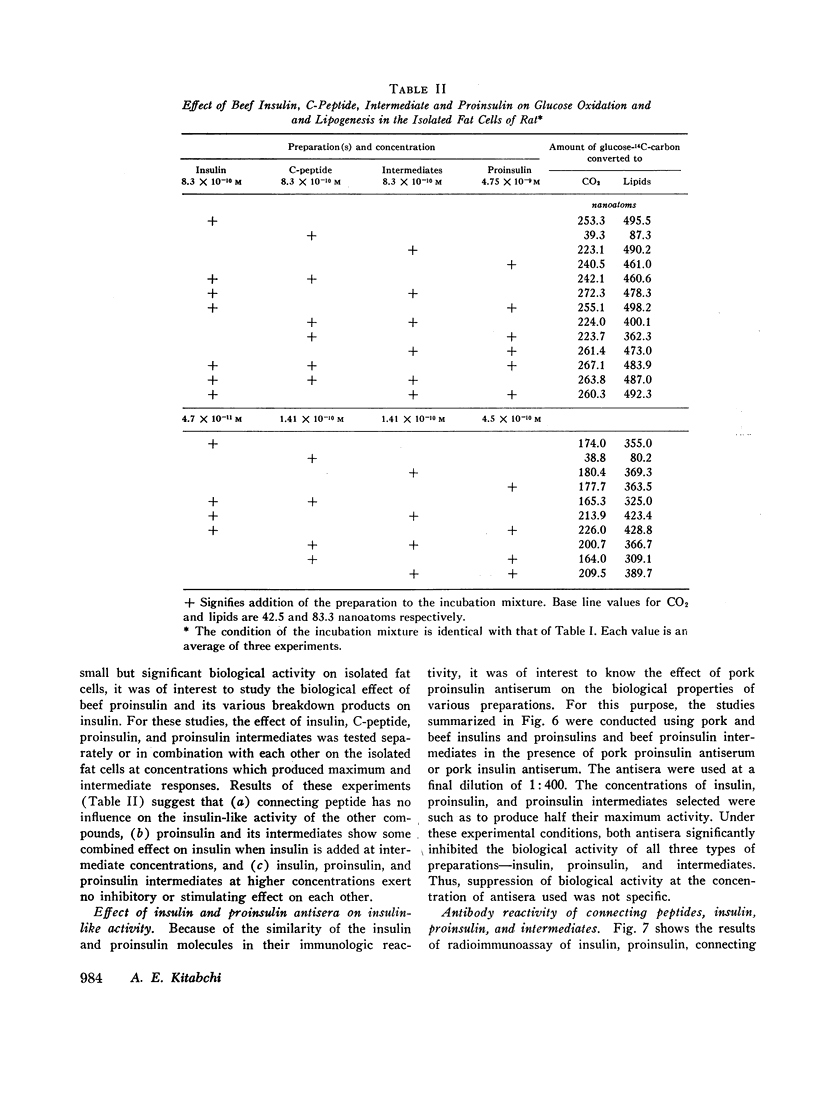
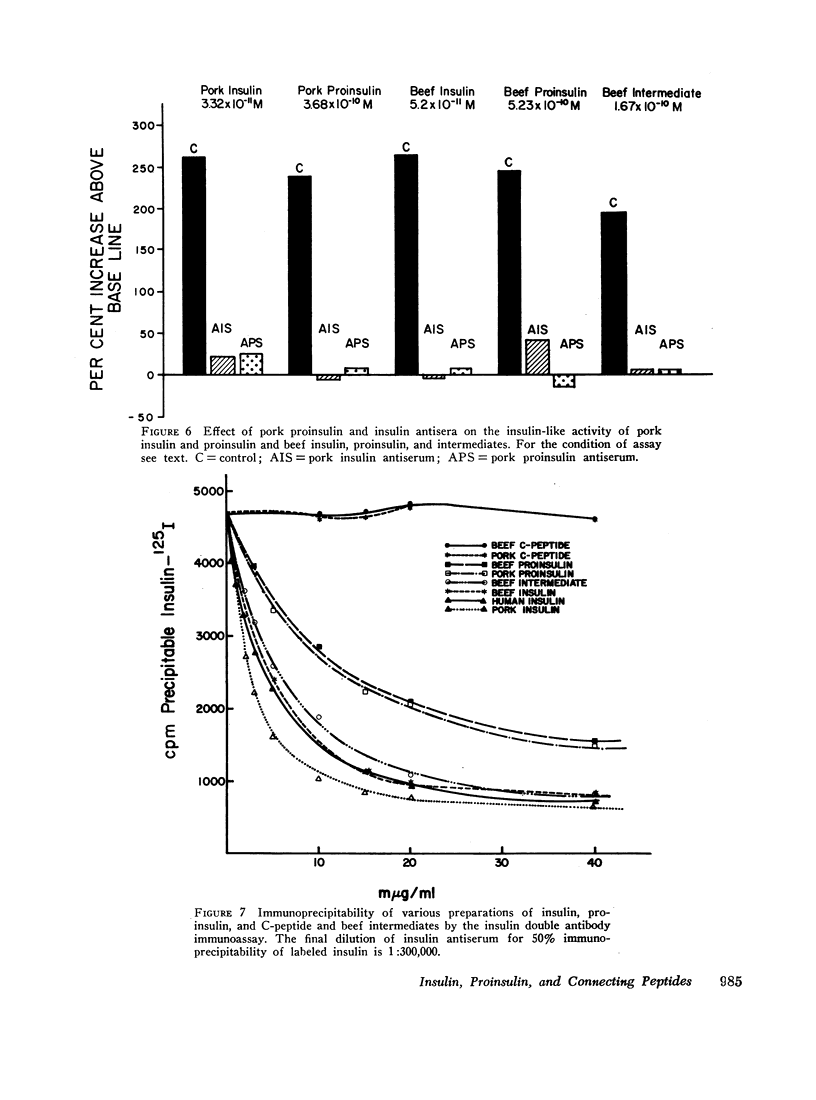
The recently discovered hormone precursors, pork and beef proinsulins, their respective connecting peptides, and beef proinsulin intermediates have been compared to insulin in their ability to stimulate the conversion of glucose-U-14C to 14CO2 and lipids in isolated fat cells. The concentrations of beef and pork proinsulins required to achieve the same biological effect were respectively, 15 and 10 times that of insulin. Beef proinsulin intermediates required only 2.6 times the concentration of insulin for the same effect. Pork and beef connecting peptides in high or low concentrations alone or in combination with proinsulin, insulin, or proinsulin intermediates showed no biological effect on the isolated fat cell system. The insulin-like activity of beef and pork proinsulins on the isolated fat cell system was not abolished with pancreatic trypsin or kallikrein inhibitors. Pork insulin antiserum inhibited the biological activity of pork insulin and proinsulin as well as that of beef insulin or proinsulin. Pork proinsulin antiserum also inhibited the insulin-like activity of both pork insulin and proinsulin. By the radioimmunoassay method, pork insulin antiserum bound only ¼ to [unk] as much proinsulin as insulin. Beef proinsulin intermediates, on the other hand, were found to react with the pork insulin antiserum to an extent nearly equal to that of insulin. These data suggest that (a) proinsulin exhibits its effect on the isolated fat cells independent of its conversion to insulin, (b) connecting peptides have no biological effect under present experimental conditions, and (c) in comparison to insulin, immunological reactivity of proinsulin is greater than its biological activity using our pork insulin antiserum; thus, the comparison of antibody specificity with the fat cell receptor specificity suggests that the biological site of action is different from the immunologic site.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chance R. E., Ellis R. M., Bromer W. W. Porcine proinsulin: characterization and amino acid sequence. Science. 1968 Jul 12;161(3837):165–167. doi: 10.1126/science.161.3837.165. [DOI] [PubMed] [Google Scholar]
- Clark J. L., Cho S., Rubenstein A. H., Steiner D. F. Isolation of a proinsulin connecting peptide fragment (C-peptide) from bovine and human pancreas. Biochem Biophys Res Commun. 1969 May 22;35(4):456–461. doi: 10.1016/0006-291x(69)90367-2. [DOI] [PubMed] [Google Scholar]
- Gliemann J. Insulin-like activity of dilute human serum assayed by an isolated adipose cell method. Diabetes. 1965 Oct;14(10):643–649. doi: 10.2337/diab.14.10.643. [DOI] [PubMed] [Google Scholar]
- Gorden P., Roth J. Circulating insulins. "Big" and "little". Arch Intern Med. 1969 Mar;123(3):237–247. [PubMed] [Google Scholar]
- Kono T. Destruction of insulin effector system of adipose tissue cells by proteolytic enzymes. J Biol Chem. 1969 Apr 10;244(7):1772–1778. [PubMed] [Google Scholar]
- Lavis V. R., Kitabchi A. E., Williams R. H. Lipid peroxidation in vitro by isolated fat cells of rats. Correlation with total lipolysis, glucose utilization, and dietary tocopherol. J Biol Chem. 1969 Aug 25;244(16):4382–4386. [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Rubenstein A. H., Melani F., Pilkis S., Steiner D. F. Proinsulin. Secretion, metabolism, immunological and biological properties. Postgrad Med J. 1969 Jul;45(Suppl):476–481. [PubMed] [Google Scholar]
- Rubenstein A. H., Steiner D. F., Cho S., Lawrence A. M., Kirsteins L. Immunological properties of bovine proinsulin and related fractions. Diabetes. 1969 Sep;18(9):598–605. doi: 10.2337/diab.18.9.598. [DOI] [PubMed] [Google Scholar]
- Rudman D., Garcia L. A., Del Rio A., Akgun S. Further observations on the cleavage of bovine insulin by rat adipose tissue. Biochemistry. 1968 May;7(5):1864–1874. doi: 10.1021/bi00845a034. [DOI] [PubMed] [Google Scholar]
- SAMOLS E., BILKUS D. A COMPARISON OF INSULIN IMMUNOASSAYS. Proc Soc Exp Biol Med. 1964 Jan;115:79–84. doi: 10.3181/00379727-115-28836. [DOI] [PubMed] [Google Scholar]
- Shaw W. N., Chance R. E. Effect of porcine proinsulin in vitro on adipose tissue and diaphragm of the normal rat. Diabetes. 1968 Dec;17(12):737–745. doi: 10.2337/diab.17.12.737. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Hallund O., Rubenstein A., Cho S., Bayliss C. Isolation and properties of proinsulin, intermediate forms, and other minor components from crystalline bovine insulin. Diabetes. 1968 Dec;17(12):725–736. doi: 10.2337/diab.17.12.725. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Oyer P. E. The biosynthesis of insulin and a probable precursor of insulin by a human islet cell adenoma. Proc Natl Acad Sci U S A. 1967 Feb;57(2):473–480. doi: 10.1073/pnas.57.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner D. F. Proinsulin and the biosynthesis of insulin. N Engl J Med. 1969 May 15;280(20):1106–1113. doi: 10.1056/NEJM196905152802008. [DOI] [PubMed] [Google Scholar]