Highlights
-
•
Lichen sclerosus in females primarily involves the hairless anogenital skin.
-
•
Skin tissue outside this area is constitutionally not at risk for lichen sclerosus.
-
•
Transplantation into the vulvar field may turn skin susceptible to lichen sclerosus.
-
•
Tissue inherent positional information might affect lichen sclerosus susceptibility.
Keywords: Lichen sclerosus, Vulvar cancer, Dermatoses, Positional information, Pudendal thigh skin flap
1. Introduction
Lichen sclerosus (LS) is a chronic inflammatory skin disease which preferably inflicts the anogenital area. Women are affected more frequently than men, the female to male ratio has been estimated to be between 6 and 10:1 (Powell and Wojnarowska, 1999). Clinically, affected skin areas exhibit pallor, atrophy, wrinkles, erosions or fissures, sclerosis and scarring, ultimately destroying skin texture, tissue compliance and normal architecture of the vulva. The pathogenesis of LS is incompletely understood, however, increasing evidence points to an autoimmune disorder involving both T-cell mediated and humoral mechanisms (Moyal-Barracco and Wendling, 2014).
In women, the lesions have a predilection for hairless genital skin areas such as the clitoris, labia minora, prepuce, interlabial sulci including the inner sides of the labia majora and the perineum. The hairy skin of the ridges of the labia majora, their lateral sides and the abutting genitocrural regions are involved rarely and only in extensive lesions of far advanced stages of the disease. Importantly, vulvar LS is associated with vulvar cancer in ca. 4–5% of cases (Powell and Wojnarowska, 1999).
First line treatment of LS typically consists of topical application of potent steroids. If this treatment approach fails, local administration of tacrolimus may be helpful (Cruickshank and Hay, 2011). In the long term, surgical excision is a treatment option for debilitating disease.
Several surgical techniques have been described for female patients, however, only small studies have been performed to evaluate these treatment strategies, as an operative approach is usually not pursued in cases of LS unless vulvar cancer develops. In one study, out of 5458 women who presented to a specialized vulvar disorder clinic only 25 were treated surgically for LS (Gurumurthy et al., 2012). Operative procedures to release contracture bands such as Fenton's procedure (median perineotomy) or a V-Y plasty were the most common ones performed in this patient cohort (Gurumurthy et al., 2012). More extensive surgical treatment such as vulvectomy are generally performed only in women with LS who develop vulvar cancer, although cases of vulvectomy and subsequent anatomical reconstruction for the treatment of LS have been reported (Abramov et al., 1996, Rojavin et al., 2008). We here provide a unique demonstration of LS recurrence in a pudendal thigh flap, illustrating the importance of tissue position in the pathogenesis of LS.
2. Case
A 66-year-old woman who had been suffering from vulvar LS was treated for squamous cell cancer of the vulva pT1b by radical wide excision of her left labium minus. The soft tissue defect was substituted by a pudendal thigh island flap raised from the left lateral labium majus. At that time the hairless vulvar skin surrounding the vulvar cancer exhibited clinical and histological features of LS but no VIN. The hairy darker pigmented skin island and the cutaneous donor site were normal. On a regular follow-up visit three months postoperatively the skin flap had healed completely without any signs of lichenification (Fig. 1A), which was proven histologically.
Fig. 1.
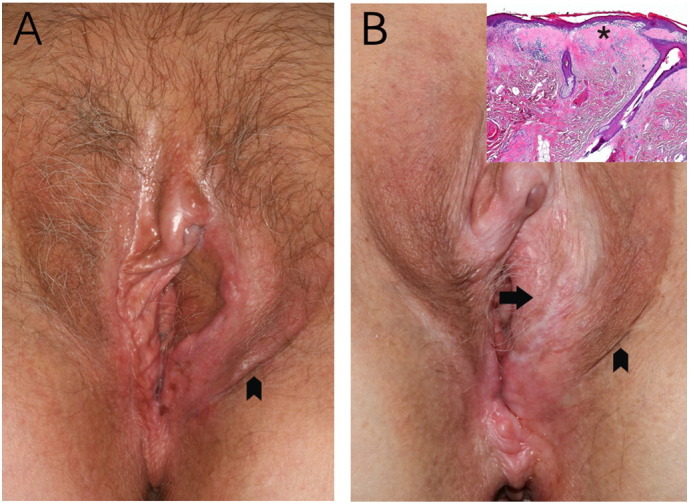
A: Three months after the flap surgery, the hairy darker pigmented skin of the flap (arrowhead) is normal corresponding to its donor site (arrowhead). The hairless vulvar skin exhibits clinical signs of lichen sclerosus. B: Eight years later, the lichen of the hairless vulva skin had progressed involving parts of the anus as well. The hairy skin island (arrow) is completely diseased, whereas the skin of the donor region (arrowhead) remains normal. Inset: Microscopic features of LS on the hairy skin of the cutaneous flap: thinning of the epidermis with loss of the rete ridges and dermal hyalinisation (*) involving the superficial reticular dermis, accompanied by scattered infiltration of lymphocytes.
Eight years later LS had progressed to include parts of the anus. A second squamous cell cancer of the right inferior labium minus pT1b was detected on routine examination and excised with primary skin closure. Strikingly, the hairy skin island previously transposed from the left lateral labium majus was now observed to be completely diseased by lichen sclerosus (Fig. 1B). This was confirmed by histopathological examination (Fig. 1B, inset). The skin at the flap's donor site remained normal. Informed consent for publication of this case report was obtained from the patient.
3. Discussion
The rarely performed surgical treatment for LS can be categorized into (i) procedures aiming to remove diseased tissue (e.g. wide excision or vulvectomy with or without reconstruction) and (ii) procedures to relieve symptoms from strictures (cryosurgery, laser ablation and classic surgical interventions such as Fenton's procedure) or clitoral adhesions with pseudocyst formation. Recurrence after surgical removal of diseased skin by vulvectomy has been reported to be as high as 40–50% (Abramov et al., 1996). In one case, LS recurred after excision and subsequent defect closure with a myocutaneous gracilis flap (Di Paola et al., 1982).
Our case also clearly demonstrates the recurrence of vulvar LS in a pudendal thigh skin flap. It should be expected, that LS does not affect hairy skin elevated from the thigh, since this skin type is usually spared by LS. Obviously, so far unknown mechanisms render previously resistant skin susceptible to LS once it is transferred to the skin region which is usually affected by LS.
It may be hypothesised that positional information instrumental for morphogenesis, regeneration and repair may also be involved in the pathogenesis of LS (Wolpert, 2011). Although environmental features such as the local microbiome or irritants such as urine, sweat or transudation cannot be ruled out as pathogenetic factors, it is difficult to explain that the manifestation of the disease is confined to precisely delineated tissue borders.
Our case shows in a unique way the importance of tissue position as pathogenic factor for LS.
References
- Abramov Y., Elchalal U., Abramov D., Goldfarb A., Schenker J.G. Surgical treatment of vulvar lichen sclerosus: a review. Obstet. Gynecol. Surv. 1996;51(3):193–199. doi: 10.1097/00006254-199603000-00023. [DOI] [PubMed] [Google Scholar]
- Cruickshank M.E., Hay I. Royal College of Obstetricians and Gynaecologists; 2011. The Management of Vulval Skin Disorders. Green Top Guideline No 58. [Google Scholar]
- Di Paola G.R., Rueda-Leverone N.G., Belardi M.G. Lichen sclerosus of the vulva recurrent after myocutaneous graft. A case report. J. Reprod. Med. 1982;27(10):666–668. [PubMed] [Google Scholar]
- Gurumurthy M., Morah N., Gioffre G., Cruickshank M.E. The surgical management of complications of vulval lichen sclerosus. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012;162(1):79–82. doi: 10.1016/j.ejogrb.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Moyal-Barracco M., Wendling J. Vulvar dermatosis. Best. Pract. Res. Clin. Obstet. Gynaecol. 2014;28(7):946–958. doi: 10.1016/j.bpobgyn.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Powell J.J., Wojnarowska F. Lichen sclerosus. Lancet. 1999;353(9166):1777–1783. doi: 10.1016/s0140-6736(98)08228-2. [DOI] [PubMed] [Google Scholar]
- Rojavin Y., Salgado C.J., Hsu P.W., Liu J., Aikins J.K. The surgical management of vulvar lichen sclerosus refractory to medical management. J. Plast. Reconstr. Aesthet. Surg. 2008;61(7):848–849. doi: 10.1016/j.bjps.2007.10.076. [DOI] [PubMed] [Google Scholar]
- Wolpert L. fourth ed. Oxford University Press; Oxford: 2011. Principles of Development. [Google Scholar]


