Highlights
-
•
Hepatitis B vaccination coverage dropped significantly after Adverse Events Following Immunization in Viet Nam in 2013.
-
•
We estimated 17,456 hepatitis B-related deaths would occur due to the drop in vaccination coverage.
-
•
Swift responses to reported adverse events following immunization are needed to maintain consumer confidence in vaccination.
Keywords: Adverse events following immunization, Hepatitis B, Vaccination coverage, Viet Nam, Disease burden
Abstract
Adverse Events Following Immunization in Viet Nam in 2013 led to substantial reductions in hepatitis B vaccination coverage (both the birth dose and the three-dose series). In order to estimate the impact of the reduction in vaccination coverage on hepatitis B transmission and future mortality, a widely-used mathematical model was applied to the data from Viet Nam. Using the model, we estimated the number of chronic infections and deaths that are expected to occur in the birth cohort in 2013 and the number of excessive infections and deaths attributable to the drop in immunization coverage in 2013. An excess of 90,137 chronic infections and 17,456 future deaths were estimated to occur in the 2013 birth cohort due to the drop in vaccination coverage. This analysis highlights the importance of maintaining high vaccination coverage and swiftly responding to reported Adverse Events Following Immunization in order to regain consumer confidence in the hepatitis B vaccine.
1. Introduction
Hepatitis B virus (HBV) infection is a major public health concern in Viet Nam. In adults born before the hepatitis B vaccine was introduced, 7–24% were chronically infected according to several studies conducted across the country [1], [2], [3]. Perinatal transmission from infected mothers to infants is common, and more than half of the population has been exposed to HBV [2], [3], [4]. People who are infected in infancy usually don’t have symptoms, but 80–90% will develop chronic infection that lasts into adulthood [5]. It is estimated that 20–30% of adults who are chronically infected will develop liver cancer or liver cirrhosis [5]. Viet Nam has one of the highest liver cancer incidence rates in the world, with an annual rate of 37.9 per 100,000 population in males [6]. Liver cancer is the most common cause of cancer deaths in Viet Nam, causing over 20,000 deaths each year [7].
Despite the heavy burden of HBV-related death, new infections can be effectively prevented through hepatitis B vaccination. If given within 24 h of birth, the vaccine is highly effective in preventing mother-to-child transmission [8], [9]. The complete series of 3 doses can provide long term protection [10]. In Viet Nam, hepatitis B vaccine was introduced in 1997, and was expanded nationwide in 2002 [11]. A birth dose was added to the immunization schedule in 2003 [11]. A dramatic reduction in prevalence of chronic hepatitis B has been observed in children after the introduction of hepatitis B vaccine. A national survey conducted in Viet Nam in 2011 estimated that children born in 2000–2003 have significantly higher prevalence of chronic HBV infection than children born in 2007 to 2008 (3.64% vs. 1.64%) [11]. The same study found that hepatitis B vaccination coverage increased from 46.1% to 84.0% for the 3 dose series and from 22.0% to 30.4% for the birth dose during the same time period [11]. Hence it is evident that the prevalence of chronic HBV infection can be reduced by achieving high vaccination coverage. The reduction in hepatitis B transmission is expected to result in a corresponding reduction in liver cancer and liver cirrhosis as the new birth cohorts enter adulthood.
Achieving high vaccination coverage requires both a strong system to deliver the vaccine and high demand for the vaccine. Public demand for vaccine is often influenced by media reports on illness or deaths after vaccination. Adverse Events Following Immunization (AEFIs) denotes “any untoward medical occurrence” that follows immunization, which does not necessarily indicate causal relationship with vaccination [12]. The events can be purely coincidental, but can lead to widespread fears over vaccine safety and major reductions in vaccine demand.
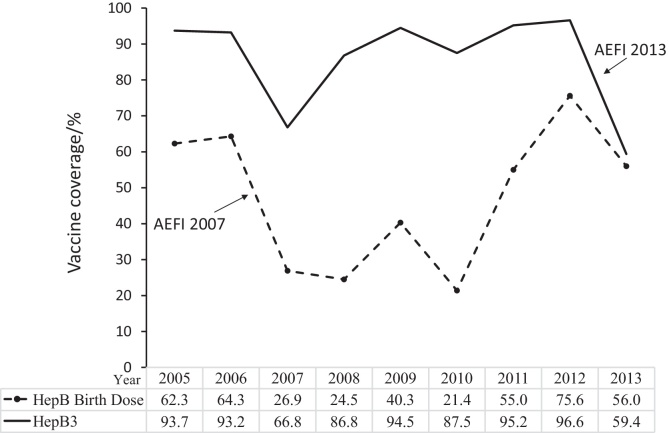
Despite strong efforts to increase hepatitis B vaccination coverage in Viet Nam, publicized news of Adverse Events Following Immunization (AEFIs) have led to fluctuations in hepatitis B vaccination coverage in recent years (Fig. 1). In 2007, the birth dose coverage of hepatitis B vaccine dropped from 64.3% in the previous year to 26.9%, partially attributable to media reports of AEFIs. In 2013, several AEFIs occurred involving both the hepatitis B monovalent vaccine used for the birth dose and the pentavalent vaccine used for the 3-dose series. Following these AEFIs, the pentavalent vaccine, Quinvaxem (DTwP-HepB-Hib) was suspended from May to October 2013 [13]. The monovalent hepatitis B vaccine was not suspended. While investigations of the AEFIs did not identify problems with the vaccine safety, the widespread media coverage of these events led to a major reduction in consumer confidence in the hepatitis B vaccine. As the public concern over vaccine safety became pervasive, the hepatitis B birth dose coverage decreased from 75.6% in 2012 to 56.0% in 2013. This paper estimates the impact of the reduction in vaccination coverage in 2013 on the number of new infections and future deaths related to hepatitis B.
Fig. 1.
Administrative coverage of Hepatitis B Vaccine (%) in Viet Nam.
2. Methods
2.1. Model overview
A published model developed by Goldstein et al. in 2005 was used to estimate the expected number of HBV infections and deaths in children born in 2013 under the reported vaccination coverage [14]. Since the risk of infection and outcome of infection is dependent on age, the model assumes all infections occur in three age periods: (1) perinatal infection in infancy, (2) transmission in early childhood (<5 years old), and (3) transmission later in life (>5 years old). The number of perinatal infections was calculated from the number of infants exposed to HBV at birth and the probability of transmission. The prevalences reported in studies conducted before vaccine introduction were used to estimate annual rate of infection without vaccination. The number of early childhood infections was calculated based on the prevalence of anti-HBc at 5 years of age, a marker that reflects both past and current infection, after excluding the number of children infected at birth. For infection among individuals aged five years and above, a constant proportion of infections occurring each year before the age of 30 was assumed, after accounting for age-specific all-cause mortality. The rate of loss of chronic infection (HBsAg) was set to 0.5% each year beginning at the age of 20, and we assumed these people who lost HBsAg are no longer at risk of HBV-related complications. The probabilities of progressing to acute or chronic infection was assumed to be only dependent on age and children have much higher probability of developing chronic infection than adults. Age specific-mortality rate of liver cirrhosis and liver cancer was applied to calculate number of deaths related to chronic hepatitis B. Vaccine efficacy was set to 95% for both birth dose and the 3-dose series in the base analysis. The description of the model and parameters can be found in this paper by Goldstein et al. [14].
2.2. Model input
A literature review was conducted to determine the sero-prevalence of HBV infection in children, pregnant women and adults before the introduction of vaccine using the key words of the sero-markers and the country's name. A total of 207 articles were retrieved from Pubmed, and data from 41 articles that reported prevalence of the sero-markers were extracted. In the 41 studies, 26 were conducted among Vietnamese immigrants and 15 were conducted in Viet Nam. Of those conducted in Viet Nam, 7 out of 15 were conducted among repeated blood donors or liver disease patients. The remaining 8 studies were among people who are not expected to be at substantially increased or decreased risk of hepatitis B infection including 5 community-based studies, 1 study among potential blood donors, 1 study among non-liver disease patients and 1 among women who attend antenatal care (Table 1) [1], [2], [3], [4], [15], [16], [17], [18]. None of the studies that reflect baseline prevalences were nationally representative. Only one study conducted in 1999 reported all the data required for the model and this study was from a community-based random sample consisting of both children and adults [16]. In this study, 228 children aged 4 to 6 years and 596 adults aged 25 to 40 years, including 114 females, were randomly selected from two districts of Thanh Hoa province [16]. We used the results of this study as model inputs for base analysis. As reported in this study, the prevalence of HBsAg in women of reproductive age was 15.8%, the prevalence of HBeAg in HBsAg positive women was 27.8%, the prevalence of anti-HBc at the age of 5 was 36.4%, and the prevalence of anti-HBc at the age of 30 was 79.2% [16]. Reported prevalence from the other studies in Table 1 were used for sensitivity analysis.
Table 1.
Prevalence of HBV serological markers from studies conducted in Viet Nam.a
| Authors | Year of survey | Study population | Overall sample size | HBsAg(+) in women of reproductive age (%) | HBeAg(+) in HBsAg(+) women (%) | Anti-HBc (+) in children aged 5 (%) | Anti-HBc(+) in adults (%) |
|---|---|---|---|---|---|---|---|
| Do et al. [4] | 2012 | Adults randomly selected in Binh Thuan province | 509 | 20b | NA | NA | 71.7 |
| Le Viet et al. [15] | 2007 | Potential voluntary blood donors in two rural communities in Quang Tri province | 1200 | 7.9 | NA | NA | 51.7 |
| Nguyen et al. [3] | 2002 | Adults randomly selected in two rural districts in Thai Binh province | 837 | 24c | 21.4c | NA | 68.2 |
| Duong et al. [2] | 2006 | Adults randomly selected in Linhson village, Thainguyen province | 383 | 8.8 | 38.2e | NA | 66.2 |
| Lan et al. [1] | 2006 | Married women aged 18–39 years in FilaBavi | 606 | 7.1 | NA | NA | NA |
| Goto et al. [18] | 2003 | Pregnant women 15–49 years of age attending antenatal care in 4 districts in Nghe An Province | 505 | 10 | NA | NA | NA |
| Hipgrave et al. [16] | 1998 | Children and adults randomly selected from two districts of Thanh Hoa province | 1579 | 15.8 | 27.8 | 36.4 | 79.2 |
| Nakata et al. [17] | 1993 | Pregnant women and patients without liver diseases in Hanoi and Ho Chi Minh | 591 | 14d | NA | 34.8f | NA |
Where necessary, recalculations from the original publications were conducted to obtain estimates of interest.
Estimate included both men and women aged 20–39 years.
Estimate included both men and women aged 20–29 years.
Estimate included both men and women aged 16 years and above.
Estimate includes all HBsAg positive participants (men and women aged 18–70 years).
The estimate presented is a proxy using the sum of anti-HBs positive or HBsAg positive participants aged 1–10 years who were born before vaccine introduction in Viet Nam.
The numbers of surviving infants and vaccination coverage were extracted from WHO/UNICEF Joint Reporting Form (JRF) from Viet Nam of 2013 [19]. According to the JRF reported to WHO and UNICEF by Viet Nam Ministry of Health, the total number of surviving infants in 2013 was 1782,720. The hepatitis B vaccination coverage for the birth dose was 56.0% and that for the complete series of at least three doses (HepB3) was 59.4% [19].
General mortality rate was derived from World Population Prospects 2012 Revision, published by United Nations Population Division. The estimated age-specific death rates in Viet Nam in 2005–2010 were used [20].
2.3. Sensitivity analysis
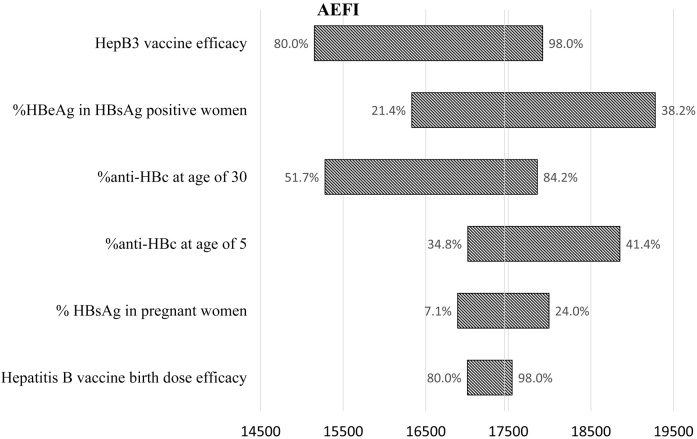
A univariate sensitivity analysis was performed to examine the impact of uncertainty in the model inputs and vaccine efficacy: HBsAg prevalence in pregnant women, HBeAg prevalence in HBsAg positive women, anti-HBc prevalence at the age of 5 years and at the age of 30 years, birth dose vaccine efficacy and efficacy of the complete series. Each parameter was changed one at a time, while others remained constant. The highest and lowest reported prevalence in the studies presented in the supplementary table were used to calculate the high and low estimates of chronic HBV infection and deaths caused by HBV infection. When the prevalence estimates in the base analysis are the highest among all reported values, in the case of anti-HBc prevalence at 5 years of age and anti-HBc at 30 years of age, 5% was added to the base prevalence to calculate the high estimate. Based on published trials [8], [9], the lower estimate of vaccine efficacy was set to 80% for both the birth dose and the 3-dose series, and the upper limit of vaccine efficacy for both birth dose and the 3-dose series was set to 98%, considering chances of break-through infections and non-response.
3. Results
The model estimated the number of chronic infections and deaths that are expected with varied vaccination coverage in children born in 2013 (Table 2). Without vaccination, among children born in 2013, a total of 293,676 chronic HBV infections and 56,655 deaths caused by HBV are expected in their lifetime if they pass through their life under the currently observed mortality rates. In 2013, the reported birth dose coverage is 56.0% and HepB3 coverage is 59.4%. With the reported coverage, 130,675 chronic infections and 25,197 deaths are expected among this birth cohort as they pass through their life. Among them, 29.3% of chronic infections and 28.6% of expected deaths are due to perinatal infection, 55.7% of chronic infections and 54.9% of expected deaths are due to horizontal transmission before the age of 5, and 16.6% of chronic infections and 15.0% of expected deaths are due to horizontal transmission after the age of 5. This suggests catch-up immunization activities in these young children could prevent over half of the expected deaths.
Table 2.
Number of chronic hepatitis B infections and deaths expected with varied vaccination coverage*.
| Vaccination coverage | Without vaccination | Regional target | Maintaining 2012 coverage | 2013 coverage after AEFI |
|---|---|---|---|---|
| Birth dose | 0.0% | 95.0% | 75.6% | 56.0% |
| HepB3 | 0.0% | 95.0% | 96.6% | 59.4% |
| Number of chronic infections | 293,676 | 28,633 | 40,538 | 130,675 |
| Number of deaths | ||||
| Acute | 1181 | 115 | 98 | 515 |
| Liver cirrhosis | 28,126 | 2742 | 3874 | 12,514 |
| Hepatocellular carcinoma | 27,348 | 2666 | 3770 | 12,168 |
| Total deaths | 56,655 | 5524 | 7741 | 25,197 |
Assuming HBsAg% in women of reproductive age = 15.8%, HBeAg% in HBsAg positive women = 27.8%, anti-HBc% among 5-year olds = 36.4%, anti-HBc% among 30-year olds = 79.2%, birth dose efficacy = 95% and complete vaccine series efficacy = 95%.
If the vaccination coverage had been maintained at the same level as in 2012 (birth dose coverage 75.6%, HepB3 coverage 96.6%), 40,538 chronic infections and 7741 deaths are expected. This suggests 90,137 excessive chronic infections and 17,456 deaths would occur due to the drop in vaccination coverage in 2013, if no catch-up immunization is to be conducted in this birth cohort.
If the birth dose coverage and HepB3 coverage could both reach the regional target of 95% while the size of birth cohorts remains constant, 51,131 deaths could be prevented and 265,043 chronic infections could be avoided every year through immunization.
Fig. 2 presents the number of excessive deaths from the sensitivity analysis, which means the deaths that could have been prevented if the vaccination coverage had been maintained at 2012 level. Assuming no catch-up immunization, the number of excessive deaths due to the drop in vaccination coverage ranged from 15,151 to 19,279, and the number of excessive chronic infections ranged from 78,304 to 97,703 as the model input changed between lowest and highest estimates.
Fig. 2.
Sensitivity analysis: number of excess deaths expected due to AEFI.
4. Discussion
Based on the model, an estimated 130,675 chronic HBV infections and 25,197 HBV-related deaths would occur in children born in 2013. If the hepatitis B vaccination coverage had been maintained at the same level as in 2012, 90,137 chronic infections and 17,456 deaths would have been avoided. The results suggest a dramatic increase in hepatitis B disease burden caused by the drop in vaccination coverage.
Although the hepatitis B vaccine is one of the safest vaccines available, media coverage of AEFIs can lead to major reductions in vaccination coverage. Following the 2013 AEFI investigation, which determined that the AEFIs were the result of human error, the National Institute of Hygiene and Epidemiology held several meetings with journalists from major newspapers to develop accurate and easily understood messages for the public. However it was not possible to meet with all media and some media sources reported that the AEFIs were due to the vaccine. In 2013 the pervasive news reports of the AEFIs in Viet Nam led to a widespread reduction in hepatitis B vaccine demand. This study demonstrates the devastating consequences of a loss in public confidence in the hepatitis B vaccine. To avoid the future burden caused by this preventable disease, it is imperative to restore public confidence in vaccination.
The timing of the first dose of the vaccine, in the first 24 h of life, coincides with the most fragile period in infancy. Infant mortality in Viet Nam is estimated to be 16 per 1000 live births [20]. This equates to 28,523 infant deaths in 2013. It is easy for concerned parents or the media to suggest that vaccination is responsible for infant deaths. Major efforts are needed to mitigate the impact of media reports of AEFIs on vaccine demand. These include educating the media on vaccine safety, swiftly investigating reported AEFI cases, and openly informing the public of the results of the investigation. One challenge to conducting AEFI investigations is the lack of evidence about causes of death in cases when no autopsy is done (as was the case for the 2007 AEFIs). It is not easy to communicate with the public without sound evidence about the causes of the AEFIs. The National Institute of Hygiene and Epidemiology therefore advises parents to immediately bring children with severe symptoms after immunization to a hospital for diagnosis and treatment.
The results suggest the great potential of hepatitis B control through immunization: if Viet Nam could reach the target of 95% coverage of both birth dose and HepB3, about 51,131 future deaths and 265,043 chronic infections can be prevented each year by vaccination. The national sero-survey in children conducted in 2011 showed the significantly lower prevalence of chronic hepatitis B after the introduction of hepatitis B vaccine. The experience from other countries in the region demonstrates that the regional goal to reduce prevalence of chronic hepatitis B infection to less than 1% in children can be achieved by maintaining high vaccination coverage.
So far there has not been a national sero-survey among adults in Viet Nam. The model parameters used in the base analysis were derived from one survey in two rural districts in northern Viet Nam, which can lead to potential bias. The two districts were inhabited by two ethnic groups, Kinh and Muong. The study found high prevalence of chronic hepatitis B among adults in both the national dominant Kinh ethnic group (19.6%) and the minority Muong (17.8%). Varied prevalences of hepatitis B have been reported in different provinces in Viet Nam [1], [2], [3], [11], [15], [16], [17], [18]. The base analysis used the HBsAg prevalence of 15.8% among women, which is around the midpoint of the reported range (7.1% to 24%) in studies that sampled general population from communities. The sensitivity analysis suggests the model is robust with large changes in model parameters. When the model inputs changed from lowest to highest estimates, the number of excessive deaths changed from 15,151 to 19,279.
Uncertainties exist in vaccination coverage and size of birth cohort. We noticed different numbers of birth cohort size were reported in the JRF and United Nations Population Division estimates, and the birth dose coverage reported in JRF is different from that in WHO and UNICEF Estimates of National Immunization Coverage (WUENIC) [19], [20], [21]. Estimating numbers of births and infant deaths is challenging in many low and middle-income countries including Viet Nam, where vital registration is incomplete [22]. We used number of surviving infants reported by JRF in the model since we consider this better reflects local situation. Administrative coverage of birth dose reported in JRF was used in the analysis since recent coverage estimated by national representative surveys is still not available, and the calibration in the WUENIC derived from a 2008 survey is less relevant to the situation in 2013.
The model does not take into account the protective effect of incomplete vaccine series or the effect of the birth dose against early childhood transmission, which may lead to underestimate of impact of vaccination. The model only considers the effect of full vaccine series in preventing transmission after birth, while the dropout between the first dose and the third dose of the pentavalent vaccine was particularly large (24%) in 2013. These children who received less than 3 doses may develop some partial immunity from incomplete series received before their vaccination was interrupted by the events. However, since limited data are available on efficacy of incomplete vaccination, the effect of incomplete vaccine series was not quantified by the model. The model may overestimate the impact of birth dose, since it assume a single birth dose to be 95% efficacious of preventing perinatal infection, regardless of whether subsequent doses are received. Yet our sensitivity analysis suggests, even if the birth dose efficacy is 80%, 17, 004 excessive deaths are still attributable to the drop in vaccination coverage.
As additional data become available, the accuracy of model input may be improved and the estimates can be compared to measured chronic hepatitis B prevalence and hepatitis B-related mortality. Yet, our result suggests even with the most conservative parameters, more than 15,000 lives could have been saved in the 2013 birth cohort in Viet Nam if the vaccination coverage had been maintained at the 2012 level.
These findings may be used in different channels to communicate with policy makers and the public on the benefits of vaccination and consequences of non-vaccination. Some suggested uses include presenting the findings at workshops and trainings with health workers and EPI staff, sending letters explaining findings to policy makers, mass media communication (press releases, workshops, TV talk shows, coverage in daily newspapers, Q&A on websites), dissemination to professional societies such as the Vietnam Association of Preventive Medicine, presenting the findings in government meetings (such as Parliament Committee of Social Affairs, Prime Minister, Government Cabinet, and Finance Ministry), and sharing the findings with international partners in the Interagency Coordinating Committee meetings (WHO, UNICEF, GAVI, WB, ADB, JICA, and PATH).
Acknowledgements
The authors would like to thank Susan Goldstein, Fangjun Zhou, and Minal Patel for the useful discussions of the published model on the global burden of hepatitis B.
Footnotes
This is an Open Access article published under the CC BY NC ND 4.0 IGO license which permits users to download and share the article for non-commercial purposes, so long as the article is reproduced in the whole without changes, and provided the original source is properly cited. This article shall not be used or reproduced in association with the promotion of commercial products, services or any entity. There should be no suggestion that WHO endorses any specific organisation, products or services. The use of the WHO logo is not permitted. This notice should be preserved along with the article's original URL.
References
- 1.Lan P., Lundborg C.S., Phuc H., Sihavong A., Unemo M., Chuc N. Reproductive tract infections including sexually transmitted infections: a population-based study of women of reproductive age in a rural district of Vietnam. Sex Transm Infect. 2008;84(2):126–132. doi: 10.1136/sti.2007.027821. [DOI] [PubMed] [Google Scholar]
- 2.Duong T.H., Nguyen P.H., Henley K., Peters M. Risk factors for hepatitis B infection in rural Vietnam. Asian Pac J Cancer Prev. 2009;10(1):97. [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen V.T.T., McLaws M.L., Dore G.J. Highly endemic hepatitis B infection in rural Vietnam. J Gastroenterol Hepatol. 2007;22(12):2093–2100. doi: 10.1111/j.1440-1746.2007.05010.x. [DOI] [PubMed] [Google Scholar]
- 4.Do S.H., Yamada H., Fujimoto M., Ohisa M., Matsuo J., Akita T. High prevalences of hepatitis B and C virus infections among adults living in Binh Thuan province, Vietnam. Hepatol Res. 2014 doi: 10.1111/hepr.12350. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . World Health Organization; 2015. Hpeatitis B fact sheet. 〈http://www.who.int/mediacentre/factsheets/fs204/en/〉 (accessed on February 24). (Updated July 2014) [Google Scholar]
- 6.WHO/IARC . WHO/IARC; 2015. GLOBOCAN 2012, liver cancer: estimated incidence, all ages, both sexes. Available from 〈http://globocan.iarc.fr/old/summary_table_site-html.asp?selection=14070&title=Liver&sex=1&type=0&window=1&africa=1&america=2&asia=3&europe=4&oceania=5&build=6&sort=4&submit=%C2%A0Execute〉 (accessed on February 24, 2015) [Google Scholar]
- 7.WHO/IARC . WHO/IARC; 2015. GLOBOCAN 2012, Viet Nam: estimated cancer incidence, all ages, both sexes. Available from 〈http://globocan.iarc.fr/old/summary_table_pop-html.asp?selection=213704&title=Viet+Nam&sex=0&type=0&window=1&sort=2&submit=%C2%A0Execute〉 (accessed on February 24, 2015) [Google Scholar]
- 8.Milne A., West D.J., Chinh D.V., Moyes C.D., Poerschke G. Field evaluation of the efficacy and immunogenicity of recombinant hepatitis B vaccine without HBIG in newborn Vietnamese infants. J Med Virol. 2002;67(3):327–333. doi: 10.1002/jmv.10071. [DOI] [PubMed] [Google Scholar]
- 9.Assateerawatt A., Tanphaichitr V.S., Suvatte V., In-ngarm L. Immunogenicity and protective efficacy of low dose recombinant DNA hepatitis B vaccine in normal and high-risk neonates. Asian Pac J Allergy Immunol. 2011;9(2):89. [PubMed] [Google Scholar]
- 10.FitzSimons D., François G., Hall A., McMahon B., Meheus A., Zanetti A. Long-term efficacy of hepatitis B vaccine, booster policy, and impact of hepatitis B virus mutants. Vaccine. 2005;23(32):4158–4166. doi: 10.1016/j.vaccine.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen T.H., Vu M.H., Nguyen V.C., Nguyen L.H., Toda K., Nguyen T.N. A reduction in chronic hepatitis B virus infection prevalence among children in Vietnam demonstrates the importance of vaccination. Vaccine. 2014;32(2):217–222. doi: 10.1016/j.vaccine.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization . World Health Organization; 2015. Adverse Events Following Immunization (AEFI) 〈http://www.who.int/vaccine_safety/initiative/detection/AEFI/en/〉 (accessed on February 24) [Google Scholar]
- 13.World Health Organization . World Health Organization; 2015. Safety of Quinvaxem (DTwP-HepB-Hib) pentavalent vaccine. 〈http://www.who.int/immunization_standards/vaccine_quality/quinvaxem_pqnote_may2013/en/〉 (accessed on February 24). Updated May 10, 2013. [Google Scholar]
- 14.Goldstein S.T., Zhou F., Hadler S.C., Bell B.P., Mast E.E., Margolis H.S. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int J Epidemiol. 2005;34(6):1329–1339. doi: 10.1093/ije/dyi206. [DOI] [PubMed] [Google Scholar]
- 15.Le Viet N.T.N.L., Ty P.X., Björkvoll B., Hoel H., Gutteberg T., Husebekk A. Prevalence of hepatitis B & hepatitis C virus infections in potential blood donors in rural Vietnam. Indian J Med Res. 2012;136(1):74. [PMC free article] [PubMed] [Google Scholar]
- 16.Hipgrave D.B., Van N.T., Huong V.M., Long H.T., Do Trung D., Tuan T.N. Hepatitis B infection in rural Vietnam and the implications for a national program of infant immunization. Am J Trop Med Hyg. 2003;69(3):288–294. [PubMed] [Google Scholar]
- 17.Nakata S., Song P., Duc D.D., Quang N.X., Murata K., Tsuda F. Hepatitis C and B virus infections in populations at low or high risk in Ho Chi Minh and Hanoi, Vietnam. J Gastroenterol Hepatol. 1994;9(4):416–419. doi: 10.1111/j.1440-1746.1994.tb01265.x. [DOI] [PubMed] [Google Scholar]
- 18.Goto A., Nguyen Q.V., Pham N.M., Kato K., Cao T.P.N., Le T.H.C. Prevalence of and factors associated with reproductive tract infections among pregnant women in ten communes in Nghe An Province, Vietnam. J Epidemiol. 2005;15(5):163–172. doi: 10.2188/jea.15.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO/UNICEF . WHO/UNICEF; 2013. WHO/UNICEF joint reporting form of Viet Nam. (unpublished) [Google Scholar]
- 20.United Nations Population Division . 2015. World population prospects, the 2012 revision. <http://esa.un.org/wpp/Excel-Data/mortality.htm. Accessed on February 24>. [Google Scholar]
- 21.World Health Organization . World Health Organization; 2014. WHO and UNICEF estimates of immunization coverage: Viet Nam. 〈http://www.data.unicef.org/fckimages/uploads/1421187961_viet_nam_rev_13_FINAL.pdf〉 (accessed on February 24, 2015) [Google Scholar]
- 22.Rao C., Osterberger B., Anh T.D., MacDonald M., Chúc N.T.K., Hill P.S. Compiling mortality statistics from civil registration systems in Viet Nam: the long road ahead. Bull World Health Organ. 2010;88(1):58–65. doi: 10.2471/BLT.08.061630. [DOI] [PMC free article] [PubMed] [Google Scholar]