Summary
Background
An increase in worldwide HPV vaccination could be facilitated if fewer than three doses of vaccine are as effective as three doses. We originally aimed to compare the immunogenicity and frequency of persistent infection and cervical precancerous lesions caused by vaccine-targeted HPV after vaccination with two doses of quadrivalent vaccine on days 1 and 180 or later, with three doses on days 1, 60, and 180 or later, in a cluster-randomised trial. Suspension of the recruitment and vaccination due to events unrelated to our study meant that some enrolled girls could not be vaccinated and some vaccinated girls received fewer than the planned number of vaccinations by default. As a result, we re-analysed our data as an observational cohort study.
Methods
Our study was designed to be done in nine locations (188 clusters) in India. Participants were unmarried girls aged 10–18 years vaccinated in four cohorts: girls who received three doses of vaccine on days 1, 60, and 180 or later, two doses on days 1 and 180 or later, two doses on days 1 and 60 by default, and one dose by default. The primary outcomes were immunogenicity in terms of L1 genotype-specific binding antibody titres, neutralising antibody titres, and antibody avidity after vaccination for the vaccine-targeted HPV types 16, 18, 6, and 11 and incident and persistent infections with these HPVs. Analysis was per actual number of vaccine doses received. This study is registered with ISRCTN, number ISRCTN98283094; and with ClinicalTrials.gov, number NCT00923702.
Findings
Vaccination of eligible girls was initiated on Sept 1, 2009, and continued until April 8, 2010. Of 21 258 eligible girls identified at 188 clusters, 17 729 girls were recruited from 178 clusters before suspension. 4348 (25%) girls received three doses, 4979 (28%) received two doses on days 1 and 180 or later, 3452 (19%) received two doses at days 1 and 60, and 4950 (28%) received one dose. Immune response in the two-dose HPV vaccine group was non-inferior to the three-dose group (median fluorescence intensity ratio for HPV 16 1·12 [95% CI 1·02–1·23] and for HPV 18 1·04 [0·92–1·19]) at 7 months, but was inferior in the two-dose default (0·33 [0·29–0·38] for HPV 16 and 0·51 [0·43–0·59] for HPV 18) and one-dose default (0·09 [0·08–0·11] for HPV 16 and 0·12 [0·10–0·14] for HPV 18) groups at 18 months. The geometric mean avidity indices after fewer than three doses by design or default were non-inferior to those after three doses of vaccine. Fewer than three doses by design and default induced detectable concentrations of neutralising antibodies to all four vaccine-targeted HPV types, but at much lower concentration after one dose. Cervical samples from 2649 participants were tested and the frequency of incident HPV 16, 18, 6, and 11 infections was similar irrespective of the number of vaccine doses received. The testing of at least two samples from 838 participants showed that there was no persistent HPV 16 or 18 infections in any study group at a median follow-up of 4·7 years (IQR 4·2–5·1).
Interpretation
Despite the limitations imposed by the suspension of the HPV vaccination, our findings lend support to the WHO recommendation of two doses, at least 6 months apart, for routine vaccination of young girls. The short-term protection afforded by one dose of HPV vaccine against persistent infection with HPV 16, 18, 6, and 11 is similar to that afforded by two or three doses of vaccine and merits further assessment.
Funding
Bill & Melinda Gates Foundation.
Introduction
Persistent infection with a high-risk HPV causes cervical cancer, and worldwide 68–82% of cervical cancers are attributed to HPV types 16 and 18.1, 2, 3, 4 Prophylactic vaccines containing recombinant virus-like particles assembled from the L1 capsid proteins of HPV 16 and 18 (bivalent vaccine) and HPV 6, 11, 16, and 18 (quadrivalent vaccine) are used in HPV vaccination programmes. Over 6 months, the efficacy of three doses of vaccine (either bivalent or quadrivalent) against high-grade cervical intraepithelial neoplasia caused by vaccine-targeted HPV was almost 100% in HPV-naive populations and greater than 55% in the intention-to-treat populations.1, 2, 3
Research in context.
Evidence before this study
Two doses of the bivalent HPV vaccine given to adolescents 6 months apart has been shown to induce a non-inferior immune response to that of three doses administered on day 1, 1 month, and 6 months. One and two doses of the bivalent HPV vaccine have been shown to protect against cervical HPV 16 and 18 infections as effectively as three doses in women aged 15–25 years. In a randomised trial, two doses of quadrivalent HPV vaccine given 6 months apart to girls aged 9–13 years resulted in a non-inferior immune response at 1 month after the last dose compared with three doses given to girls aged 9–13 years and to women aged 16–26 years on day 1, 2 months, and 6 months. We searched PubMed and MEDLINE for full-length articles published between Jan 1, 2008, and June 30, 2015, using the keywords “HPV vaccination”, “less than three doses”, “alternate doses”, “one dose”, “two doses”, “three doses”, “immunogenicity”, “HPV infection”, “cervical intraepithelial neoplasia”, “clinical trials”, “randomised trials”, “follow-up studies”; we also searched ClinicalTrials.gov for ongoing clinical trials of fewer than three doses of the HPV vaccine. We assessed all potentially relevant articles and found that neither the immunogenicity after one dose of quadrivalent HPV vaccination nor the extent of protection against HPV 16 and 18 infections afforded by fewer than three doses of quadrivalent HPV vaccine has been reported.
Added value of the study
Our findings confirm that two doses of quadrivalent HPV vaccine, administered with an interval of 180 days or more, are immunologically non-inferior to the three-dose schedule and afford protection against incident and persistent HPV 16, 18, 6, and 11 infection that is similar to that afforded by three doses. We also report the first evidence to our knowledge that one dose of quadrivalent HPV vaccine induced detectable titres of HPV neutralising antibodies and that lower vaccine-induced antibody concentrations after one dose of quadrivalent HPV vaccine provide similar protection against vaccine-targeted HPV infections compared with the higher antibody concentrations induced after two or three doses.
Implications of all the available evidence
Our preliminary results suggest that future trials of HPV vaccines should include a single-dose arm. Further long-term follow-up of vaccinated women in our study will clarify whether protection afforded after one dose of quadrivalent HPV vaccine is long-lasting. Our findings after fewer than three doses by design or default are consistent with results from other studies of fewer than three doses. Administration of three doses of HPV vaccine, or two doses as recommended by WHO, remains a challenge, and if one dose proves to be as efficacious as more doses, vaccine uptake will be improved and costs will be reduced. Data on immunogenicity, durability of antibody response, and the frequency of persistent infection after one dose of vaccine will expedite the introduction of HPV vaccination in national immunisation programmes. In view of the programmatic advantages of vaccinating in one dose, assessment of the clinical efficacy of a single dose of vaccine is of great public health significance.
The administration of three doses of the HPV vaccine, or even two doses as recommended by WHO,4, 5 is a challenge, and if one dose was as effective as two or three doses then vaccine uptake would increase greatly and costs would be reduced. The high immune response in pre-adolescent girls given three doses suggests that reductions in dose number could prevent cervical neoplasia.6, 7 Data on immunogenicity, durability of antibody response, and frequency of persistent infection after one dose will enable the swift introduction of HPV vaccination in national immunisation programmes.
In 2009, we initiated a cluster-randomised trial in India to compare the effectiveness of two doses versus three doses of a quadrivalent vaccine targeting HPV types 16, 18, 6, and 11, in preventing cervical neoplasia. However, recruitment and vaccination of the girls in the trial was suspended midway due to events unrelated to our study, which meant that some enrolled girls could not be vaccinated and some vaccinated girls received fewer than the planned number of vaccinations by default. Thus, randomisation was impaired and our study became an observational study of four different vaccinated cohorts. Here, we describe the immunogenicity and infection results after suspension of vaccination.
Methods
Study design and participants
We initiated a multicentre, cluster-randomised trial in India on Sept 1, 2009, to find out whether two doses of quadrivalent HPV vaccine would be as effective in inducing non-inferior immune response and in preventing persistent vaccine-targeted HPV infection and cervical neoplasia as three doses. We recruited unmarried girls who were ambulant and in good general health aged 10–18 years, living in 188 geographical clusters in nine locations in India (Osmanabad, Dindigul, Pune, Mumbai, Ahmedabad, Delhi, Hyderabad, Gangtok in Sikkim, and Aizawl in Mizoram). We chose these regions to represent different regions of India. We excluded those in poor general health and with severe or debilitating illness.
The study was reviewed and approved by the ethics review committees and institutional review boards of the participating centres. The data safety monitoring board regularly monitored the safety and outcomes of the study. We obtained written informed consent from one of the parents or the legal guardian of the participant, with the assent of the participant. We obtained a new written informed consent from girls who became 18 years old during follow-up.
Procedures
The vaccine used was the quadrivalent HPV vaccine (Gardasil; Merck Sharp & Dohme, Whitehouse Station, NJ, USA). Girls were originally randomly assigned to the two-dose group with vaccination on days 1 and 180 (or later) or the three-dose group with vaccination on days 1, 60, and 180 (or later) by the statistician at the International Agency for Research on Cancer (IARC). At each project site, the identified clusters were randomly assigned into two groups using a computer-generated random allocation. Also using the computer-generated random allocation, the two groups in each study site were then randomly assigned to the two-dose group or the three-dose group. All eligible girls in the randomised clusters were enumerated into the study. Vaccinations occurred from study start until April 8, 2010, when the Indian authorities suspended all further recruitment and vaccination of girls in all HPV vaccination trials in India because of events unrelated to our study. The suspension meant that we had four groups of girls who were vaccinated: those on days 1, 60, and 180 or later (original three-dose group); those on days 1 and 180 or later (original two-dose group); those on days 1 and 60 by default (two-dose default group); and those with one dose only by default (one-dose default group).
At each study site, dedicated health workers and nurses were recruited and trained to identify and interview eligible girls for sociodemographic and reproductive information, explain the study to the participating girls and their parents or guardian, obtain informed consent, administer the vaccines, record adverse events, and do follow-up procedures, including obtaining blood and cervical cell samples. Medical practitioners in the study clusters were trained to know how to manage or refer vaccinated girls who reported any adverse events after vaccination. Participants could contact a study clinician if they needed urgent medical attention using a 24-h telephone help line.
Each participant was visited every year by a health worker or nurse in her household to enquire about her general health and wellbeing. Details about marriage, medically significant events, pregnancy, antenatal and postnatal events, delivery, and migration were gathered and documented through the household visits, a network of social workers, medical-care providers, hospitals, and relatives. We obtained blood and cervical cell samples during designated periods.
Blood samples to determine immunogenicity were obtained by nurses during the vaccination session at a clinic or during household visits on day 1 and at 7, 12, 18, 24, 36, 48, and 60 months from a cohort of a convenience sample of participants representing all included ages (10–18 years of age at study entry) of the vaccinated study population. Samples were treated with EDTA and analysed with Luminex (Austin, TX, USA) based multiplex serology8, 9 to assess the concentration of binding antibodies against the major capsid protein L1 of HPV 16, 18, 6, and 11 as mean median fluorescence intensity (MFI; appendix). MFI values as a measure of antibody concentration quantified by use of HPV multiplex serology are directly comparable with optical densities measured with ELISA8 and MFI values for HPV 16 in natural and vaccine-induced HPV 16-specific antibody responses are strongly correlated with endpoint titration titres in the neutralisation assay.9 Seropositivity cutoffs for seroconversion were calculated for each HPV type, based on the MFI values of serum samples obtained from the participants at baseline after allowing for 5% seropositivity in the total baseline samples. The immunogenicity measure was the geometric mean of MFI.8, 9
Antibody avidity, which indicates the degree of antibody affinity maturation, was measured with a modified version of the HPV-L1 genotype-specific binding antibody assay described above (appendix).
Antibodies specific for neutralising epitopes in HPV-L1 protein were measured with a highly sensitive, automated, high-throughput pseudovirion-based neutralisation assay (appendix).9 Bovine papillomavirus (BPV) pseudovirion assays were run as controls to verify that the test serum was not toxic to the cells; toxicity can mimic neutralisation.
Pelvic examination to obtain cervical cell samples was done in women 18 months after marriage or 6 months after delivery of their first child, whichever was earlier, and every year thereafter for 3 years. The HPV genotyping method involved HPV-type-specific E7 PCR bead-based multiplex genotyping.10, 11 The multiplex HPV-type-specific E7 PCR uses HPV type-specific primers targeting the E7 region for the detection of 19 high-risk or probable high-risk HPV types (16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68a, 68b, 70, 73, and 82), and two low-risk HPV types (6 and 11), with detection limits ranging from ten to 1000 copies of the viral genome. The method was validated at the Rajiv Gandhi Centre for Biotechnology (RGCB; Thiruvananthapuram, India) under the supervision of scientists from IARC and the cervical cell samples were tested at RGCB. Women with two or more infections were only counted at the time of the first incident infection. We defined incident infections that persisted for 12 months or longer without an HPV negative test (for the HPV type in question) between the positive tests as persistent infections.
Immunological assays and HPV testing were done at RGCB, where a dedicated laboratory was established with technology transfer and external quality assurance from the German Cancer Research Centre (DKFZ; Heidelberg, Germany) and the Infections and Cancer Biology Group at IARC (Lyon, France). Scientists from RGCB were trained at DKFZ, and the multiplex serology HPV-L1 antibody assays were done at RGCB by trained personnel, masked to the number of vaccine doses received by participants, under the technical supervision of experts from DKFZ.
Outcomes
The primary outcomes were immunogenicity outcomes: the HPV-L1 genotype-specific binding antibody concentrations using geometric mean MFI, antibody avidity induced after vaccination using the geometric mean avidity index, and antibody concentrations specific for neutralising epitopes in HPV-L1 using geometric mean neutralisation titres (GMTs); and infection outcomes: first incident and persistent infections of vaccine-targeted HPV types accumulated during follow-up. Cervical cells were taken 6 months after delivery or 18 months after marriage, whichever was earlier, and annually thereafter for 3 consecutive years.
For the HPV-L1 binding antibodies outcome, we compared the geometric mean MFIs at day 1 for all participants, at 7 months (1 month after the last dose), 18 months, 36 months, and 48 months after the first dose of vaccination in the three-dose group versus the two-dose group, and at 18 months and 36 months after the first dose of vaccination in the two-dose default and one-dose default groups versus the three-dose group. For the 12-month comparison, the MFIs in the one-dose default group were compared with those in the two-dose default group because other dose regimens were not measured at that timepoint. For the antibody avidity outcome, the geometric mean avidity indices were compared at 7 months for the two-dose and three-dose groups and at 18 months for all vaccination groups. For the neutralising antibody outcome, we compared the GMTs at 18 months for HPV 16, 18, and 6 L1 in the two-dose, two-dose default, and one-dose default groups with those in the three-dose group.
The secondary outcomes were cross-protection against non-vaccine targeted high-risk HPV types: first incident and persistent non-targeted high-risk HPV infections accumulated during follow-up.
The study will be monitored and assessed over 20 years. Long-term outcomes, such as the frequency of cervical intraepithelial neoplasia grades 2 and 3 and invasive cancer, will be assessed by screening of participating women and using data from population-based cancer registries.
Statistical analysis
We aimed to recruit 20 000 girls from nine locations in India (7000 girls from Osmanabad, 3000 each from Dindigul, Pune, and Mumbai, 1000 each from Ahmedabad, Delhi, and Hyderabad, and 500 each from Gangtok in Sikkim, and Aizawl in Mizoram), with 10 000 girls living in 100 clusters randomly allocated to the two-dose group and 10 000 girls in 88 clusters to the three-dose group. To test for non-inferiority of antibody concentrations in different dose groups, we used log-transformed mean MFIs in linear regression models to obtain MFI ratios and their corresponding 95% CIs. In keeping with other HPV immunogenicity studies, non-inferiority of a vaccination group was concluded if the lower bound of the 95% CI for its MFI ratio was greater than 0·5.12, 13, 14 In a post-hoc power calculation, using the lowest limit of 95% CI with a sample size of 40 individuals per group, we had 90% power for non-inferiority testing between each of the observational groups and the three-dose group.
The antibody avidity index was calculated by dividing the MFI values of urea-treated samples by the MFI values of the untreated samples and multiplied by 100%. To compare the fewer than three-dose regimens with the three-dose regimen, log-transformed avidity index values were used in linear regression models to obtain avidity index ratios and their 95% CIs. Non-inferiority of the fewer than three-dose regimens to that of the three-dose regimen was concluded if the 95% CI lower bound of the avidity ratio index was greater than 0·5. Type-specific seroprevalence for neutralisation titres is reported as a proportion. The neutralisation GMTs and their 95% CIs were compared by use of the log-transformed titre values in linear regression models. Non-inferiority of the GMT was concluded if the 95% CI lower bound of the neutralisation GMT ratio was greater than 0·5.
Because of the initial cluster randomisation design, responses within the clusters might be more correlated than those between the clusters. For this reason, all regression analyses were adjusted for the cluster design by specifying the option in the regression models that allows the variance-covariance estimator to include the clustering effect.
HPV infection outcomes are reported as frequencies of the detection of first incident infection and persistent infections of vaccine-targeted and non-targeted high-risk HPV infections accumulated during the follow-up.
For both the primary and secondary outcomes, analysis was done per the actual number of vaccine doses a participant received. The statistical analyses were done with Stata (version 13.1).
This study is registered with ISRCTN, number ISRCTN98283094, and with ClinicalTrials.gov, number NCT00923702.
Role of the funding source
The funder had no role in the design, conduct, monitoring, and assessment of the study, analysis of data, or writing of the report. Other than the donation of the vaccines, Merck did not have any role in design, protocol development, study conduct, monitoring, and assessment, analysis of data, or writing of the report. AM, BMN, EL, EZ, GJ, KJ, MP, MM, MT, NB, POE, PRP, PS, RM, MRP, RS, SS, SJ, SGM, SSS, TG, URRP, and YV had access to the raw data. The corresponding author had full access to all the data in the study and all the authors had final approval for the decision to submit.
Results
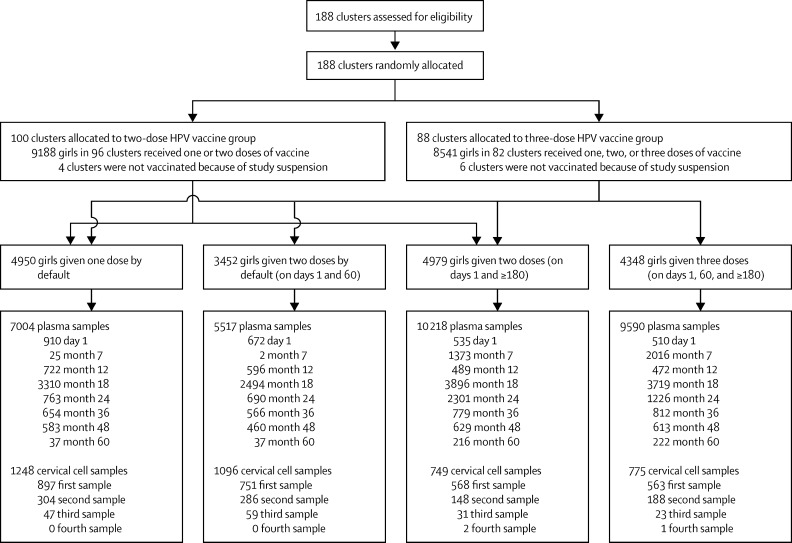
Vaccination of eligible girls was initiated on Sept 1, 2009, and continued until April 8, 2010. Of the 21 258 eligible girls identified for recruitment in the 188 clusters, 17 729 (83%) from 178 clusters were vaccinated at least once: 4348 (25%) girls received three doses on days 1, 60, and 180 or later (three-dose group); 4979 (28%) received two doses on days 1 and 180 or later (two-dose group); 3452 (19%) received two doses on days 1 and 60 by default (two-dose default group); and 4950 (28%) received one dose by default (one-dose default group; figure 1; appendix). The baseline characteristics of participants in the four groups show more or less similar age distribution, whereas we noted some differences in average monthly household income and education (table 1). Local and systemic reactions within 15 days of administering 34 856 vaccine doses in the study included injection-site pain (number of reactions; n=1092), low-grade fever (n=293), injection-site swelling (n=124), dizziness (n=64), headache (n=49), nausea (n=30), skin rash (n=15), diarrhoea (n=13), abdominal cramps (n=13), and fainting attacks (n=10). No serious adverse events were attributable to the vaccine. Median follow-up was 4·7 years (IQR 4·2–5·1).
Figure 1.
Study profile
Table 1.
Baseline characteristics
| Three-dose group | Two-dose group | Two-dose default group | One-dose default group | ||
|---|---|---|---|---|---|
| Number of participants | 4348 | 4979 | 3452 | 4950 | |
| Age at recruitment (years) | |||||
| 10–14 | 2833 (65%) | 3184 (64%) | 2081 (60%) | 2970 (60%) | |
| 15–18 | 1515 (35%) | 1795 (36%) | 1371 (40%) | 1980 (40%) | |
| Type of house | |||||
| Thatched | 399 (9%) | 361 (7%) | 329 (10%) | 673 (14%) | |
| Tiled | 2844 (65%) | 3299 (66%) | 2657 (77%) | 3412 (69%) | |
| Concrete | 1019 (23%) | 1129 (23%) | 463 (13%) | 842 (17%) | |
| Unknown | 86 (2%) | 190 (4%) | 13 (<1%) | 23 (<1%) | |
| Household income (INR per month) | |||||
| <2000 | 1451 (33%) | 1454 (29%) | 588 (17%) | 957 (19%) | |
| 2000–4999 | 1808 (42%) | 2103 (42%) | 2291 (66%) | 3186 (64%) | |
| 5000–9999 | 777 (18%) | 1009 (20%) | 421 (12%) | 674 (14%) | |
| ≥10 000 | 308 (7%) | 412 (8%) | 149 (4%) | 128 (3%) | |
| Unknown | 4 (<1%) | 1 (<1%) | 3 (<1%) | 5 (<1%) | |
| Participant's education | |||||
| None | 46 (1%) | 35 (<1%) | 26 (<1%) | 47 (<1%) | |
| Primary | 364 (8%) | 443 (9%) | 182 (5%) | 629 (13%) | |
| Middle | 2018 (46%) | 2383 (48%) | 1174 (34%) | 1773 (36%) | |
| High | 1464 (34%) | 1556 (31%) | 1442 (42%) | 1746 (35%) | |
| College | 456 (10%) | 561 (11%) | 626 (18%) | 755 (15%) | |
| Unknown | 0 | 1 (<1%) | 2 (<1%) | 0 | |
Data are number or number (%). INR=Indian Rupees.
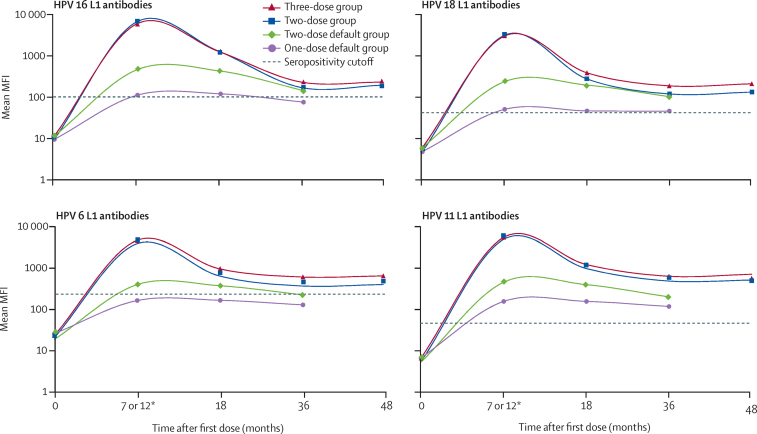
Results of the HPV-L1 binding antibody analyses are in the appendix. The MFI threshold values for seroconversion for HPV 16, 18, 6, and 11 L1 were 100, 41, 240, and 48, respectively. Analysis of 625 plasma samples (308 in the three-dose group and 317 in the two-dose group) obtained at month 7 for peak HPV-L1 binding antibody concentrations indicated that all girls in both groups had seroconverted and almost all girls in the two-dose group had MFIs equal to or higher than the lowest MFI in the three-dose group for all four vaccine-targeted HPV types (appendix). The peak antibody concentrations at 7 months for vaccine-targeted HPV types in the two-dose cohort were non-inferior to the MFIs in the three-dose group (appendix). MFI ratios for the two-dose versus three-dose group were 1·12 (95% CI 1·02–1·23) for HPV 16 L1, 1·04 (0·92–1·19) for HPV 18 L1, 1·04 (0·97–1·13) for HPV 6 L1, and 1·12 (1·06–1·19) for HPV 11 L1.
The HPV-L1 binding antibody concentrations for the vaccine-targeted HPV types were similar for the three-dose and two-dose groups over 48 months (figure 2; appendix). The antibody concentrations in the two-dose vaccine group had similar decay kinetics and were non-inferior to those in the three-dose group at 7 months, 18 months, 36 months, and 48 months, over the 48-month period (appendix). At least 124 (98%) of 127 girls in the two-dose group had antibody concentrations equal to or greater than the lowest antibody MFI in the three-dose group at 48 months (appendix).
Figure 2.
Mean MFI values for HPV types 16, 18, 6, and 11 L1 antibodies
Dashed lines show the threshold (cutoff) values for seroconversion. MFI=median fluorescence intensity. *MFI values for month 7 were used for the three-dose and two-dose vaccine groups, whereas MFI values for month 12 were used for the two-dose default and one-dose default groups.
The HPV-L1 binding antibody concentrations for the vaccine-targeted HPV types in the two-dose default and one-dose default groups were inferior to the concentrations in the three-dose group at 18 months and 36 months. At month 18, the number of samples analysed was 313 in the three-dose group, 449 in the two-dose default group, and 476 in the one-dose default group. At month 36, the number of samples analysed was 271 in the three-dose group, 513 in the two-dose default group, and 510 in the one-dose default group (appendix). At month 18, MFI ratios of the two-dose default group compared with the three-dose group were 0·33 (95% CI 0·29–0·38) for HPV 16 L1, 0·51 (0·43–0·59) for HPV 18 L1, 0·48 (0·42–0·55) for HPV 6 L1, and 0·40 (0·35–0·45) for HPV 11 L1. For the one-dose default group compared with the three-dose group, month 18 MFI ratios were 0·09 (0·08–0·11) for HPV 16 L1, 0·12 (0·10–0·14) for HPV 18 L1, 0·17 (0·15–0·20) for HPV 6 L1, and 0·12 (0·11–0·14) for HPV 11 L1 (figure 2; appendix). For the two-dose default group versus the three-dose group, month 36 MFI ratios were 0·62 (0·54–0·71) for HPV 16 L1, 0·55 (0·47–0·64) for HPV 18 L1, 0·46 (0·40–0·53) for HPV 6 L1, and 0·39 (0·34–0·45) for HPV 11 L1. For the one-dose default group compared with the three-dose group, month 36 MFI ratios were 0·33 (0·28–0·37) for HPV 16 L1, 0·25 (0·21–0·29) for HPV 18 L1, 0·21 (0·18–0·24) for HPV 6 L1, and 0·18 (0·16–0·21) for HPV 11 L1 (figure 2; appendix). At 36 months, 511 (>99%) of 513 girls in the two-dose default group and 462 (91%) of 510 in the one-dose default group had antibody concentrations equal to or higher than the lowest MFI value in the three-dose group (appendix). There was no correlation between age and antibody titres in the one-dose default group (correlation coefficient=0·067).
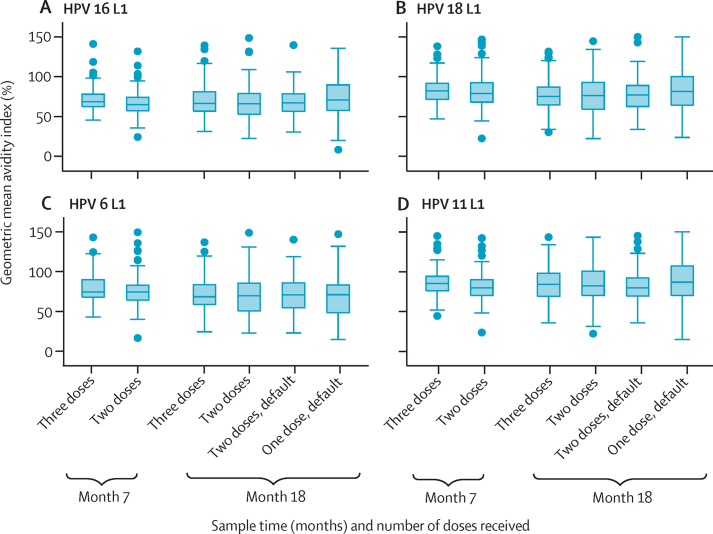
The values for geometric mean avidity index for HPV types 16, 18, 6, and 11 after the two-dose schedule at 7 months and after the two-dose, two-dose default, and one-dose default schedules at 18 months, were non-inferior to the value after the three-dose regimen. For example, the avidity index ratio of the one-dose default group compared with the three-dose group for HPV 16 L1 was 1·10 (95% CI 1·01–1·19; figure 3; appendix).
Figure 3.
Box plots of the avidity index of MFI for HPV types 16 (A), 18 (B), 6 (C), and 11 (D) L1 antibodies at 7 months and 18 months after the first dose
MFI=median fluorescence intensity.
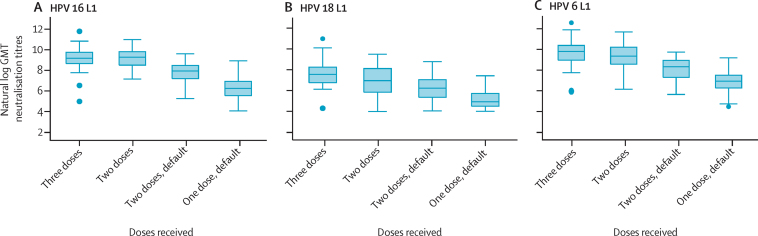
The GMTs of neutralising antibodies to HPV types 16 and 6 at 18 months after the first dose in the two-dose group were non-inferior to the GMTs in the three-dose group, but were inferior for HPV 18. The GMT ratio of HPV 16 L1 neutralisation titres was 1·00 (95% CI 0·69–1·45) for the two-dose group compared with the three-dose group, whereas for HPV 18 L1 neutralisation titres, it was 0·42 (0·27–0·65) for the two-dose group compared with the three-dose group (figure 4; appendix). The GMT after the two-dose default and one-dose default schedules for HPV types 16, 18, and 6 were inferior to the GMT of the three-dose group (figure 4; appendix). The GMT ratio of HPV 16 L1 neutralisation titres was 0·23 (0·16–0·34) for the two-dose default group compared with the three-dose group.
Figure 4.
Box plots of neutralisation titres of HPV types 16 (A), 18 (B), and 6 (C) L1 antibodies at 18 months after the first dose
Samples without neutralising activity were not included in the GMT analyses. GMT=geometric mean neutralisation titre.
The median time between first vaccination and first cervical sample collection from 2649 participants was 3·9 years (IQR 3·0–4·7). The medians were 4·1 years (IQR 3·3–4·8) for the three-dose group, 4·3 years (3·3–4·8) for the two-dose group, 3·7 years (2·9–4·6) for the two-dose default group, and 3·7 years (2·9–4·5) for the one-dose default group. The incidence of vaccine-targeted and non-vaccine-targeted HPV infections is shown in table 2. In 838 women for whom two or more samples were available for analysis, there were no persistent HPV 16 and 18 infections in any of the four study groups at a median follow-up of 4·7 years (IQR 4·2–5·1; table 2). Higher frequencies of persistent vaccine non-targeted HPV infections were noted in the four study groups as compared with vaccine-targeted HPV infections.
Table 2.
Incident HPV infection and HPV infection status
|
HPV incidence |
HPV infection status |
||||||
|---|---|---|---|---|---|---|---|
| Women assessed | Women with incident infections | Incidence (95% CI) | Remained negative | Negative turned positive* | Cleared | Persistent | |
| HPV 16 or 18 infections | |||||||
| Three doses of vaccine | 536 | 2 | 0·4% (0·0–1·3) | 171 (31·9%) | 1 (0·2%) | 0 | 0 |
| Two doses of vaccine | 526 | 4 | 0·8% (0·2–1·9) | 128 (24·3%) | 1 (0·2%) | 0 | 0 |
| Two doses of vaccine by default | 717 | 9 | 1·3% (0·6–2·4) | 257 (35·8%) | 2 (0·3%) | 1 (0·3%) | 0 |
| One dose of vaccine by default | 870 | 10 | 1·1% (0·6–2·1) | 268 (30·8%) | 8 (0·9%) | 1 (0·1%) | 0 |
| HPV 16 infection | |||||||
| Three doses of vaccine | 536 | 2 | 0·4% (0·0–1·3) | 171 (31·9%) | 1 (0·2%) | 0 | 0 |
| Two doses of vaccine | 526 | 3 | 0·6% (0·1–1·7) | 128 (24·3%) | 1 (0·2%) | 0 | 0 |
| Two doses of vaccine by default | 717 | 8 | 1·1% (0·5–2·2) | 257 (35·8%) | 2 (0·3%) | 1 (0·1%) | 0 |
| One dose of vaccine by default | 870 | 9 | 1·0% (0·5–2·0) | 268 (30·8%) | 8 (0·9%) | 1 (0·1%) | 0 |
| HPV 18 infection | |||||||
| Three doses of vaccine | 536 | 0 | 0 | 172 (32·1%) | 0 | 0 | 0 |
| Two doses of vaccine | 526 | 1 | 0·2% (0·0–1·1) | 129 (24·5%) | 0 | 0 | 0 |
| Two doses of vaccine by default | 717 | 1 | 0·1% (0·0–0·8) | 260 (36·3%) | 0 | 0 | 0 |
| One dose of vaccine by default | 870 | 1 | 0·1% (0·0–0·6) | 277 (31·8%) | 0 | 0 | 0 |
| HPV 6 or 11 infections | |||||||
| Three doses of vaccine | 536 | 1 | 0·2% (0·0–1·0) | 171 (31·9%) | 0 | 1 (0·2%) | 0 |
| Two doses of vaccine | 526 | 1 | 0·2% (0·0–1·1) | 129 (24·5%) | 0 | 0 | 0 |
| Two doses of vaccine by default | 717 | 5 | 0·7% (0·2–1·6) | 256 (35·7%) | 1 (0·1%) | 2 (0·3%) | 1 (0·1%) |
| One dose of vaccine by default | 870 | 4 | 0·5% (0·1–1·2) | 275 (31·6%) | 0 | 2 (0·2%) | 0 |
| HPV 16 or 18 or 6 or 11 infections | |||||||
| Three doses of vaccine | 536 | 3 | 0·6% (0·1–1·6) | 170 (31·7%) | 1 (0·2%) | 1 (0·2%) | 0 |
| Two doses of vaccine | 526 | 5 | 1·0% (0·3–2·2) | 128 (24·3%) | 1 (0·2%) | 0 | 0 |
| Two doses of vaccine by default | 717 | 14 | 2·0% (1·1–3·3) | 253 (35·3%) | 3 (0·4%) | 3 (0·4%) | 1 (0·1%) |
| One dose of vaccine by default | 870 | 14 | 1·6% (0·9–2·7) | 266 (31%) | 8 (0·9%) | 3 (0·3%) | 0 |
| Non-vaccine-targeted HPV 31 or 33 or 45 infections | |||||||
| Three doses of vaccine | 536 | 32 | 6·0% (4·1–8·3) | 155 (29%) | 8 (0·3%) | 9 (1·7%) | 0 |
| Two doses of vaccine | 526 | 26 | 4·9% (3·3–7·2) | 123 (23·4%) | 1 (0·2%) | 5 (1·0%) | 0 |
| Two doses of vaccine by default | 717 | 33 | 4·6% (3·2–6·4) | 239 (33·3%) | 8 (1·1%) | 12 (1·7%) | 1 (0·3%) |
| One dose of vaccine by default | 870 | 77 | 8·9% (7·0–10·9) | 230 (26·4%) | 8 (0·9%) | 35 (4·0%) | 4 (0·5%) |
| Non-vaccine-targeted HPV infections excluding 31, 33, and 45 | |||||||
| Three doses of vaccine | 536 | 74 | 13·8% (11·0–17·0) | 139 (26·9%) | 12 (2·2%) | 17 (3·2%) | 4 (0·7%) |
| Two doses of vaccine | 526 | 48 | 9·1% (6·8–11·9) | 113 (21·5%) | 8 (1·5%) | 7 (1·3%) | 1 (0·9%) |
| Two doses of vaccine by default | 717 | 68 | 9·5% (7·4–11·9) | 223 (31·1%) | 11 (1·5%) | 22 (3·1%) | 4 (0·6%) |
| One dose of vaccine by default | 870 | 118 | 13·6% (11·4–16·0) | 222 (25·5%) | 19 (2·2%) | 32 (3·7%) | 4 (0·5%) |
Data are number (%), unless otherwise indicated. HPV incidence assessed in women with at least one sample tested. HPV infection status assessed in women with at least two samples tested at 12 months apart.
Incident HPV infections with no subsequent sample to assess persistence.
Discussion
Our findings suggest that two doses of quadrivalent HPV vaccine, administered with an interval of 180 days or more between doses, are immunologically non-inferior to the three-dose schedule and afford protection against incident and persistent HPV 16, 18, 6, and 11 infections that is similar to that afforded by three doses. Low frequencies of premarital sexual activity, smoking, drinking, oral contraceptive use, and HPV infection have been reported for pre-adolescent and adolescent, unmarried girls in India.15, 16, 17, 18, 19 The frequency of any HPV infection in young married women in India ranges from 7% to 19%, and the frequency of HPV types 16 and 18 infections range from 3% to 9%.20, 21, 22, 23 In this context, our initial results provide new evidence that one and two doses of quadrivalent vaccine prevent incident and persistent cervical infections with HPV types 16, 18, 6, and 11, similar to the protection afforded after a three-dose schedule during a median follow-up of 4·7 years (IQR 4·2–5·1).
To our knowledge, our study is the first in which immunogenicity and vaccine-targeted HPV infection frequencies are reported after one dose of quadrivalent vaccine. Although the antibody concentrations after one dose were lower than those in the two-dose and three-dose groups, the titres were stable and the mean avidity index after one dose was non-inferior to that after three doses. Vaccine-induced HPV L1 binding antibody concentrations measured with multiplex serology correlated well with the concentrations of serum HPV-neutralising activity after the different dose regimens. Additionally, the frequencies of incident and persistent vaccine-targeted HPV infections after one dose were similar to those after three and two doses. One-dose vaccination also induced detectable titres of neutralising antibodies to all four vaccine-targeted HPV types in most of the participants that were assessed, but at a much lower concentration. There is no known minimum threshold concentration of antibodies that is protective; seroconversion is an arbitrary cutoff and does not represent the minimum threshold for protection.1, 24
Our immunogenicity findings after three and two doses of vaccine are consistent with the results from a Canadian randomised trial25 in which 261 girls aged 9–13 years were allocated to vaccine with three doses of quadrivalent vaccine at day 1, 2 months, and 6 months; 259 girls also aged 9–13 years were allocated to vaccine with two doses at day 1 and 6 months; and 310 women aged 16–26 years were allocated to vaccine with three doses also at day 1, 2 months, and 6 months. The antibody concentrations for all four vaccine-targeted HPV types in the girls aged 9–13 years given two doses were non-inferior to those in women aged 16–26 years who received three doses during a 36-month period. Similarly, antibody concentrations after two doses of bivalent vaccine administered on days 1 and 180 were non-inferior to the concentrations after the three-dose schedule in several studies.26, 27, 28
In a non-randomised comparison of the bivalent HPV vaccine in the context of the Costa Rica Vaccine and PATRICIA trials, one and two doses were as protective as three doses in preventing persistent HPV 16 or 18 infections.29, 30 The non-significant differences in the frequencies of incident and persistent infections with HPV types 16, 18, 6, and 11 after the four different dose schedules shown in our study is new evidence for the protective efficacy of two doses and one dose of quadrivalent vaccine in the short term. The frequencies of HPV 16 and 18 infections in our vaccinated study groups are substantially lower than the 3–9% frequency of such infections reported in unvaccinated young women in India.20, 21, 22, 23 Distinguishing between a positive HPV DNA result due to an incident infection and that due to a recent exposure is not possible. Our results suggest that the low vaccine-induced antibody concentrations after one dose afford similar protection against vaccine-targeted HPV infections as the high antibody concentrations from two or three doses during a median follow-up of 4·7 years (IQR 4·2–5·1). The similar frequency of incident non-vaccine HPV types in each dose group (table 2) implies that HPV exposure was similar across the different groups and participants' sexual behaviours were not related to the number of doses received. A follow-up of these women is planned over 20 years to assess the efficacy endpoints for the incidence of cervical intraepithelial neoplasia grades 2 and 3 and invasive cervical cancer caused by vaccine-targeted and non-targeted HPV types in the different dose groups by screening of participating women and using data from population-based cancer registries. We have also recruited and are following up an age-matched and residence-matched cohort of unvaccinated women (n=1540).
The protection afforded by virus-like particle vaccines is believed to be mediated by neutralising antibodies produced by plasma cells,31, 32 most of which have short lifespans, leading to declines in peak antibody concentrations within a few months. By contrast, some antibody-secreting cells become long-lived plasma cells. Concentrations of antibodies measured 12–18 months after the last dose of a virus-like particle vaccine generally represent the activity of long-lived plasma cells and are the best predictors of antibody persistence.
The patterns of antibody responses after one dose of vaccine in our study and in the Costa Rica and PATRICIA trials27, 30 seem to be similar to the pattern after a live attenuated vaccine, which usually affords long-lasting protection. The stable antibody response after one dose of the HPV L1 vaccine might be due to the structure of the HPV-like particles and the antigens it displays to the immune system might eliminate the need for doses after the priming dose.27, 33, 34 Notably, in a cohort study35 in which girls aged 10–24 years and women received three doses (n=98 252), two doses by default (n=112 555), and one dose by default (n=119 046) of the quadrivalent vaccine, the incidence rate ratios for the occurrence of condylomas were 0·18, 0·29, and 0·31, respectively, implying that one dose and two doses with a short interval were associated with significant reduction in condyloma risk compared with 926 119 unvaccinated women.
Due to the disruption of recruitment and randomisation because of the suspension of vaccination midway in our planned two-arm randomised trial, our study has essentially become an observational cohort study of participants in four dose groups with similar sociodemographic characteristics. Vaccination was suspended in our study in response to instructions from the Indian Council of Medical Research in the context of all HPV vaccination studies in India pending an enquiry into the causes of death of five girls (subsequently proven unrelated to HPV vaccination) recruited for an HPV vaccination demonstration project in the states of Andhra Pradesh and Gujarat. Major limitations include the non-randomised comparison of the vaccination dose groups, an absence of memory B-cell response data, limitations in interpreting early results for girls given incomplete dose schedules, and biases that might have been introduced after vaccination interruption and loss of randomisation. Despite these limitations, our study of HPV vaccination is representative of generalisable real-world conditions: it had varying dose schedules and a broad age range of participants in addition to the difficulties and challenges inherent in running HPV vaccination studies in low-income and middle-income countries. Moreover, the inclusion of sexually naive girls aged 10–18 years (the primary and secondary target age groups for vaccination), the possibility of long-term follow-up, and the technology transfer that allowed validated and quality assured execution of antibody assays and HPV genotyping and the resultant independence in assessing outcomes without having to rely on pharmaceutical industry laboratories or laboratories abroad are important strengths of our study.
Our preliminary results after one dose of quadrivalent vaccine by default, although promising, should be interpreted with caution and suggest that future trials of HPV vaccines should include a single dose arm. Further long-term follow-up of vaccinated women in our study will clarify lasting protection after one dose. Because of the programmatic advantages of one dose in terms of cost savings, logistics, and coverage, and the high proportion of participants who default after the first dose in immunisation programmes, particularly in developed countries,36, 37 assessment of the clinical efficacy of a single dose is of substantial public health significance. A single dose of HPV vaccine, providing strong and sustained protection against HPV infection in the long term, is the best way to overcome the barriers to vaccine uptake globally.
Our findings support the WHO recommendation to use two doses separated by 6 months or more for vaccination of young girls, and indicate that a single dose of the HPV vaccine merits further assessment.
Acknowledgments
Acknowledgments
The Bill & Melinda Gates Foundation provided a grant for this study. Merck provided the vaccines used in the study through a memorandum of understanding with WHO. Establishment of the Luminex-based assays at RGCB was partly supported by the European Commission-Seventh Framework Programme grant HPV-AHEAD (FP7-HEALTH-2011-282562). We thank the Bill & Melinda Gates Foundation for their generous financial support without which this study would not have been possible; Peter Dull (Integrated Clinical Vaccine Development, Bill & Melinda Gates Foundation) for his valuable support, encouragement, and advice; Merck Sharp Dohme for donation of HPV vaccines for use in the study; current and past members of the data safety monitoring board: Lynette Denny, Lutz Gissmann, Peter Sasieni, Thangarajan Rajkumar, Doreen Ramogola-Masire, Raul Murillo, the late Arun P Kurkure, and Rolando Herrero for their valuable advice and monitoring of the study safety and outcomes; Jan Agosti (formerly at Bill & Melinda Gates Foundation and now at the University of Seattle) for her valuable support, encouragement, and advice; Union for International Cancer Control for the award of International Cancer Technology Transfer fellowships that helped technology transfer and quality assurance for immunogenicity and HPV genotyping studies at RGCB; the district administrative, civic, education, and health authorities, and medical practitioners in the districts of India where the study sites are located for their cooperation, facilitation, and assistance in implementing the study; the study participants, their parents, families, and legal guardians for their understanding, cooperation, excellent and continuing participation in the study, and follow-up procedures despite the challenges and misinformation after suspension of HPV vaccination; our field staff at our project sites in India and scientists and technicians at RGCB and at DKFZ for their tremendous effort and dedication; Christopher P Wild, Walter Prendiville, and Partha Basu (all IARC) for their valuable suggestions on a draft version of this manuscript; and Evelyn Bayle and Krittika Guinot (both IARC) for their help in preparing this manuscript.
Contributors
RS had the initial idea for the study, and was responsible for the conception, design, and conduct, monitoring, and supervision of the study, and acquisition, analysis, and interpretation of the data. PRP was responsible for the analysis of sera samples in the Indian central laboratory. MP was responsible for the overall supervision of laboratory analyses on sera and technology transfer to staff from the Indian laboratory. TG was a co-investigator, and was responsible for the overall supervision of the laboratory analyses of the cervical cell samples, and technology transfer to staff from the Indian central laboratory. NB was the study representative for the Indian authorities on behalf of all participants; she was responsible for the on-site conduct of the study in Delhi, participated in monitoring, supervision, acquisition, and interpretation of the data, and the provision of clinical services at the study site. RM was responsible for the statistical analysis and interpretation of the data, and monitoring of the study. KJ was responsible for the overall supervision and technical support for the on-site conduct of the study in Barshi, and the analysis and interpretation of results. EL was responsible for generating project questionnaires, software for data entry and analysis, and training on-site staff in the use of the software. AM and JB were responsible for the laboratory analyses on sera and technology transfer to staff from the Indian laboratory. JMBV was responsible for genotyping and serology laboratory analyses in the Indian central laboratory. SS was responsible for the database entries of the results from the laboratory analyses in the Indian central laboratory and participated in the genotyping of the cervical HPV infection. TPRAK and RV participated in the genotyping and serology laboratory analyses in the Indian central laboratory. UD was responsible for the overall on-site conduct of the study in Pune, ST in Ambilikkai, and GJ in Ahmedabad. MW-F, TW, and MM were responsible for the supervision of laboratory analyses on sera and technology transfer to staff from the Indian laboratory. PS was responsible for the laboratory analyses on sera and technology transfer to staff from the Indian laboratory. SH participated in monitoring, acquisition, analysis, and interpretation of the data for the site in Barshi. AK assisted in the on-site conduct of the study in Delhi. RJ was responsible for on-site conduct of the study in Pune and participated in the provision of clinical services at the study site. CS assisted in data analysis and interpretation. MT was responsible for the overall supervision of laboratory analyses of cervical cell samples and technology transfer to staff from the central laboratory in India. MRP was the principal investigator and was responsible for the overall supervision of laboratory analyses at the central laboratory in India. BMN and SGM were responsible for the on-site conduct of the study in Barshi, POE in Ambilikkai, SJ in Pune, URRP in Hyderabad, PJ in Ahmedabad, YV and MS in Sikkim, EZ and MS in Mizoram, and SSS, GM, and SP in Mumbai, and they participated in the monitoring, supervision, acquisition, and interpretation of the data, and the provision of clinical services at their respective study sites.
Declaration of interests
MP and MM are inventors on a patent application by DKFZ on the high-throughput pseudovirion-based neutralisation assay. PS is co-inventor on a patent application by DKFZ on the high-throughput pseudovirion-based neutralisation assay. NB has received research funding through her Institute from GlaxoSmithKline and Merck. SJ received funds from GlaxoSmithKline through the Jehangir Clinical Development Center to do an HPV vaccine study. The other authors declare no competing interests.
Supplementary Material
References
- 1.Schiller JT, Castellsague X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine. 2012;30(suppl 5):F123–F138. doi: 10.1016/j.vaccine.2012.04.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosch FX, Broker TR, Forman D. Comprehensive control of human papillomavirus infections and related diseases. Vaccine. 2013;31(suppl 5):F1–31. doi: 10.1016/j.vaccine.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Erickson BK, Landers EE, Huh WK. Update on vaccination clinical trials for HPV-related disease. Clin Ther. 2014;36:8–16. doi: 10.1016/j.clinthera.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 4.WHO Summary of the SAGE April 2014 meeting. http://www.who.int/immunization/sage/meetings/2014/april/report_summary_april_2014/en/ (accessed Sept 16, 2015).
- 5.World Health Organization Evidence based recommendations on Human Papilloma Virus (HPV) Vaccines Schedules. Background paper for SAGE discussions. 2014. http://www.who.int/immunization/sage/meetings/2014/april/1_HPV_Evidence_based_recommendationsWHO_with_Appendices2_3.pdf (accessed Sept 16, 2015).
- 6.Block SL, Nolan T, Sattler C. Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in male and female adolescents and young adult women. Pediatrics. 2006;118:2135–2145. doi: 10.1542/peds.2006-0461. [DOI] [PubMed] [Google Scholar]
- 7.Pedersen C, Petaja T, Strauss G. Immunization of early adolescent females with human papillomavirus type 16 and 18 L1 virus-like particle vaccine containing AS04 adjuvant. J Adolesc Health. 2007;40:564–571. doi: 10.1016/j.jadohealth.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 8.Waterboer T, Sehr P, Michael KM. Multiplex human papillomavirus serology based on in situ-purified glutathione s-transferase fusion proteins. Clin Chem. 2005;51:1845–1853. doi: 10.1373/clinchem.2005.052381. [DOI] [PubMed] [Google Scholar]
- 9.Sehr P, Rubio I, Seitz H. High-throughput pseudovirion-based neutralization assay for analysis of natural and vaccine-induced antibodies against human papillomaviruses. PLoS One. 2013;8:e75677. doi: 10.1371/journal.pone.0075677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitt M, Dondog B, Waterboer T, Pawlita M, Tommasino M, Gheit T. Abundance of multiple high-risk human papillomavirus (HPV) infections found in cervical cells analyzed by use of an ultrasensitive HPV genotyping assay. J Clin Microbiol. 2010;48:143–149. doi: 10.1128/JCM.00991-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gheit T, Landi S, Gemignani F. Development of a sensitive and specific assay combining multiplex PCR and DNA microarray primer extension to detect high-risk mucosal human papillomavirus types. J Clin Microbiol. 2006;44:2025–2031. doi: 10.1128/JCM.02305-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reisinger KS, Block SL, Lazcano-Ponce E. Safety and persistent immunogenicity of a quadrivalent human papillomavirus types 6, 11, 16, 18 L1 virus-like particle vaccine in preadolescents and adolescents: a randomized controlled trial. Pediatr Infect Dis J. 2007;26:201–209. doi: 10.1097/01.inf.0000253970.29190.5a. [DOI] [PubMed] [Google Scholar]
- 13.Neuzil KM, Canh DG, Thiem VD. Immunogenicity and reactogenicity of alternative schedules of HPV vaccine in Vietnam: a cluster randomized noninferiority trial. JAMA. 2011;305:1424–1431. doi: 10.1001/jama.2011.407. [DOI] [PubMed] [Google Scholar]
- 14.Wang WW, Mehrotra DV, Chan IS, Heyse JF. Statistical considerations for noninferiority/equivalence trials in vaccine development. J Biopharm Stat. 2006;16:429–441. doi: 10.1080/10543400600719251. [DOI] [PubMed] [Google Scholar]
- 15.Sankaranarayanan R, Bhatla N, Gravitt PE. Human papillomavirus infection and cervical cancer prevention in India, Bangladesh, Sri Lanka and Nepal. Vaccine. 2008;26(suppl 12):M43–M52. doi: 10.1016/j.vaccine.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Hussain S, Bharadwaj M, Nasare V. Human papillomavirus infection among young adolescents in India: impact of vaccination. J Med Virol. 2012;84:298–305. doi: 10.1002/jmv.22261. [DOI] [PubMed] [Google Scholar]
- 17.Santhya KG, Ram U, Acharya R. Pre-marital sexual relations among youth in India: findings from the Youth in India, Situations and Needs Study. XXVI International Union for the Scientific Study Population Conference; Marrakech, Morocco; Sept 27, to Oct 2, 2009. http://iussp2009.princeton.edu/papers/92480 (accessed Sept 16, 2015).
- 18.Kelkar DS, Patwardhan M, Joshi VD. Prevalence and causalities of tobacco consumption (TC) among adolescents: a cross sectional study at Pune. J Assoc Physicians India. 2013;61:174–178. [PubMed] [Google Scholar]
- 19.Madaan M, Agrawal S, Puri M, Meena J, Kaur H, Trivedi SS. Health profile of urban adolescent girls from India. Int J Adolesc Med Health. 2014;26:233–237. doi: 10.1515/ijamh-2013-0513. [DOI] [PubMed] [Google Scholar]
- 20.Datta P, Bhatla N, Dar L. Prevalence of human papillomavirus infection among young women in North India. Cancer Epidemiol. 2010;34:157–161. doi: 10.1016/j.canep.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 21.Datta P, Bhatla N, Pandey RM. Type-specific incidence and persistence of HPV infection among young women: a prospective study in North India. Asian Pac J Cancer Prev. 2012;13:1019–1024. doi: 10.7314/apjcp.2012.13.3.1019. [DOI] [PubMed] [Google Scholar]
- 22.Dutta S, Begum R, Mazumder ID. Prevalence of human papillomavirus in women without cervical cancer: a population-based study in eastern India. Int J Gynecol Pathol. 2012;31:178–183. doi: 10.1097/PGP.0b013e3182399391. [DOI] [PubMed] [Google Scholar]
- 23.Sharma K, Kathait A, Jain A. Higher prevalence of human papillomavirus infection in adolescent and young adult girls belonging to different Indian tribes with varied socio-sexual lifestyle. PLoS One. 2015;10:e0125693. doi: 10.1371/journal.pone.0125693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joura EA, Kjaer SK, Wheeler CM. HPV antibody levels and clinical efficacy following administration of a prophylactic quadrivalent HPV vaccine. Vaccine. 2008;26:6844–6851. doi: 10.1016/j.vaccine.2008.09.073. [DOI] [PubMed] [Google Scholar]
- 25.Dobson SR, McNeil S, Dionne M. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA. 2013;309:1793–1802. doi: 10.1001/jama.2013.1625. [DOI] [PubMed] [Google Scholar]
- 26.Romanowski B, Schwarz TF, Ferguson LM. Immune response to the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose or 3-dose schedule up to 4 years after vaccination: results from a randomized study. Hum Vaccin Immunother. 2014;10:1155–1165. doi: 10.4161/hv.28022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Safaeian M, Porras C, Pan Y. Durable antibody responses following one dose of the bivalent human papillomavirus L1 virus-like particle vaccine in the Costa Rica Vaccine Trial. Cancer Prev Res (Phila) 2013;6:1242–1250. doi: 10.1158/1940-6207.CAPR-13-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lazcano-Ponce E, Stanley M, Munoz N. Overcoming barriers to HPV vaccination: non-inferiority of antibody response to human papillomavirus 16/18 vaccine in adolescents vaccinated with a two-dose vs. a three-dose schedule at 21 months. Vaccine. 2014;32:725–732. doi: 10.1016/j.vaccine.2013.11.059. [DOI] [PubMed] [Google Scholar]
- 29.Kreimer AR, Rodriguez AC, Hildesheim A. Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J Natl Cancer Inst. 2011;103:1444–1451. doi: 10.1093/jnci/djr319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kreimer AR, Struyf F, Del Rosario-Raymundo MR, for the Costa Rica Vaccine Trial and the PATRICIA study groups Efficacy of fewer than three doses of an HPV-16/18 AS04-adjuvanted vaccine: combined analysis of data from the Costa Rica Vaccine and PATRICIA trials. Lancet Oncol. 2015;16:775–786. doi: 10.1016/S1470-2045(15)00047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Breitburd F, Kirnbauer R, Hubbert NL. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J Virol. 1995;69:3959–3963. doi: 10.1128/jvi.69.6.3959-3963.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Day PM, Kines RC, Thompson CD. In vivo mechanisms of vaccine-induced protection against HPV infection. Cell Host Microbe. 2010;8:260–270. doi: 10.1016/j.chom.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schiller JT, Lowy DR. Raising expectations for subunit vaccine. J Infect Dis. 2015;211:1373–1375. doi: 10.1093/infdis/jiu648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol. 2010;10:787–796. doi: 10.1038/nri2868. [DOI] [PubMed] [Google Scholar]
- 35.Herweijer E, Leval A, Ploner A. Association of varying number of doses of quadrivalent human papillomavirus vaccine with incidence of condyloma. JAMA. 2014;311:597–603. doi: 10.1001/jama.2014.95. [DOI] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention National and state vaccination coverage among adolescents aged 13–17 years—United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:685–693. [PMC free article] [PubMed] [Google Scholar]
- 37.Bertaut A, Chavanet P, Aho S, Astruc K, Douvier S, Fournel I. HPV vaccination coverage in French girls attending middle and high schools: a declarative cross sectional study in the department of Cote d'Or. Eur J Obstet Gynecol Reprod Biol. 2013;170:526–532. doi: 10.1016/j.ejogrb.2013.07.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.