Abstract
Glutamate is an inhibitor of phosphate dependent glutaminase (PDG), and renal cortical glutamate is decreased in metabolic acidosis. It has been postulated previously that the rise in renal production of ammonia from glutamine in metabolic acidosis is due primarily to activation of cortical PDG as a consequence of the fall in glutamate. The decrease in cortical glutamate has been attributed to the increase in the capacity of cortex to convert glutamate to glucose in acidosis.
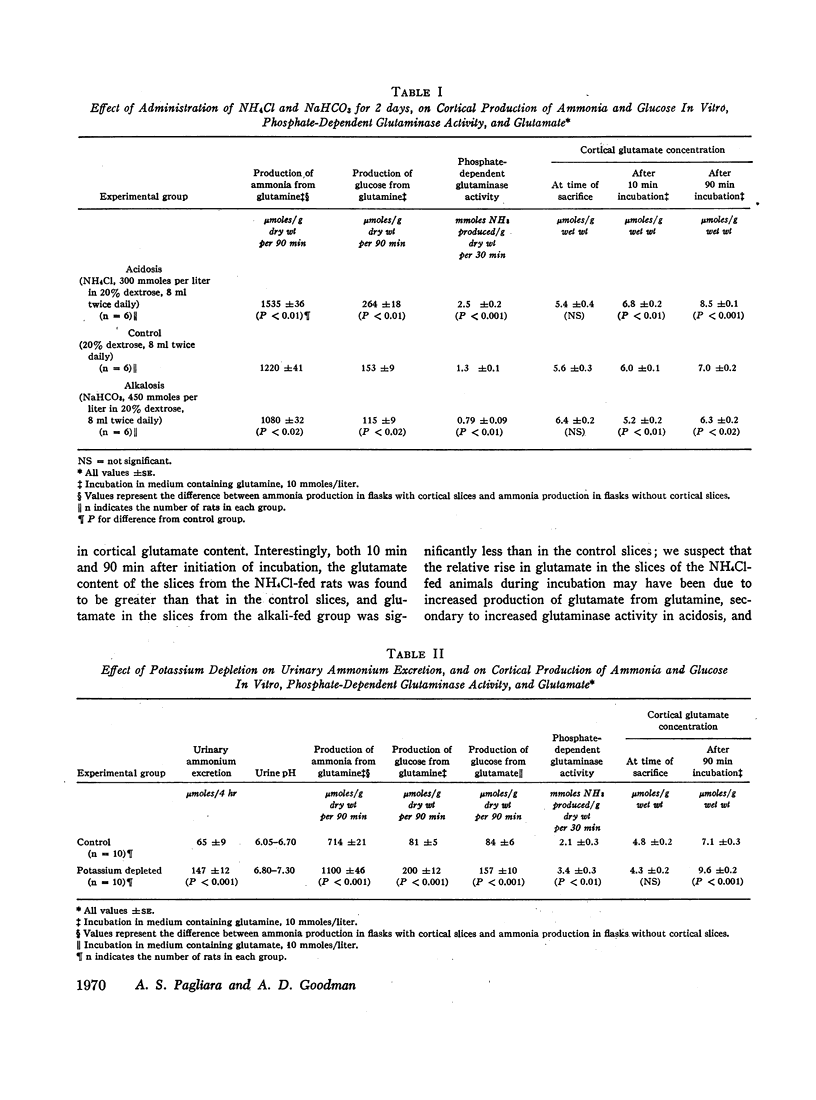
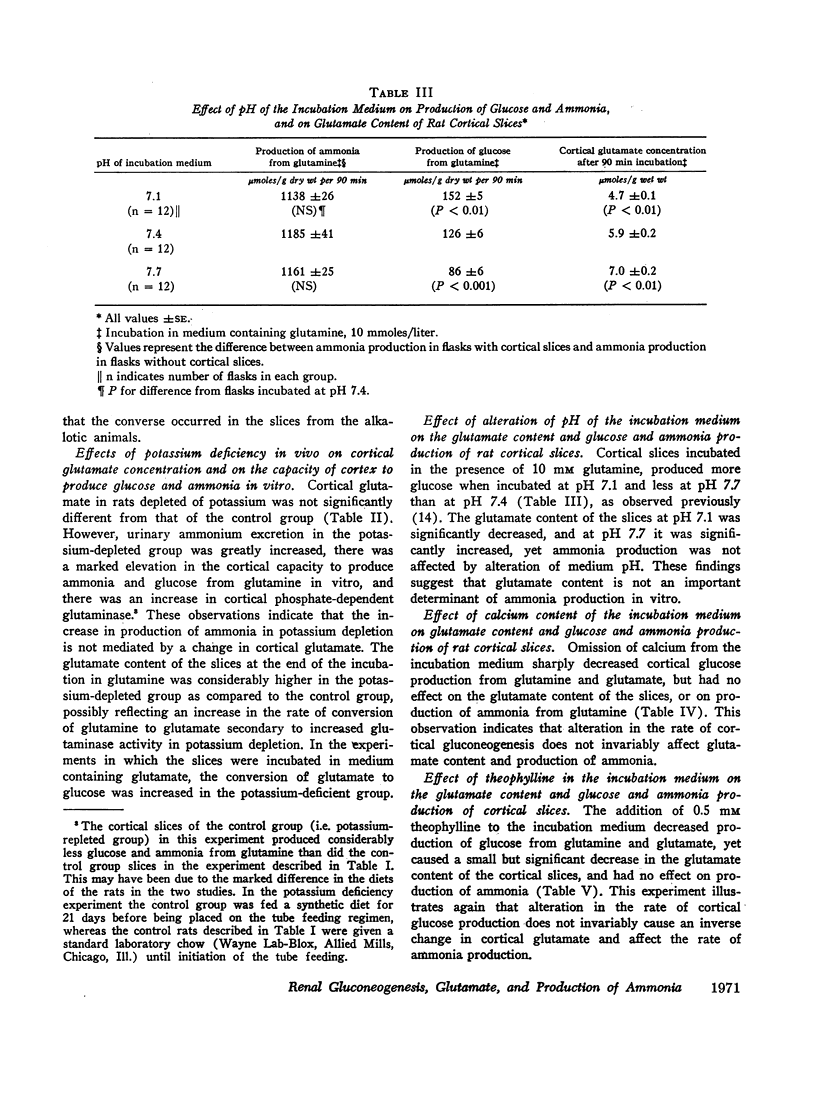
In the present study, administration of ammonium chloride to rats in an amount inadequate to decrease cortical glutamate increased the capacity of cortex to produce ammonia from glutamine in vitro and increased cortical PDG. Similarly, cortex from potassium-depleted rats had an increased capacity to produce ammonia and an increase in PDG, but glutamate content was normal. The glutamate content of cortical slices incubated at pH 7.1 was decreased, and that at 7.7 was increased, compared to slices incubated at 7.4, yet ammonia production was the same at all three pH levels. These observations suggest that cortical glutamate concentration is not the major determinant of ammonia production.
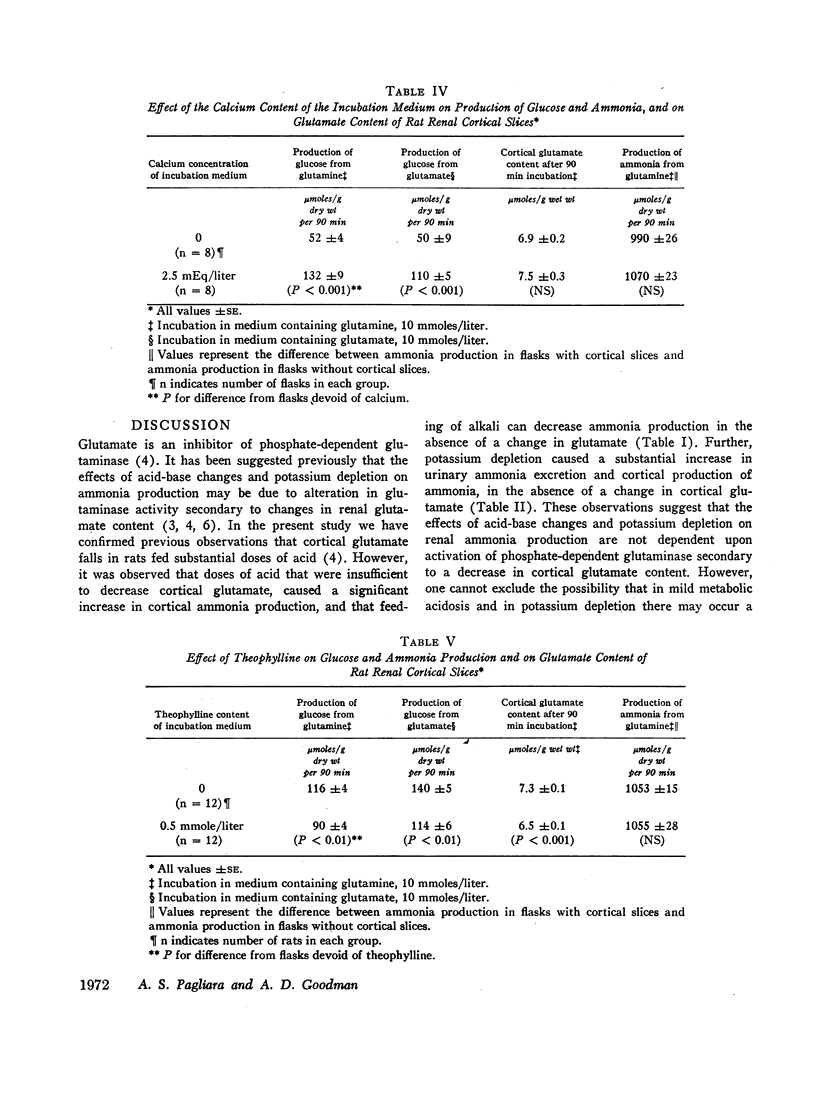
In potassium-depleted rats there was a 90% increase in the capacity of cortex to convert glutamate to glucose, yet cortical glutamate was not decreased. In vitro, calcium more than doubled conversion of glutamate to glucose by cortical slices without affecting the glutamate content of the slices, and theophylline suppressed conversion of glutamate to glucose yet decreased glutamate content. These observations indicate that the rate of cortical gluconeogenesis is not the sole determinant of cortical glutamate concentration.
The increase in cortical gluconeogenesis in acidosis and potassium depletion probably is not the primary cause of the increase in ammonia production in these states, but the rise in gluconeogenesis may contribute importantly to the maintenance of increased ammoniagenesis by accelerating removal of the products of glutamine degradation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alleyne G. A., Scullard G. H. Renal metabolic response to acid base changes. I. Enzymatic control of ammoniagenesis in the rat. J Clin Invest. 1969 Feb;48(2):364–370. doi: 10.1172/JCI105993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dies F., Lotspeich W. D. Hexose monophosphate shunt in the kidney during acid-base and electrolyte imbalance. Am J Physiol. 1967 Jan;212(1):61–71. doi: 10.1152/ajplegacy.1967.212.1.61. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN L., COPENHAVER J. H., Jr Relation of glutaminase I activity to glutamic acid concentration in the rat kidney. Am J Physiol. 1960 Feb;198:227–229. doi: 10.1152/ajplegacy.1960.198.2.227. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN L. RELATION OF RENAL GLUTSMINE TRANSEMINASE-OMEGA-AMIDASE ACTIVITY TO AMMONIA EXCRETION IN THE RAT. Nature. 1964 Mar 21;201:1229–1230. doi: 10.1038/2011229a0. [DOI] [PubMed] [Google Scholar]
- Goldstein L. Pathways of glutamine deamination and their control in the rat kidney. Am J Physiol. 1967 Oct;213(4):983–989. doi: 10.1152/ajplegacy.1967.213.4.983. [DOI] [PubMed] [Google Scholar]
- Goldstein L. Relation of glutamate to ammonia production in the rat kidney. Am J Physiol. 1966 Mar;210(3):661–666. doi: 10.1152/ajplegacy.1966.210.3.661. [DOI] [PubMed] [Google Scholar]
- Goodman A. D., Fuisz R. E., Cahill G. F., Jr Renal gluconeogenesis in acidosis, alkalosis, and potassium deficiency: its possible role in regulation of renal ammonia production. J Clin Invest. 1966 Apr;45(4):612–619. doi: 10.1172/JCI105375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicki R. H., Goldstein L. Glutamine synthetase and renal ammonia metabolism. Am J Physiol. 1969 May;216(5):1107–1110. doi: 10.1152/ajplegacy.1969.216.5.1107. [DOI] [PubMed] [Google Scholar]
- KREBS H. A., BENNETT D. A., DE GASQUET P., GASQUET P., GASCOYNE T., YOSHIDA T. Renal gluconeogenesis. The effect of diet on the gluconeogenic capacity of rat-kidney-cortex slices. Biochem J. 1963 Jan;86:22–27. doi: 10.1042/bj0860022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamm D. E., Fuisz R. E., Goodman A. D., Cahill G. F., Jr Acid-base alterations and renal gluconeogenesis: effect of pH, bicarbonate concentration, and PCO2. J Clin Invest. 1967 Jul;46(7):1172–1177. doi: 10.1172/JCI105610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEONARD E., ORLOFF J. Regulation of ammonia excretion in the rat. Am J Physiol. 1955 Jul;182(1):131–138. doi: 10.1152/ajplegacy.1955.182.1.131. [DOI] [PubMed] [Google Scholar]
- MOYED H. S., UMBARGER H. E. Regulation of biosynthetic pathways. Physiol Rev. 1962 Jul;42:444–466. doi: 10.1152/physrev.1962.42.3.444. [DOI] [PubMed] [Google Scholar]
- PITTS R. F., DEHAAS J., KLEIN J. Relation of renal amino and amide nitrogen extraction to ammonia production. Am J Physiol. 1963 Feb;204:187–191. doi: 10.1152/ajplegacy.1963.204.2.187. [DOI] [PubMed] [Google Scholar]
- PITTS R. F. RENAL PRODUCTION AND EXCRETION OF AMMONIA. Am J Med. 1964 May;36:720–742. doi: 10.1016/0002-9343(64)90182-2. [DOI] [PubMed] [Google Scholar]
- Pagliara A. S., Goodman A. D. Effect of adenosine 3',5'-monophosphate on production of glucose and ammonia by renal cortex. J Clin Invest. 1969 Aug;48(8):1408–1412. doi: 10.1172/JCI106106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss H. G. Renal glutamate metabolism in acute metabolic acidosis. Nephron. 1969;6(3):235–246. doi: 10.1159/000179731. [DOI] [PubMed] [Google Scholar]
- Rognstad R. Gluconeogenesis in the kidney cortex. Flow of malate between compartments. Biochem J. 1970 Feb;116(3):493–502. doi: 10.1042/bj1160493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELIGSON D., HIRAHARA K. The measurement of ammonia in whole blood, erythrocytes, and plasma. J Lab Clin Med. 1957 Jun;49(6):962–974. [PubMed] [Google Scholar]
- Steiner A. L., Goodman A. D., Treble D. H. Effect of metabolic acidosis on renal gluconeogenesis in vivo. Am J Physiol. 1968 Jul;215(1):211–217. doi: 10.1152/ajplegacy.1968.215.1.211. [DOI] [PubMed] [Google Scholar]



