Abstract
With few exceptions, immunization supply chains in developing countries continue to face chronic difficulties in providing uninterrupted availability of potent vaccines up to service delivery levels, and in the most efficient manner possible. As these countries struggle to keep pace with an ever growing number of vaccines, more and more Ministries of Health are considering options of engaging the private sector to manage vaccine storage, handling and distribution on their behalf. Despite this emerging trend, there is limited evidence on the benefits or challenges of this option to improve public supply chain performance for national immunization programmes. To bridge this knowledge gap, this study aims to shed light on the value proposition of outsourcing by documenting the specific experience of the Western Cape Province of South Africa. The methodology for this review rested on conducting two key supply chain assessments which allowed juxtaposing the performance of the government managed segments of the vaccine supply chain against those managed by the private sector. In particular, measures of effective vaccine management best practice and temperature control in the cold chain were analysed. In addition, the costs of engaging the private sector were analysed to get a better understanding of the economics underpinning outsourcing vaccine logistics. The results from this analysis confirmed some of the theoretical benefits of outsourcing to the private sector. Yet, if the experience in the Western Cape can be deemed a successful one, there are several policy and practice implications that developing countries should be mindful of when considering engaging the private sector. While outsourcing can help improve the performance of the vaccine supply chain, it has the potential to do the reverse if done incorrectly. The findings and lessons learnt from the Western Cape experience can serve as a step towards understanding the role of the private sector in immunization supply chain and logistics systems for developing countries.
Keywords: Supply chain and logistics, Outsourcing, Private sector, Vaccine management, Costing, South Africa
1. Introduction
With few exceptions, immunization supply chains in developing countries continue to face chronic difficulties in providing uninterrupted availability of potent vaccines up to service delivery levels, and in the most efficient manner possible. Combined with the struggles to keep pace with an ever increasing number of vaccines to introduce, many government-managed systems remain crippled by inefficiencies [1], [2], [3], [4]. Recent WHO and UNICEF assessments in 65 low and lower-middle income countries have revealed that few countries meet minimum standards for effective vaccine storage, distribution, handling, and stock management [5], [6]. Without significant investments to upgrade government-run immunization supply chains that already tie up significant resources, the aspirations to strengthen routine immunization as a whole will remain quixotic at best. Another option to such large investments is for national government in developing countries to consider engaging the private sector to manage supply chain functions on their behalf. Supply chain theory postulates that, if managed correctly, the outsourcing of vaccine logistics to a 3rd party private sector logistics service provider can lead to a reduction in total delivery costs, better on-time delivery of vaccines, higher adherence to temperature thresholds, better vaccine management and handling practises, reduced inventory costs, and greater ability to increase volume and scale (volume flexibility) than a government managed supply chain system. While the theory suggests that outsourcing can be more cost-effective and lead to higher levels of service performance, there is limited evidence or published case studies in developing countries documenting the theory against the practice when it comes to vaccines.
This study aims to shed light on the value proposition of outsourcing immunization supply chain functions to the private sector by documenting the specific experience of the Western Cape Province of South Africa. Indeed, in 2005 the Western Cape decided to enter into a public private partnership with a private sector logistics service provider (LSP) known as the “Distribution Agreement” [7], [8], [9]. Enduring challenges of stock management, the lack of adequate cold chain storage facilities, and overall inefficiencies in the government run distribution system for vaccines, were the primary reason leading the Western Cape Department of Health (WCDH) to relegate certain supply chain functions previously managed “in-house”, to a 3rd party logistics service provider that had a comparative advantage in storing, handling and transporting vaccines at lower costs and at higher levels of service. For a fee levied on the value of the vaccines procured, the outsourced private sector LSP was responsible for guaranteeing the cold chain storage and management of vaccines for the province, and their effective transport from their provincial warehouse directly to health centres. On the basis of two key assessments conducted, the findings show that outsourcing resulted in a better vaccine management and handling practises, and higher adherence to temperature thresholds in the cold chain. In addition, the analysis of the cost of the outsourcing arrangement point to lower costs than those that would be incurred is the same logistics function were done “in-house” by the provincial government. Lastly, key lessons learnt and policy and practice implications were teased out. From this experience, it is hoped that these findings will serve as a step towards understanding the potential role of the private sector in immunization supply chain and logistics systems for developing countries.
2. Methods
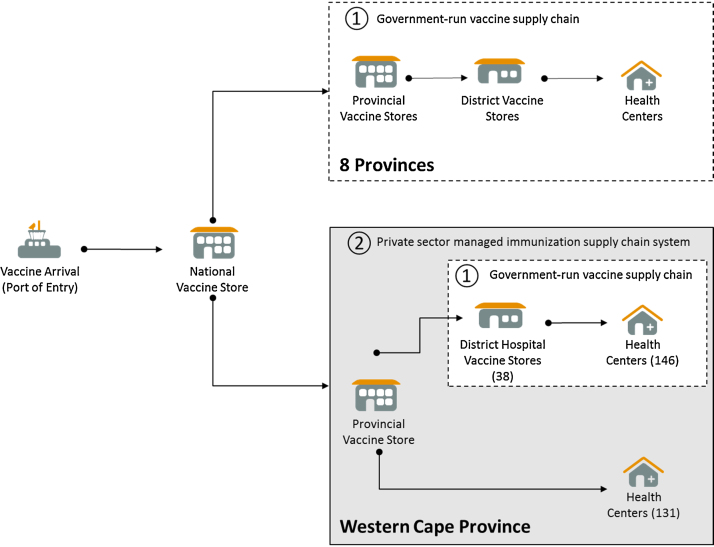
For this study, both qualitative and quantitative evidence was collect along the different vaccine supply chain segments in effect between the provincial vaccine store and health centres. One of the particularities was the presence of two types of supply chain segments that needed to be reviewed separately: a 2 tiered supply chain segment that was outsourced to the private sector LSP which covered the distribution of vaccines from provincial level to 47% of health centres in the province (or 131 Health Centres); and a 3 tiered supply chain segment where vaccines are managed by public sector district hospitals and where the district governments are responsible for storing and transporting vaccines the remaining 53% of health centres in the Western Cape Province (Fig. 1).
Fig. 1.
Schematic of the supply chain network specific of the Western Cape Province.
Data collection was carried out in both the 2 tiered (outsourced) and 3 tiered (government managed) supply chain segments during two key assessments. In addition to the provincial vaccine warehouse, a total of 10 sites were sampled for these two assessments, including 2 district hospitals and 8 health facilities. First, an EVM assessment was carried out using the standardized methodology and tool developed by WHO in order to determine whether vaccine management practices are up to international norms and standards, and to diagnose overall strengths and weaknesses in the vaccine supply chain according to eight key dimensions: temperature control in the cold chain; sufficient cold chain storage capacity; quality of the infrastructure; efficiency in the maintenance systems for the infrastructure; stock management processes; vaccine distribution practices; compliance with vaccine management policies; and use of a logistics management information systems (LMIS) to track and trace vaccines [10]. The EVM methodology aggregates the results so that each dimension is given a score between 0% and 100% at the end of the assessment. For the supply chain system to be considered up to standard on a dimension, a score of 80% or more is required [10]. Second, an in-depth temperature monitoring assessment was conducted to determine whether vaccines were being kept in the recommended temperature ranges, at all times during storage and transport to ensure that their potency was not compromised by damaging temperature excursions. The WHO study protocol for temperature monitoring in the vaccine cold chain was the basis of the methodology [11]. The temperature of the cold chain for storage was monitored over an 8 month period at the provincial vaccine warehouse and the 10 sampled sites across the 2 and 3 tiered segments of the Western Cape immunization supply chain. In addition, the temperature was monitored over the same period during the transportation of vaccines along the corresponding distribution routes [12]. A total of 24 WHO pre-qualified temperature recorders (Log Tag TRIX-8) were used for logging temperatures every 15 min, and data was reviewed against the WHO benchmarks for temperature excursion events indicating that the potency of the vaccine may have been compromised: freeze alarm corresponding to reading below −0.5 °C for 60 min or more; and heat alarm corresponding to readings above 8.0 °C for 10 h or more.
Lastly, to determine whether the cost of outsourcing was economically viable an economic analysis using standardize methods developed by the WHO was conducted for two periods of 2005 and 2010 that represent the first year of the outsourcing arrangement and the year of this study [13]. Because it was not possible to conduct two economic analyses that would have allowed a direct comparison between the cost of the outsourced system against an estimate of what it would have cost the Western Cape Department of Health to store vaccine at provincial level and distribute these up to health centre, the costs of the outsourced system were compared to cost of logistics benchmarks from other studies [20], [23].
3. Results
3.1. Effective vaccine management
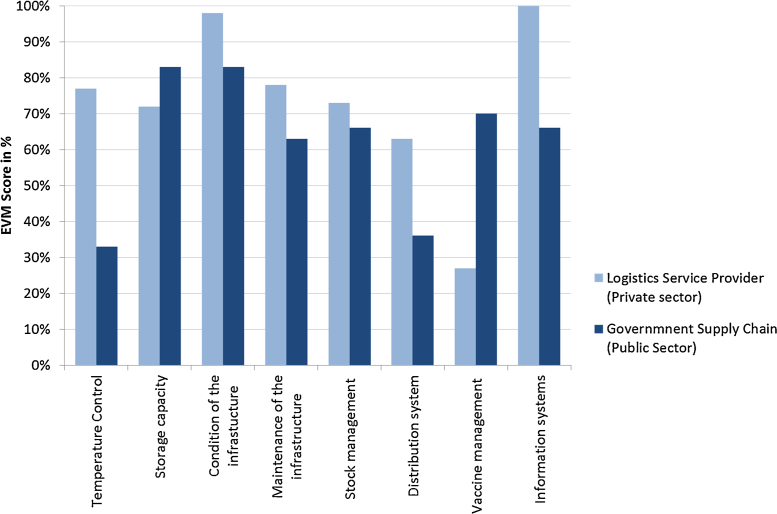
The effective vaccine management assessment results have highlighted the extent to which the outsourced logistics service provider outperformed the government run supply chain system on all relevant EVM dimensions [14]. The overall EVM score for the segments of the immunization supply chain covered by the outsourcing arrangement exceeded the 80% benchmark level when averaging across all 8 vaccine management dimensions. In contrast, this overall score was 63% for the supply chain segments managed by the government. The main differences in performance are most pronounced on the criteria for temperature (the lack of basic knowledge on managing temperatures for vaccines); distribution (due to the absence of frozen water-pack conditioning to prevent freezing vaccines during transport); shortcoming on the vaccine logistics information systems (the lack of clear procedures for forecasting and ordering vaccines, for recording key indicators like vaccine wastage and for monitoring temperature during transport) (Fig. 2).
Fig. 2.
Comparative performance levels on effective vaccine management.
It is noteworthy that the private sector LSP performed less well than the government-run segments of the vaccine supply chain for two EVM dimensions relating to sufficient cold chain storage capacity and compliance with vaccine management policies.
3.2. Temperature control
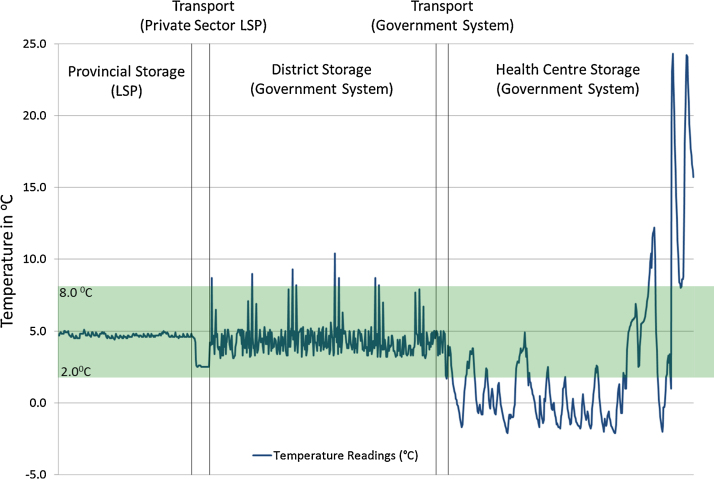
The findings on the temperature monitoring study confirmed the ability of the outsourced LSP in maintaining vaccines within WHO recommended temperature range of 2–8 °C during both storage and transport. Over the 8 month monitoring period, the temperature averaged 4.9 °C (4.4–5.6 °C range) for storage at the provincial level warehouse. No temperature alarms or excursions that would expose vaccines to damaging freezing or heat temperatures occurred. On the transport side, the findings show that in 85% of deliveries, vaccines were transported under good temperature control conditions. The reasons why the LSP did not achieve 100% was due to the occasional use of a 4th party courier services to transport vaccines to certain parts of the province where distances were large against a small order of vaccines. Since it was not cost-effective for the outsourced LSP to do the transportation themselves in these instances, they resorted to pay a courier service for delivering vaccines. This poor practise was to their detriment since their overall temperature performance was penalised by the inability of the courier service to maintain adequate cold chain temperatures during transport and vaccine were put at risk.
For the 3 tiered segment of the supply chain managed by the government, the system was unable to maintain the same level of temperature control during storage and transport. This is illustrated in Fig. 3 that represents the full temperature profile for the vaccines that reached health centres from provincial level via segments of the supply chain managed by the government. Maintaining adequate temperature in the cold chain was most challenging at health centre level. While the average temperature inside all the health centre fridges monitored averaged 3.1 °C over the 8 month period, the range of temperatures around this average was 0.9–9.2 °C, and suggests that temperature breaches occurred on both ends of the recommended 2.0–8.0 °C range. A deeper analysis indicated that in 30% of health centres refrigerators, vaccines were exposed to temperature below 0 °C and were freeze alarms were triggered. The main reason is the poor quality of the cold chain equipment provided for the immunization programme which has shown to be largely inadequate for storing vaccines [12], [15]. On the government run transport system, the findings indicated that vaccines were more at risk from heat exposure than if they were transported directly from the outsourced LSP although no heat damage had occurred during these temperature excursions [12].
Fig. 3.
Illustrative temperature profile for the segment of the vaccine supply chain managed by the government. (a) The x-axis is indicative of time as the vaccines move from the provincial warehouse to health centres refrigerators. The peaks and troughs in the temperature curve at health centre level reflect the poor performance of the vaccine refrigerators equipping government run health clinics and their inability to adequately maintain stable temperatures irrespective diurnal or seasonal changes in ambient temperatures over the 8 months study period for temperature monitoring.
3.3. Economic analysis
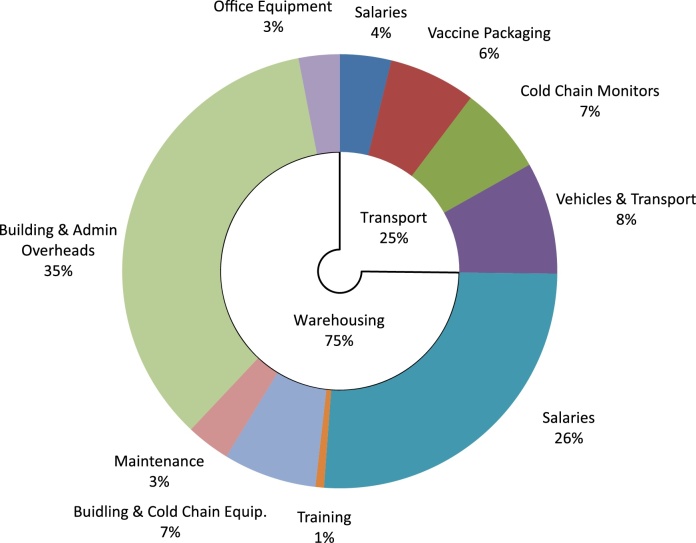
The costing analysis indicates that the total cost of the private sector LSP to perform outsourced services amounted to US$ 822,429 for the year 2010. These costs represented 5.7% of the overall procurement cost of vaccines and is slightly less than the 6.0% service fee charged to the government in the outsourcing contract. The warehousing costs for the vaccine is by far the largest cost component representing 75% of the overall costs to the LSP whereas the cost of transporting vaccines from province to health centres accounted for the remaining 25% (Fig. 4).
Fig. 4.
Cost breakdown for the outsourcing fee in 2010.
Since the start of the outsourcing arrangement in 2005, the costs to private sector LSP rose due to the significant increase in the volume of vaccines to manage (see Table 1). Between 2005 and 2010, the logistics cost per child experienced a five-fold increase from $1.3 to $7.9. Likewise, the logistics cost per dose administered rose by a factor of 10 from $0.06 to $0.71. These increases are the consequence of important investments that the private sector LSP needed to make to accommodate the 3 new vaccines introduced—the size of the provincial warehouse was expanded (77% increase in surface area); more staff were recruited to manage the growing workload (from 3 to 16 full-time equivalents) and the cost of warehousing overheads increased substantially (due rising cost of electricity) [16]. The combined effect of all these factors meant that their cost of doing business went from US$ 120,750 in 2005 to US$ 822,429 in 2010.
Table 1.
Evolution of key immunization supply chain cost indicators—2005 and 2010.
| 2005 | 2010 | |
|---|---|---|
| Vaccine turnover ($US) | US$ 2.0 million | US$ 14.5 million |
| Vaccine volume (cm3 per fully immunized child) | 46.2 cm3 | 5780.2 cm3 |
| LSP service fee | 6.0% | 6.0% |
| Public sector service fee (from the CMD)* | 5.0% | 5.0% |
| Cost to the LSP of providing the service ($US) | US$ 120,750 | US$ 822,429 |
| Per child under one year of age | $1.3 | $7.9 |
| Per dose administered | $0.061 | $0.706 |
| Per US$1000 worth of vaccines | $58.4 | $56.9 |
| % value of vaccines | 5.8% | 5.7% |
Represents the fee charged by the central medical depot in the Western Cape Province. This fee is charged to the Western Cape Department of Health for the provincial storage of non-cold chain pharmaceutical products and does not include any transportation to sub-provincials stores or health centres.
While the cost in value terms rose, the fee charged by the private sector LSP remained unchanged at 6% between 2005 and 2010. And relative to benchmark figures from other studies, the 6% fee charged by the private sector LSP is considered economical. Within the Western Cape province, one comparator is the 5% fee charged to the provincial government by the semi-private Central Medical Depot for the provincial storage of non-temperature sensitive pharmaceuticals. This fee does not include cold chain storage nor any transportation of these pharmaceuticals to sub-provincials stores or health centres win the Western Cape.
In benchmarking against what it would cost the government to manage the vaccine supply chain, various figures are of interest in presenting. A assessment of the vaccine supply chain and logistics system in Thailand indicate that the cost of the government managed system represents 31% of the value of vaccine procured [20]. This figure is consistent with another estimate of 28% based on the average cost of a government-run supply chain system for vaccines in Eastern and Southern Africa countries [24]. Lastly, the benchmark logistics cost for government managed health supply chains for none temperature sensitive pharmaceuticals have been shown to range anywhere between 13% and 44% of the procurement cost of the health commodity in many African countries [23].
4. Discussion
When reviewed against effective vaccine management, temperature and economic performance measures, the results from this outsourcing case study confirm some of the theoretical benefits of outsourcing vaccine supply chain functions to the private sector. Overall, the findings suggest that performance was greater for the 2 tiered segments of the supply chain that were outsourced (province to health centre), than the 3 tiered segments managed by the provincial government (province to district hospitals to health centre). Furthermore, the 6% fee charged by the private sector LSP is considered economical, particularly given that the fee remained unchanged after three new vaccines were introduced into the Western Cape Province in 2010—these significantly raised the value and volume of vaccines that the private sector LSP had to warehouse and distribute.
If the experience in the Western Cape can be deemed a successful one on the basis of performance measures assessed, there are several limitations to this case-study that need to be mentioned [16]. The first relates to the inability to make direct comparisons between the private and public sector performance measures in the Western Cape Province system. These required alternative approaches to juxtapose the two systems. For the EVM and temperature monitoring results, the comparisons were made by contrasting the measures for the segments of the supply chain covered by the outsourcing arrangement with those where the provincial government choose to manage vaccines themselves independently of the agreement—these segments of the supply chain are close but not identical. Arguably, the analysis would have been more robust if the comparator was another province in South Africa where the supply chain is entirely managed by the provincial government. For the economic analysis, the comparison of the 6% service charge for the outsourcing arrangement against other benchmark figures is a clear limitation. A direct comparison between the cost of the outsourced system against an estimate of what it would have cost the Western Cape Department of Health to perform the same logistics function “in-house” would have been more robust.
The second limitation is the inability to expand on the qualitative aspects of the outsourcing experience documented for the Western Cape that highlight a darker side to the outsourcing arrangement. Given the policy and practice implications of these qualitative aspects, it is important to summarize the five key shortcomings that are primarily attributable to: (i) the lack of management oversight of the contract by the WCDH; (ii) the weaknesses in the outsourcing contract itself with insufficiently detailed service level agreements; (iii) the lack of a monitoring and evaluation framework with key performance indicators; (iv) the decentralized and sluggish government clearance on financial transactions to purchase vaccines that resulted in important delays in payment to the LSP; and (v) the lack of a process to share critical planning information between both parties—particularly on vaccine forecasting. Unfortunately, all of these drawback were present in the Western Cape experience. While these shortcomings don’t undermine the theoretical benefits of outsourcing, they do speak to the importance of ensuring that the government provides ongoing contractual oversight and maintain a functional relationship with the private sector logistics service provider [16].
On the basis of the Western Cape outsourcing experience, there are clear lessons learned for developing countries. While outsourcing to the private sector can help improve the performance of the vaccine supply chain, the success of engaging the private sector will hinge on ensuring that: (i) all stakeholders are consulted in advance of a decision to outsource vaccine logistics to discuss the possible benefits and constraints, especially at the lowest levels (district and health centre levels); (ii) important supply chain performance assessments of the government managed vaccine supply chain are conducted upfront to ensure that the right choice of what supply chain functions to outsource; (iii) the costs of outsourcing are conducted and compared with the cost of keeping the functions “in house”; and (iv) the right private sector 3rd party LSP is selected and a strong contract is established with a sufficiently detailed service level agreement; a strong monitoring and evaluation framework with key performance indicators; and a robust process within the government health authorities to oversee and manage the outsourcing contract.
Even if our understanding of how to couple private sector outsourcing to a government managed supply chain system for vaccines remains limited for developing country settings, the review of the Western Cape Province experience provides an incremental step towards filling the knowledge gap. The lessons learned from this case study highlight many considerations that should be carefully weighed before any decision is made by other countries to outsource to the private sector. Beyond recommending that more evidence on the benefits and challenges of outsourcing be generated, there is need to develop a framework and approach for countries on how to consider and select outsourcing opportunities as a means of improving public sector vaccine supply chain performance [17]. In addition, there is a need to develop guidance and tools for analysing the potential cost and performance of these outsourcing opportunities that can support the design and assessment of an outsourcing options [18].
While outsourcing has enormous potential to increase supply chain performance of an existing system when done correctly [19], [20], it is not a panacea. Venturing down this path will require new sets of skills and will come with a host of new sets of challenges [21]. If the trend continues and more and more developing country governments consider engaging the private sector in vaccine supply chains [22], it will be imperative to bridge the knowledge gaps to ensure countries can be adequately advised and supported so that benefits of outsourcing in the setting can be leveraged effectively.
Acknowledgements
This review would not have been possible without the support from the Private Sector Logistics Service Provider, their administration and management, the Western Cape Department of Health, the District Health Systems Management, the Health Programme Management, Pharmaceutical Services, all the District Managers, Facility Managers and the District Pharmacists. The authors would like to pay special thanks to the following people whose support for this study has been invaluable: Carl Raubenheimer, Venesha Shunmugam, Hanif Parker, Velasco Voigt, Naomi Wasserman, and Sister Lisa.
References
- 1.Kaufman J.R., Miller R., Cheyne J. Vaccine supply chains need to be better funded and strengthened or lives will be at risk. Health Aff. 2011;30(6):1113–1121. doi: 10.1377/hlthaff.2011.0368. [DOI] [PubMed] [Google Scholar]
- 2.Humphreys G. Vaccination: rattling the supply chain. Bull World Health Organ. 2011;89:324–325. doi: 10.2471/BLT.11.030511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sabot O., Yadav P., Zaffran M. National Bureau of Asian Research; Seattle: 2011. Maximizing every dose and dollar: the imperative of efficiency in vaccine delivery. [Google Scholar]
- 4.Zaffran M., Vandelaer J., Kristensen D., Melgaard B., Yadav P., Antwi-Agyei K.O. The imperative for stronger vaccine supply and logistics systems. Vaccine. 2013;31(Suppl. 2):B73–B80. doi: 10.1016/j.vaccine.2012.11.036. [DOI] [PubMed] [Google Scholar]
- 5.Garnett A. Optimize project landscape analysis report. WHO; 2008. Analysis of vaccine management assessments (VMA) indicators in 22 countries. (October, unpublished WHO report) [Google Scholar]
- 6.Colrain P. Final report. WHO; 2011. Analysis of effective vaccine management (EVM) assessment results and improvement plans in 65 low and lower-middle income countries. (September, unpublished WHO report) [Google Scholar]
- 7.Western Cape Department of Health . Department of Health of South Africa; 2005. Memorandum of agreement for vaccine warehousing and distribution with the [private sector logistics service provider] [Google Scholar]
- 8.Western Cape Department of Health . Department of Health of South Africa; 2009. Supply and distribution agreement between the Western Cape Provincial Depart of Health and the [private sector logistics service provider] (unpublished document) [Google Scholar]
- 9.Government of South Africa . Department of Health of South Africa; 2003. Final supply agreement between the National Department of Health and the [private sector logistics service provider] (unpublished document) [Google Scholar]
- 10.World Health Organization, (WHO) Immunization, Vaccine and Biologicals Department (IVB); 2010. Effective vaccine management initiative. [Google Scholar]
- 11.World Health Organization, (WHO) Immunization, Vaccine and Biologicals Department (IVB); 2005. Study protocol for temperature monitoring in the vaccine cold chain. (with revision in October 2011 WHO/IVB/05.01) [Google Scholar]
- 12.Raubenheimer T. Collaborative Center for Cold Chain Management (CCCCM); 2010. Temperature monitoring study in the Western Cape. (unpublished WHO report for project Optimize) [Google Scholar]
- 13.World Health Organization, (WHO) Immunization, Vaccine and Biologicals Department (IVB); 2008. WHO guide for standardization of economic evaluations of immunization programmes. (WHO/IVB/08.14) [Google Scholar]
- 14.Raubenheimer T. Collaborative Center for Cold Chain Management (CCCCM); 2011. Effective vaccine management in the Western Cape. (unpublished WHO report for project Optimize) [Google Scholar]
- 15.World Health Organization (WHO) World Health Organization (WHO); 2011. Report of the post-introduction evaluation of new vaccines in South Africa. (July, unpublished report) [Google Scholar]
- 16.World Health Organization, (WHO). The Western Cape Experience in South Africa. WHO; Geneva: 2011. Outsourcing the vaccine supply chain and logistics. System to the private sector. [Google Scholar]
- 17.Watson N., Forster G., Hasselback L. MIT-International Zaragoza Logistics Programme at Zaragoza Logistics Center (ZLC); Zaragoza, Spain: 2011. Framework on distribution outsourcing in government-run distribution systems. [Google Scholar]
- 18.Private Sector Engagement . Technical report. United-Nations Commission on Life-Saving Commodities; 2014. A guidance document for health supply chains in the modern context. (February) [Google Scholar]
- 19.Copeland Michael. Smarter medicine. 52. Strategy & Business; 2008. How the US centers for disease control and prevention revolutionized the way vaccines are delivered. (Autumn) [Google Scholar]
- 20.PATH, World Health Organization (WHO), Health Systems Research Institute, Mahidol University . PATH; Seattle: 2011. An assessment of vaccine supply chain and logistics systems in Thailand. [Google Scholar]
- 21.Emerging Trends in Supply Chain Management . Emerging Trends in Supply Chain Management; 2010. Outsourcing public health logistics in developing countries. (July, USAID | DELIVER PROJECT) [Google Scholar]
- 22.Private Sector Role in Health Supply Chains . Technical partner paper 13. Rockefeller Foundation, Dalberg and MIT-Zaragoza; 2008. Review of the role and potential for private sector engagement in developing country health supply chains. [Google Scholar]
- 23.Sarley David, Linda Allain, Anup Akkihal. Technical Brief; 2009. Estimating the global in-country supply chain costs of meeting the MDGs by 2015. (July, USAID | DELIVER PROJECT) [Google Scholar]
- 24.PATH . PATH; 2012. Outsourcing vaccine supply chain and logistics to the private, sector. Evidence brief series. Project optimize. (September) [Google Scholar]