Abstract
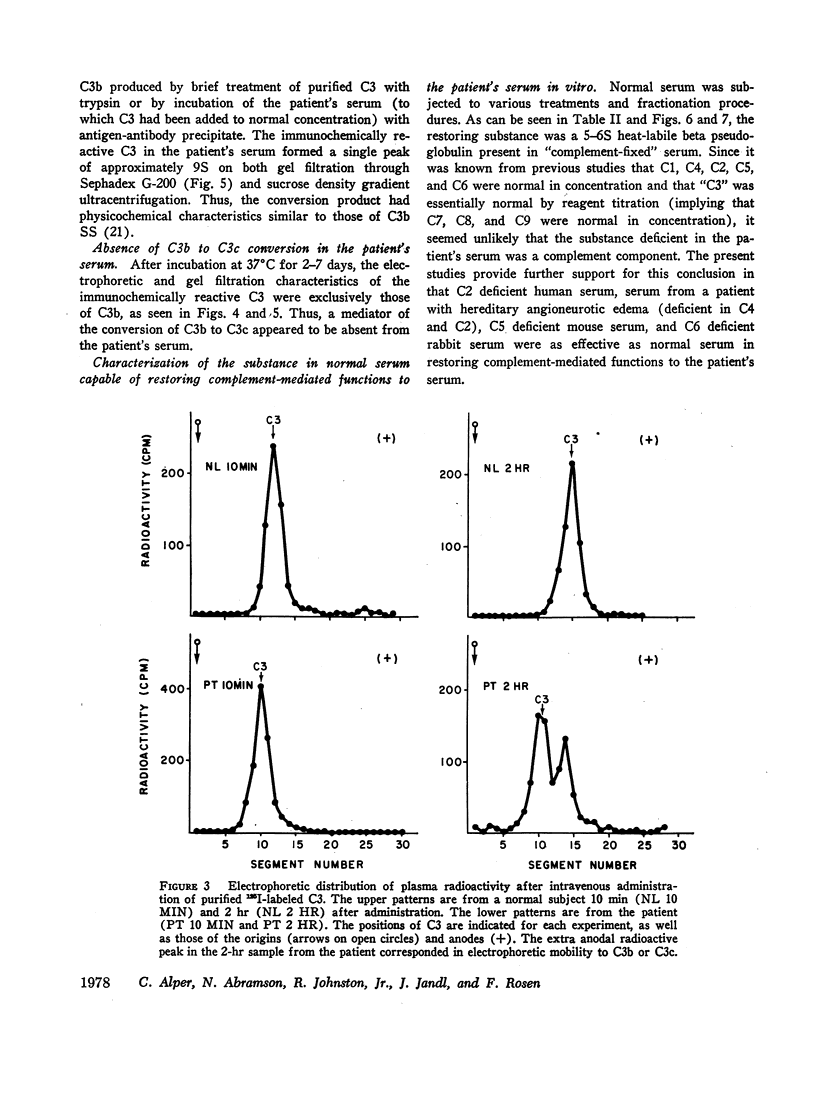
In a patient with increased susceptibility to infection, lowered serum C3 concentration, and continuously circulating C3b, it was shown that purified 125I-labeled C3 was converted to labeled C3b shortly after intravenous administration. The fractional catabolic rate of C3 was approximately five times normal at 10% of the plasma pool per hr. The synthesis rate and pool distribution of C3 were normal. Despite this evidence of C3 instability in vivo, no accelerated inactivation of C3 was found in vitro. Similarly, no free proteolytic activity could be detected in the patient's serum, and serum concentrations of known protease inhibitors were normal.
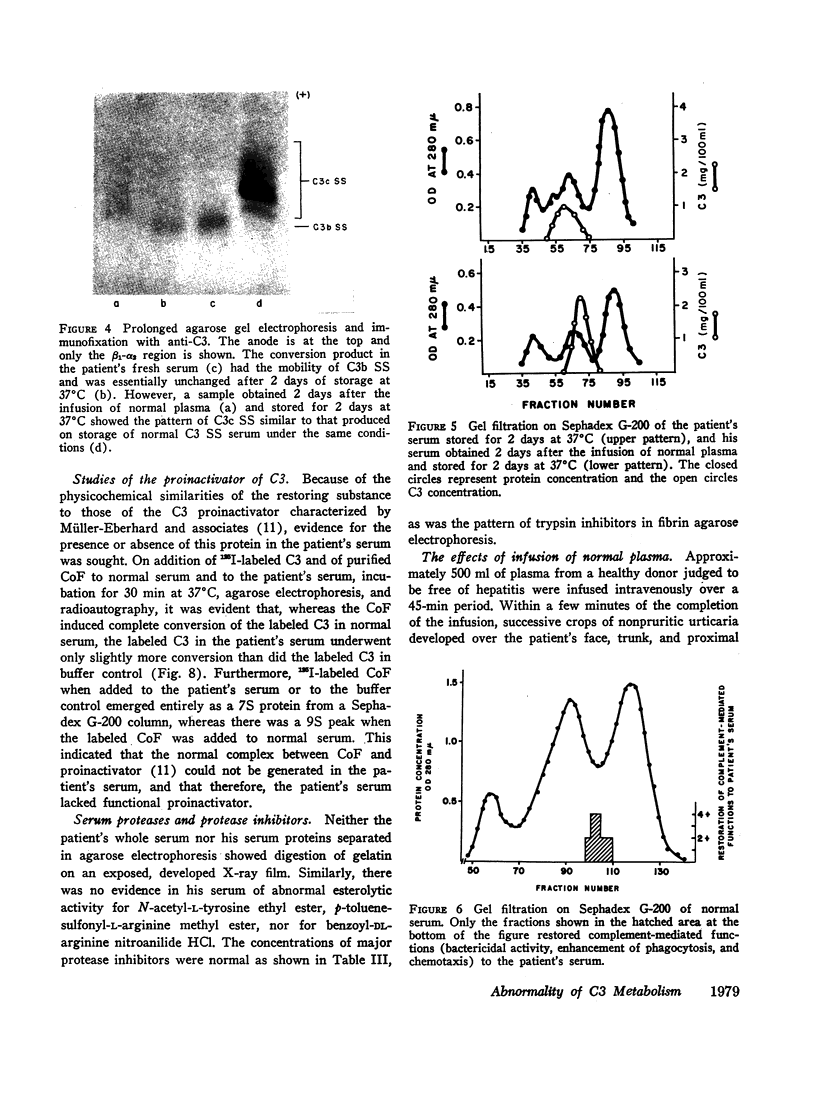
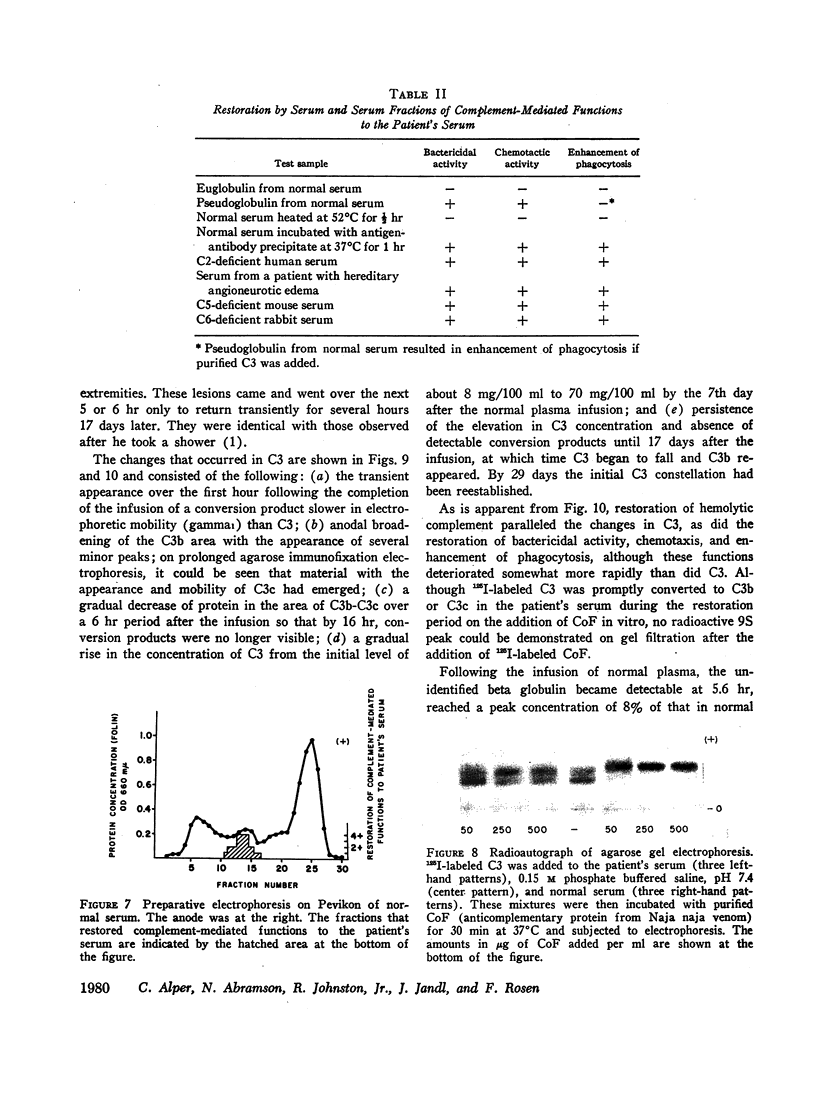
Complement-mediated functions, which were markedly deficient in the patient's serum, could be restored partially or completely by the addition of a 5-6S heat-labile beta pseudoglobulin from normal serum. The C3 proinactivator, which has these physicochemical characteristics, was also shown to be either absent or nonfunctional in the patient's serum. An unidentified 6S beta pseudoglobulin to which a monospecific antiserum was available was not detectable in the patient's serum. This last protein appeared not to be a complement component, nor was it the C3 inactivator or proinactivator. Finally, the substance or substances necessary for the conversion of C3b to C3c were missing from the patient's serum.
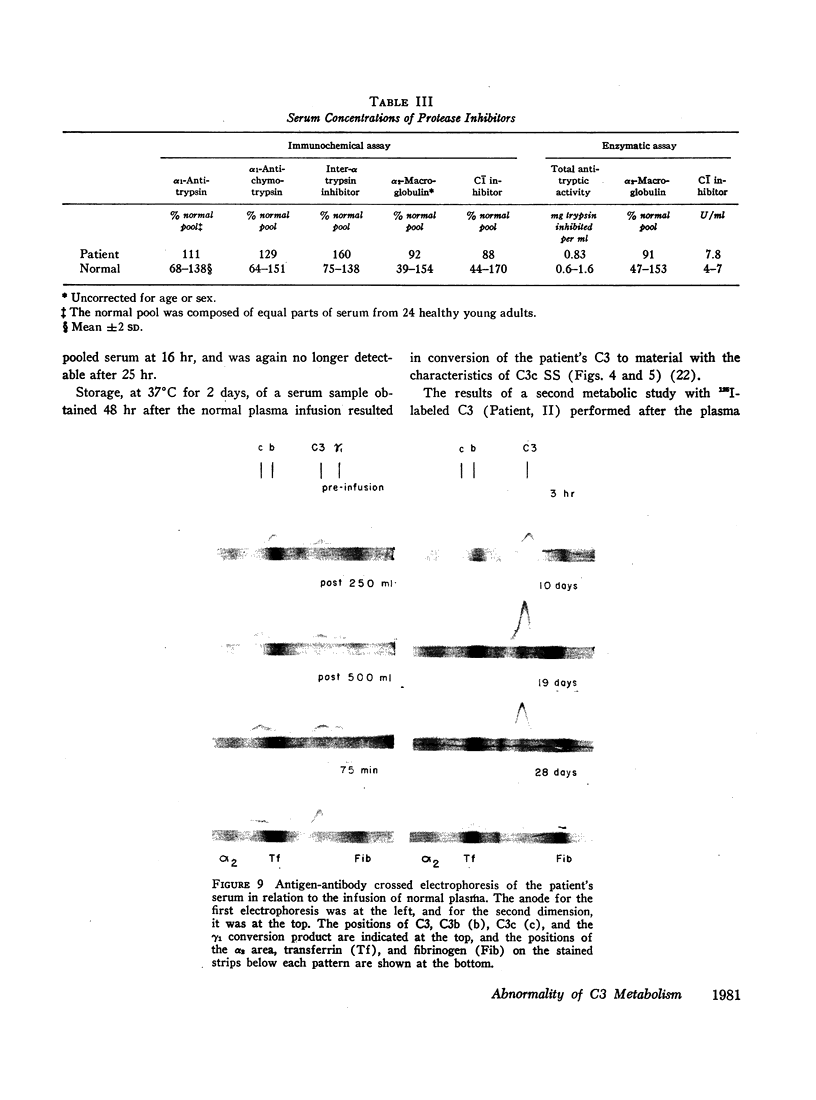
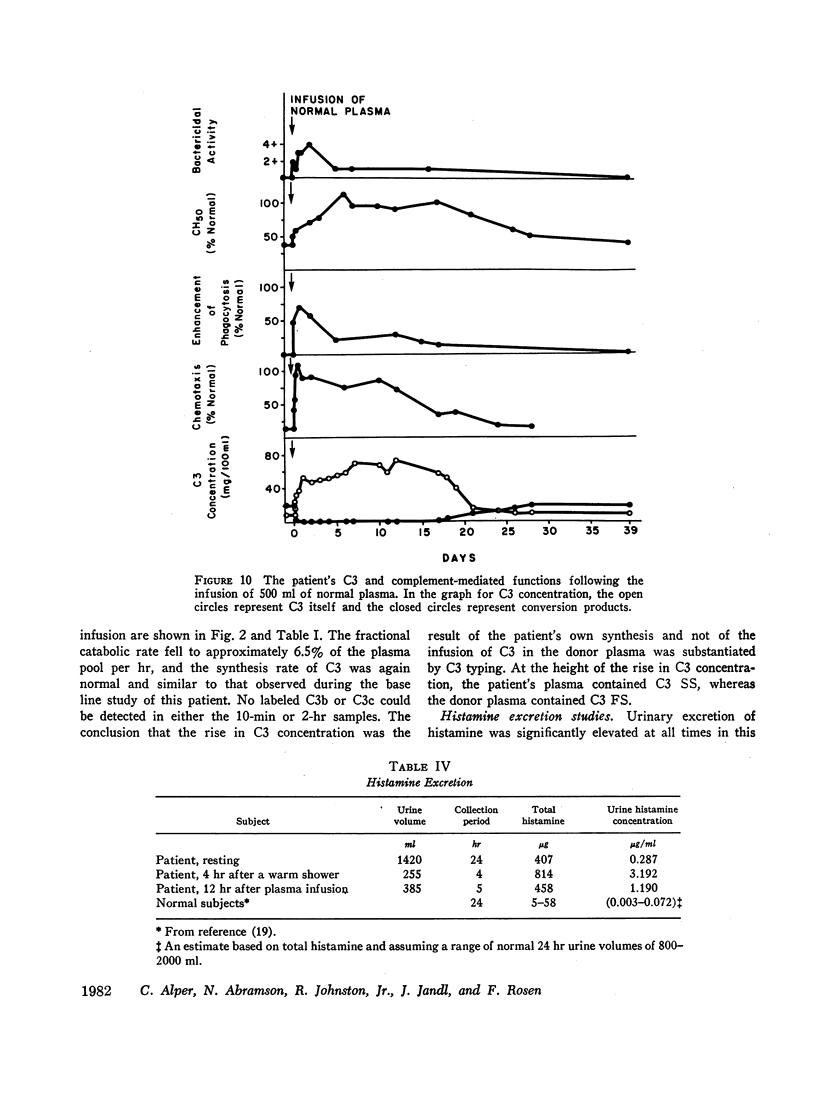
The administration of 500 ml of normal plasma to the patient corrected all of his abnormalities partially or completely for as long as 17 days. The changes in C3 were dramatic; serum concentration rose from 8 to 70 mg/100 ml, and C3b could no longer be detected. A second metabolic study during this normalization period showed a decrease in fractional catabolic rate toward normal.
The patient's histamine excretion was constantly elevated but increased further after a warm shower and after receiving normal plasma; at both times he had urticaria. These observations were consistent with the endogenous production of C3a and the resulting histamine release from mast cells. The inactivating mechanism for C3a was apparently intact in the patient's serum.
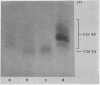
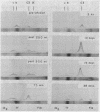
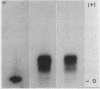
The difference in the electrophoretic mobilities of C3b and C3c was shown as well as the electrophoretic heterogeneity of C3c. Suggestive evidence was also presented that the form of C3 with an activated combining site for red cells, previously postulated by others, is a transient C3 conversion product with an electrophoretic mobility slower than that of C3 on agarose electrophoresis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper C. A., Johnson A. M. Immunofixation electrophoresis: a technique for the study of protein polymorphism. Vox Sang. 1969 Nov;17(5):445–452. doi: 10.1111/j.1423-0410.1969.tb00414.x. [DOI] [PubMed] [Google Scholar]
- Alper C. A., Propp R. P. Genetic polymorphism of the third component of human complement (C'3). J Clin Invest. 1968 Sep;47(9):2181–2191. doi: 10.1172/JCI105904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper C. A., Rosen F. S. Alper CA, Rosen FS: Studies of the in vivo behavior of human C'3 in normal subjects and patients. J Clin Invest. 1967 Dec;46(12):2021–2034. doi: 10.1172/JCI105691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLOMBACK M., JORPES J. E., NILSSON I. M. von Willebrand's disease. Am J Med. 1963 Feb;34:236–241. doi: 10.1016/0002-9343(63)90057-3. [DOI] [PubMed] [Google Scholar]
- Boenisch T., Alper C. A. Isolation and properties of a glycine-rich gamma-glycoprotein of human serum. Biochim Biophys Acta. 1970 Jul 27;214(1):135–140. doi: 10.1016/0005-2795(70)90077-2. [DOI] [PubMed] [Google Scholar]
- DONALDSON V. H., EVANS R. R. A BIOCHEMICAL ABNORMALITY IN HEREDIATRY ANGIONEUROTIC EDEMA: ABSENCE OF SERUM INHIBITOR OF C' 1-ESTERASE. Am J Med. 1963 Jul;35:37–44. doi: 10.1016/0002-9343(63)90162-1. [DOI] [PubMed] [Google Scholar]
- DONALDSON V. H., ROSEN F. S. ACTION OF COMPLEMENT IN HEREDITARY ANGIONEUROTIC EDEMA: THE ROLE OF C'1-ESTERASE. J Clin Invest. 1964 Nov;43:2204–2213. doi: 10.1172/JCI105094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias Da Silva W., Lepow I. H. Complement as a mediator of inflammation. II. Biological properties of anaphylatoxin prepared with purified components of human complement. J Exp Med. 1967 May 1;125(5):921–946. doi: 10.1084/jem.125.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERLANGER B. F., KOKOWSKY N., COHEN W. The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys. 1961 Nov;95:271–278. doi: 10.1016/0003-9861(61)90145-x. [DOI] [PubMed] [Google Scholar]
- Ganrot P. O. Determination of alpha-2-macroglobulin as trypsin-protein esterase. Clin Chim Acta. 1966 Oct;14(4):493–501. doi: 10.1016/0009-8981(66)90037-4. [DOI] [PubMed] [Google Scholar]
- Huff J. A., Davis V. E., Brown H. A simplified technique for the estimation of histamine in blood and urine. J Lab Clin Med. 1966 Jun;67(6):1044–1047. [PubMed] [Google Scholar]
- Johnston R. B., Jr, Klemperer M. R., Alper C. A., Rosen F. S. The enhancement of bacterial phagocytosis by serum. The role of complement components and two cofactors. J Exp Med. 1969 Jun 1;129(6):1275–1290. doi: 10.1084/jem.129.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAURELL C. B. ANTIGEN-ANTIBODY CROSSED ELECTROPHORESIS. Anal Biochem. 1965 Feb;10:358–361. doi: 10.1016/0003-2697(65)90278-2. [DOI] [PubMed] [Google Scholar]
- LEVY L. R., LEPOW I. H. Assay and properties of serum inhibitor of C'l-esterase. Proc Soc Exp Biol Med. 1959 Aug-Sep;101:608–611. doi: 10.3181/00379727-101-25034. [DOI] [PubMed] [Google Scholar]
- LUNDH B. THE EFFECT OF INCUBATION WITH HYDRAZINE AND IMMUNOPRECIPITATE ON BETA-1C-GLOBULIN. Acta Pathol Microbiol Scand. 1965;63:266–274. doi: 10.1111/apm.1965.63.2.266. [DOI] [PubMed] [Google Scholar]
- Lachmann P. J., Müller-Eberhard H. J. The demonstration in human serum of "conglutinogen-activating factor" and its effect on the third component of complement. J Immunol. 1968 Apr;100(4):691–698. [PubMed] [Google Scholar]
- Laurell C. B., Niléhn J. E. A new type of inherited serum albumin anomaly. J Clin Invest. 1966 Dec;45(12):1935–1945. doi: 10.1172/JCI105498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Lundh B., Laurell C. B. Charge and size of the conversion products of serum beta-1C-globulin. Immunology. 1967 Dec;13(6):649–655. [PMC free article] [PubMed] [Google Scholar]
- MOLLER B., KRISTENSEN H. S. ON THE TREATMENT OF TETANUS. Acta Med Scand. 1965 Jan;177:1–6. doi: 10.1111/j.0954-6820.1965.tb01796.x. [DOI] [PubMed] [Google Scholar]
- MULLER-EBERHARD H. J. A new supporting medium for preparative electrophoresis. Scand J Clin Lab Invest. 1960;12:33–37. [PubMed] [Google Scholar]
- Muller-Eberhard H. J., Nilsson U. R., Dalmasso A. P., Polley M. J., Calcott M. A. A molecular concept of immune cytolysis. Arch Pathol. 1966 Sep;82(3):205–217. [PubMed] [Google Scholar]
- Müllerèberhard H. J., Dalmasso A. P., Calcott M. A. The reaction mechanism of beta-1C-globulin (C'3) in immune hemolysis. J Exp Med. 1966 Jan 1;123(1):33–54. doi: 10.1084/jem.123.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NILSSON I. M., BLOMBACK M., BLOMBACK B. v. Willebrand's disease in Sweden; its pathogenesis and treatment. Acta Med Scand. 1959 Jun 30;164:263–278. [PubMed] [Google Scholar]
- NILSSON U. R., MUELLER-EBERHARD H. J. ISOLATION OF BETA IF-GLOBULIN FROM HUMAN SERUM AND ITS CHARACTERIZATION AS THE FIFTH COMPONENT OF COMPLEMENT. J Exp Med. 1965 Aug 1;122:277–298. doi: 10.1084/jem.122.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura N., Nelson R. A., Jr Three naturally-occurring inhibitors of components of complement in guinea pig and rabbit serum. J Immunol. 1967 Sep;99(3):582–589. [PubMed] [Google Scholar]
- da Silva W. D., Eisele J. W., Lepow I. H. Complement as a mediator of inflammation. 3. Purification of the activity with anaphylatoxin properties generated by interaction of the first four components of complement and its identification as a cleavage product of C'3. J Exp Med. 1967 Dec 1;126(6):1027–1048. doi: 10.1084/jem.126.6.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]