Abstract
Objective Report routes of skull base invasion for head and neck nonmelanoma skin cancers (NMSCs) and their survival outcomes.
Design Retrospective.
Participants Ninety patients with NMSC with skull base invasion between 2004 and 2014.
Major Outcome Measures Demographic, tumor characteristics, and treatments associated with different types of skull base invasion and disease-specific survival (DSS) and overall survival (OS).
Results Perineural invasion (PNI) to the skull base occurred in 69% of patients, whereas 38% had direct skull base invasion. Age, histology, orbital invasion, active immunosuppression, cranial nerve (CN) involved, and type of skull base invasion were significantly associated with DSS and OS (p < 0.05). Patients with basal cell carcinoma (BCC) had significantly improved DSS and OS compared with other histologies (p < 0.05). Patients with CN V PNI had significantly improved DSS and OS compared with CN VII PNI (p < 0.05). Patients with zone II PNI had significantly improved DSS and OS compared with those with direct invasion or zone III PNI (p < 0.05). Nonsurgical therapy was rarely used and is associated with a reduction in DSS and OS (p < 0.05).
Conclusion Patterns and survival outcomes for NMSC skull base invasion are reported. Zone II PNI, BCC, and CN V PNI are associated with improved survival outcomes.
Keywords: skin cancer, skull base invasion, direct invasion, intracranial invasion, risk factors, survival outcomes, perineural spread
Introduction
Squamous cell carcinoma and basal cell carcinoma (BCC) comprise the vast majority of nonmelanoma skin cancers (NMSCs) that occur in the head and neck region.1 Sun exposure and genetic factors play a major role in tumor pathogenesis.2 3 Several factors have been identified as markers of poor prognosis for head and neck skin cancers: primary tumor size, primary tumor location, family history of skin cancers, multiple skin cancers, and immunosuppression.4 In addition, patients who develop skull base invasion have a more severe disease with increased morbidity and mortality; however, this clinical entity is not well defined in the literature.
Skull base invasion for head and neck NMSC can occur by several routes, particularly direct invasion through the underlying soft tissue and by perineural spread (PNS) through the motor or sensory branches of cranial nerves (CNs). PNS plays an important role in extension of disease to the skull base and the type of CN involved determines the route of spread.5 Approximately 2% of all cutaneous BCCs and 3% of all squamous cell carcinomas in the head and neck region develop PNS. PNS makes complete surgical resection very challenging and has been defined as a marker of poor prognosis.6 Congenital fissure lines, orbital structures, and blood vessels are other potential routes for tumors to directly invade the skull base.
Unfortunately, when skull base invasion occurs, treatment can be morbid due to the close proximity of the orbit, CNs, and intracranial structures. In addition, many authors consider cases inoperable if there is obvious intracranial extension.7 Meticulous surgical resection with adjuvant radiation is the mainstay of treatment in cases of NMSC skull base invasion. Salvage treatment modalities are often unsuccessful if the primary treatment fails, which makes early diagnosis of skull base invasion crucial and potentially lifesaving to prevent intracranial extension.
Routes of PNS have been defined using the zonal classification illustrated in Table 1.8 9 The California staging system was used to classify the patterns of direct invasion according to the depth of invasion and the necessary surgery required for complete resection, which is summarized in Table 2.10 Skull base invasion patterns for NMSC are not well studied; particularly, correlating the type of skull base invasion with survival outcomes has not been demonstrated. Our goal is to further define routes of skull base invasion for NMSC, identify risk factors that are essential for early diagnosis and treatment, and describe how survival outcomes correlate with type of skull base invasion, as well as other patient, tumor, and treatment factors.
Table 1. The zonal classification of perineural invasion based on the anatomy of the cranial nerves8 9 .
| Area of perineural invasion | Anatomic subsites | |
|---|---|---|
| Zone I | Peripheral cranial nerves | Peripheral cranial nerve involvement |
| Zone II | Skull base | V: Tumor between the superior orbital fissure, foramen rotundum, or foramen ovale
and the trigeminal ganglion VII: Tumor between the stylomastoid foramen and the geniculate ganglion |
| Zone III | Intracranial extension | Cisternal involvement between the cranial nerve ganglion and the brain stem |
Table 2. California staging system for classification of direct skull invasion for skin cancers.
| Superficial | Tumor invasion is between the skin and the skull or skull base periosteum. |
| Intermediate | Tumor invasion involves the bone of the skull or skull base. |
| Deep | Tumor invasion extends through the bone of the skull or skull base and involves the dura or brain parenchyma. |
Materials and Methods
With institutional review board approval, we searched our database for patients with NMSC in the head and neck region and radiographic evidence of skull base invasion. The medical records of 90 consecutive patients treated between 2004 and 2014 at our tertiary care institution were reviewed retrospectively. Patient demographics and clinical data were collected, including sex, age, comorbid diseases, characteristics of the primary tumor, previous oncologic treatment modalities, skull base invasion pattern, and disease-specific survival (DSS) and overall survival (OS) rates. Treatment recommendations for each patient were determined at the multidisciplinary Head and Neck Oncology Treatment Planning Conference.
The pattern of skull base invasion for each patient was classified on the basis of computed tomography (CT) and magnetic resonance imaging (MRI) results. There were two invasion pathways to the skull base: PNS and direct invasion. The zonal classification system was used to grade PNS to the skull base, and by definition, the cases involved in this study had either zone II or III disease. In addition, each case of skull base invasion was categorized based on the CN primarily responsible for the invasion and cases with multiple CNs involved were identified. The California staging system was used to classify the patterns of direct skull base, and by definition, the cases involved in this study had intermediate and deep disease.
Treatments received were stratified into surgical resection alone, definitive chemoradiation, and combined surgical resection with adjuvant radiation. The surgical resection was completed after careful pre- and intraoperative assessments of the extent of disease, evaluating affected nerves, ganglions, and the bony skull base, including intraoperative frozen section analysis, with the goal of surgery to achieve negative final margins. Depending on the extent of disease, the surgical resection was performed either through a transfacial approach or combined head and neck/neurosurgery approach with a craniofacial resection.
Statistical analysis was performed using XLSTAT (version 2015.6.01, Addinsoft, United States). Descriptive statistics were performed by independent t-tests and the Mann-Whiney U-test for mean comparisons of variables with two groupings. For variables with groupings of three or more a one-way ANOVA (analysis of variance) test was utilized. Chi-squared and Fisher's exact tests were used to analyze categorical variables. Univariate survival estimates were generated by the Kaplan-Meier method and compared with the log-rank test. Mulitvariate survival analysis was performed using the Cox proportional hazard regression model, utilizing all variables approaching significance (p ≤ 0.2) to control for confounding covariates. All tests were two-tailed, and results were considered significant for p ≤ 0.05.
Results
There were 90 patients who met the inclusion criteria. The mean follow-up time was 36 months. Patient demographics, tumor characteristics, and treatment groups are summarized in Table 3. The average patient age at skull base invasion diagnosis was 72 years old (range: 41–98). Seventy-two (80%) patients were male and 18 (20%) were female. The vast majority of patients had recurrent tumors (82%). Sixteen (16%) patients had a history of previous radiation treatment. Eighteen (20%) patients were actively taking immunosuppressive medications at the time of diagnosis because of transplantation or a rheumatologic disease. Sixty-eight (75%) primary tumor were squamous cell carcinomas, 15 (16%) were BCCs, and 7 (7%) were categorized as other histologies. Sixty-nine (77%) patients underwent surgery followed by adjuvant radiation therapy, 15 (17%) underwent surgical resection without adjuvant radiation treatment, and 6 (7%) underwent definitive chemoradiation treatment.
Table 3. Patient demographics, tumor characteristics, and treatment groups.
| Patient and tumor factors | Number of patients (% of total) (n = 90) | |
|---|---|---|
| Sex | Male | 72 (80%) |
| Female | 18 (20%) | |
| Primary subsite | Preauricular | 15 (17%) |
| Scalp | 15 (17%) | |
| Temple | 11 (12%) | |
| Forehead | 10 (11%) | |
| Cheek | 9 (10%) | |
| Ear | 8 (9%) | |
| Eyebrow | 8 (9%) | |
| Postauricular | 5 (6%) | |
| Nose | 4 (4%) | |
| Midface | 3 (3%) | |
| Lip | 1 (1%) | |
| Neck | 1 (1%) | |
| Histology | Squamous cell carcinoma | 68 (76%) |
| Basal cell carcinoma | 15 (17%) | |
| Other | 7 (8%) | |
| Prior treatments | Surgery | 74 (82%) |
| Radiation | 15 (17%) | |
| Immunosuppression | Yes | 18 (20%) |
| No | 72 (80%) | |
| Orbital invasion | Yes | 25 (28%) |
| No | 65 (72%) | |
| Treatment groups | Surgery alone | 15 (17%) |
| Radiation alone | 6 (7%) | |
| Surgery and radiation | 69 (77%) | |
| Complications | 30-d mortality | 2 (2%) |
| Stroke | 1 (1%) | |
| Myocardial infarction | 2 (2%) | |
| CSF leak | 5 (6%) | |
| Reconstructive flap failure | 2 (2%) | |
| Hematoma | 4 (4%) | |
Abbreviation: CSF, cerebrospinal fluid.
Routes of skull base PNS are summarized in Table 4. Thirty-four (38%) patients had direct skull invasion, with 27 intermediate-stage and 7 deep-stage invasion. Sixty-two (69%) patients had skull base invasion via PNS, 39 (63%) had zone I invasion and 23 patients (37%) had zone III invasion. Figs. 1, 2 illustrate typical cases with zone III involvement that were treated surgically. Forty-one (66%) patients had skull base PNS on CN V, whereas 21 (34%) had skull base PNS on CN VII. Fourteen (23%) patients had both CN V and CN VII involvement peripherally, in zone I, but no patient had skull base PNS on both CNs. Six patients had direct skull invasion in addition to skull base PNS. For patients who underwent a surgical resection with zone II PNS, 68% of patients underwent a transfacial resection and 32% underwent a craniofacial resection. For patients who underwent a surgical resection with zone III PNS, 16% of patients underwent a transfacial resection and 84% underwent a craniofacial resection. On final pathology, 21% of patients had a central microscopic positive margin on the nerve resection for PNS, and this was managed with a boost to the adjuvant radiation delivered to this area.
Table 4. Description of the routes of skull base perineural spread.
| Route of perineural spread | Number of patients (% of total) (n = 62) | |
|---|---|---|
| Zone II | Zone III | |
| Cranial nerve V (n = 41) | – | – |
| V1 | 10 (16%) | 3 (5%) |
| V2 | 10 (16%) | 5 (8%) |
| V3 | 7 (11%) | 6 (10%) |
| Cranial nerve VII (n = 21) | 12 (19%) | 9 (15%) |
| Total | 39 (63%) | 23 (37%) |
Fig. 1.
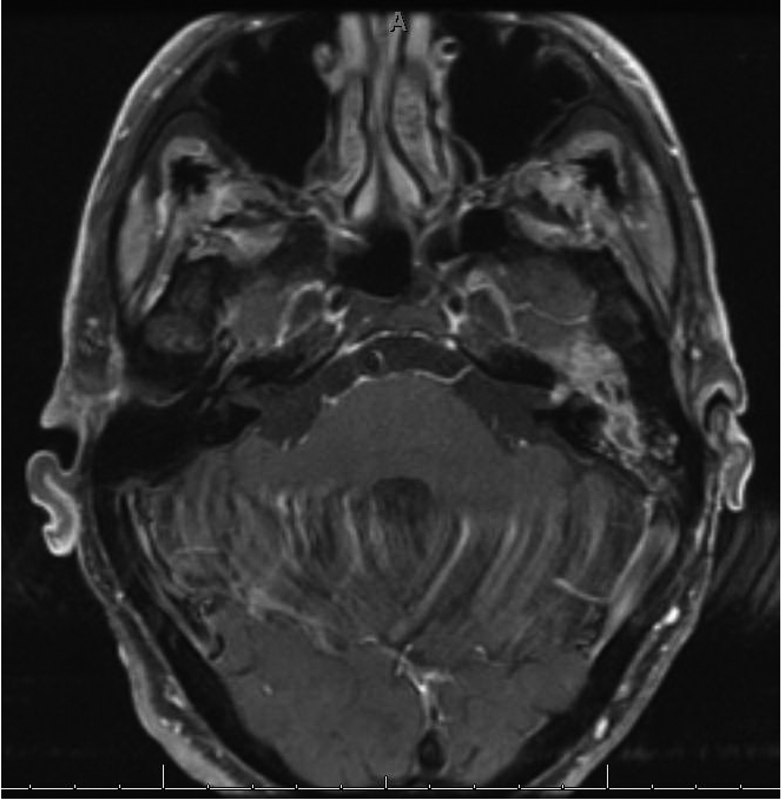
Axial T1 MRI postcontrast and fat saturated demonstrating enhancing tumor, squamous cell carcinoma, in the left mastoid tracking along the tympanic segment of the facial nerve, through the geniculate ganglion, and extending into the proximal IAC. This was resected through a translabyrinthine approach.
Fig. 2.
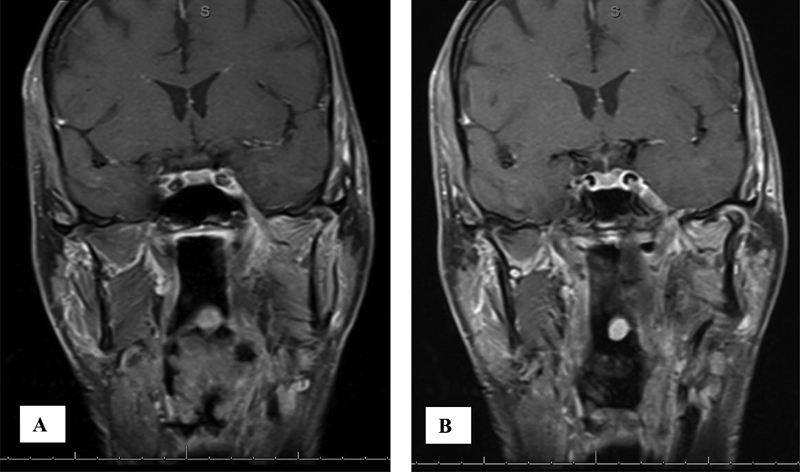
Coronal T1 MRI postcontrast and fat saturated demonstrating enhancing tumor proven to be squamous cell carcinoma (A) in the left infratemporal fossa tracking up V3 through foramen ovale and (B) extending intracranially into Meckel cave.
Mean DSS and OS were 79 and 56 months, respectively. DSS and OS at 2 years were 72% and 60%, respectively, and at 5 years 64% and 52%, respectively. Univariate analysis of OS of patient demographics, tumor characteristics, and treatment groups is summarized in Table 5. Sex, primary subsite, and type of previous treatment were not predictive of DSS or OS. Histology, active immunosuppression, orbital invasion, CN involved (V vs. VII), type of skull base invasion, and treatment group were significantly associated with DSS and OS (p < 0.05).
Table 5. Univariate analysis of mean disease-specific and overall survival (months) for patient demographics, tumor characteristics, and treatment groups (p < 0.05 in bold).
| Patient and tumor factors | Disease-specific survival (mo) | p Value | Overall survival (mo) | p Value | |
|---|---|---|---|---|---|
| Sex | Male | 65 | 0.487 | 55 | 0.624 |
| Female | 63 | 0.447 | 56 | 0.624 | |
| Primary subsite | Preauricular | 54 | 0.222 | 45 | 0.165 |
| Scalp | 69 | 0.454 | 60 | 0.697 | |
| Temple | 58 | 0.559 | 49 | 0.496 | |
| Forehead | 64 | 0.879 | 52 | 0.652 | |
| Cheek | 70 | 0.396 | 65 | 0.297 | |
| Ear | 60 | 0.497 | 51 | 0.369 | |
| Eyebrow | 68 | 0.584 | 62 | 0.586 | |
| Postauricular | 66 | 0.612 | 55 | 0.310 | |
| Nose | 60 | 0.333 | 50 | 0.586 | |
| Midface | 57 | 0.529 | 52 | 0.688 | |
| Lip | 70 | 0.686 | 72 | 0.724 | |
| Neck | 38 | 0.778 | 40 | 0.858 | |
| Histology | Squamous cell carcinoma | 49 | 0.018 | 42 | 0.039 |
| Basal cell carcinoma | 75 | 0.013 | 65 | 0.012 | |
| Other | 54 | 0.022 | 45 | 0.043 | |
| Prior treatments | Surgery | 64 | 0.749 | 54 | 0.767 |
| Radiation | 64 | 0.749 | 55 | 0.767 | |
| Immunosuppression | Yes | 50 | 0.011 | 41 | 0.004 |
| No | 71 | 0.011 | 58 | 0.004 | |
| Orbital invasion | Yes | 48 | 0.007 | 40 | 0.009 |
| No | 74 | 0.007 | 60 | 0.009 | |
| Route of skull base Invasion | Perineural zone II | 73 | 0.023 | 68 | 0.038 |
| Perineural zone III | 47 | 0.034 | 39 | 0.041 | |
| Direct invasion | 48 | 0.012 | 44 | 0.023 | |
| Cranial nerve | V involved | 70 | 0.009 | 63 | 0.016 |
| VII involved | 40 | 0.009 | 32 | 0.016 | |
| Treatment groups | Surgery alone | 70 | 0.025 | 59 | 0.002 |
| Chemoradiation | 26 | 0.038 | 17 | 0.018 | |
| Surgery and radiation | 69 | 0.021 | 60 | 0.008 | |
Multivariate analysis demonstrated that age at skull base invasion diagnosis, histology, active immunosuppression, orbital involvement, CN involved, type of skull base invasion, and treatment group were also all independent factors associated with DSS and OS (p < 0.05).
Comparing Kaplan-Meier curves for DSS and OS for histology, type of skull base invasion, and treatment group did not demonstrate different survival curve trends; therefore, only OS Kaplan-Meier curve are presented (Figs. 3 4 5 6). Patients with BBC histology had significantly improved DSS (75 months) and OS (65 months) compared with squamous cell carcinoma (49 and 42 months, respectively) and other subtypes (54 and 45 months, respectively) (p < 0.05) (Table 5, Fig. 3). Patients with zone II skull base PNS had significantly improved mean DSS (73 months) and OS (68 months) compared with zone III PNS (47 and 39, respectively) and direct invasion (48 and 44 months, respectively) (p < 0.05) (Table 5, Fig. 4).
Fig. 3.
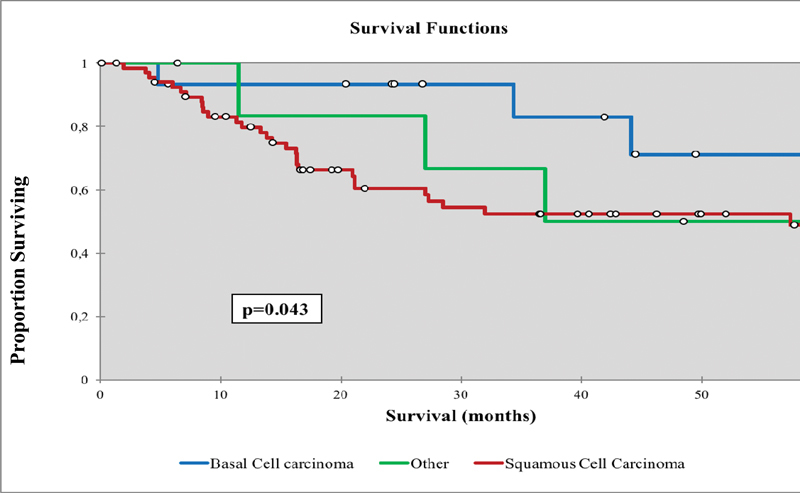
Overall survival for nonmelanoma skin cancers with skull base invasion comparing the different histologies.
Fig. 4.
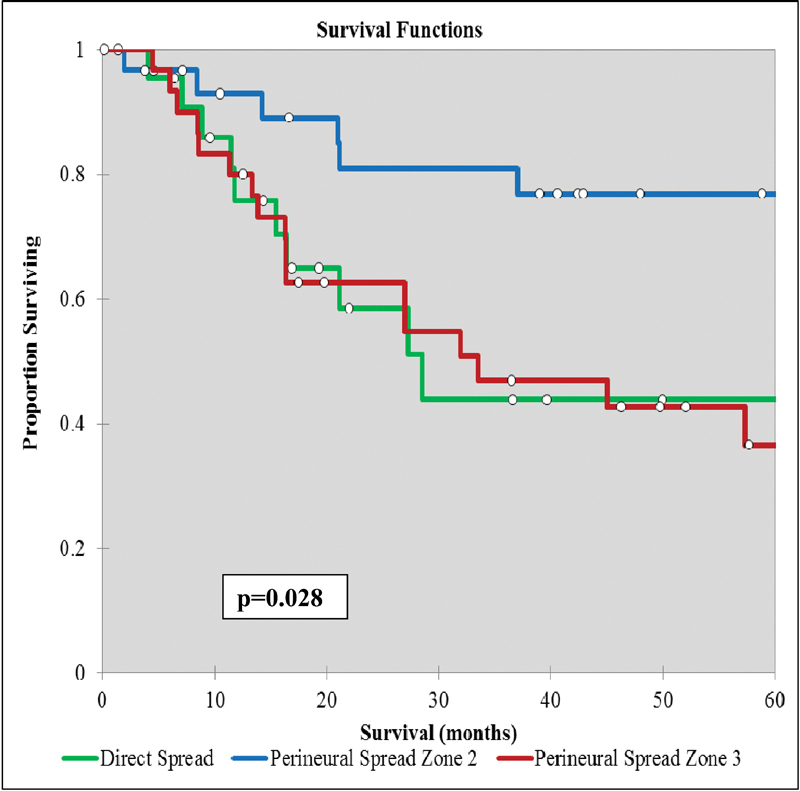
Overall survival for nonmelanoma skin cancers with skull base invasion comparing the route and extent of invasion.
Fig. 5.
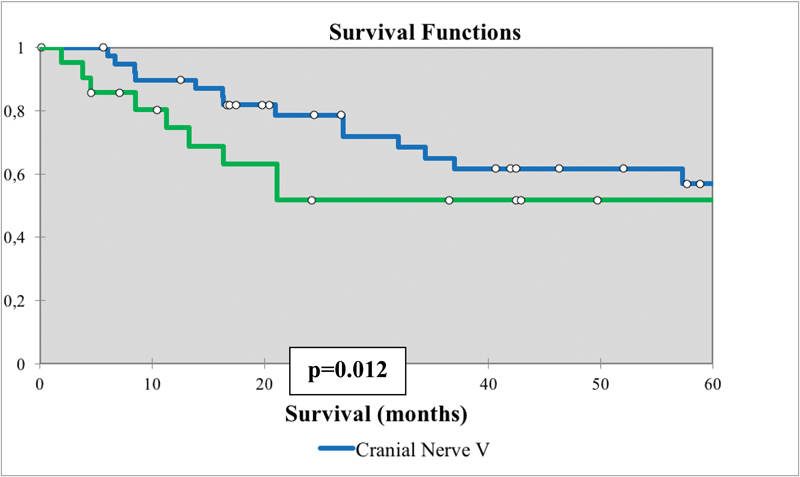
Overall survival for nonmelanoma skin cancers with skull base invasion comparing the route of invasion based on cranial nerve involved (V vs. VII).
Fig. 6.
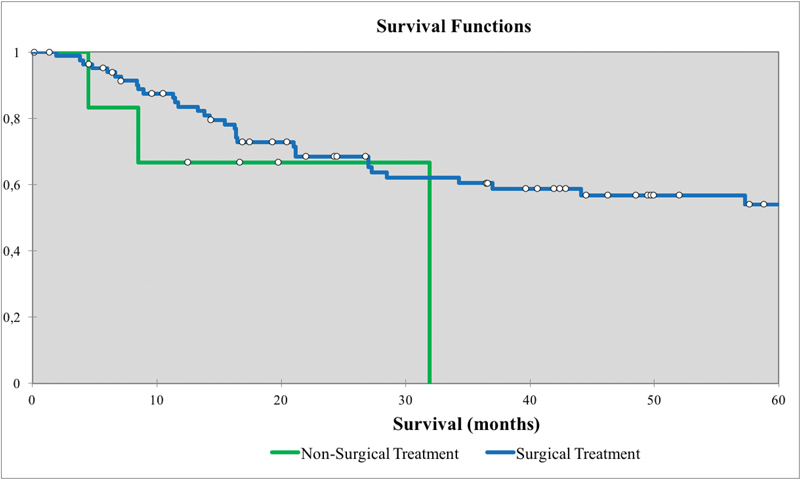
Overall survival for nonmelanoma skin cancers with skull base invasion between treatment groups (surgical vs. nonsurgical treatment).
Patterns of recurrence and treatment failure for NMSC with skull base invasion were stratified by the route and extent of invasion and are summarized in Table 6. The location of failure was classified as central, with an intracranial or leptomeningeal recurrence, locoregional, with an extracranial recurrence, or metastatic. There was a significant association between the route and extent of skull base invasion and the location of failure (p = 0.004). Direct invasion failed with metastatic disease (61%) and locoregional recurrences (33%), whereas zone II skull base PNS failed with locoregional recurrences (60%) and metastatic disease (33%). Zone III skull base PNS largely failed with central recurrences (47%) and metastatic disease (41%), and rarely failed with locoregional recurrences (12%).
Table 6. Patterns of recurrence and treatment failure for nonmelanoma skin cancers with skull base invasion stratified by the route and extent of invasion.
| Route and extent of skull base invasion | Patterns of recurrence and treatment failure | p Value | ||
|---|---|---|---|---|
| Locoregional | Central | Metastatic | ||
| Direct invasion | 6 (33%) | 1 (6%) | 11 (61%) | 0.004 |
| Zone II PNS | 9 (60%) | 1 (7%) | 5 (33%) | 0.004 |
| Zone III PNS | 2 (12%) | 8 (47%) | 7 (41%) | 0.004 |
Abbreviation: PNS, perineural spread.
Skull base PNS on CN V had significantly improved DSS (70 months) and OS (63 months) compared with patients with PNS on CN VII (40 and 32 months, respectively) (Table 5, Fig. 5). Surgical-based therapy (n = 83) had significantly improved mean DSS (70 months) and OS (59 months) compared with nonsurgical based therapy (n = 7) (26 and 17 months, respectively) (Table 5, Fig. 6).
Discussion
Skull base invasion patterns of NMSC are poorly understood and have not been extensively studied because of the rare overall incidence of this disease. There are limited data that analyzes skull base invasion routes from the skin and the clinical significance. Additionally, the current published literature to date does not comprehensively evaluate skull base invasion pathways for NMSC and the survival outcomes. Skull base invasion occurs with advanced-stage disease that is characterized with a high rate of locoregional recurrence and poor survival outcomes. The American Joint Committee on Cancer (AJCC; 2010 7th edition) classifies all cases of skull base invasion as a T4 disease.4 Treatments for skull base invasion of NMSCs usually consists of comprehensive surgical resection followed by adjuvant radiation; however, reports have been published regarding the success of definitive chemoradiotherapy.7 10
Skull base invasion of nonmelanoma head and neck skin cancers predominantly occurs by two routes: direct invasion and perineural tumor spread. Direct invasion of the skull base is the most common invasion patterns of BCCs. Perineural tumor spread with extension to the skull base predominantly occurs in squamous cell carcinomas. However, both histologies can result in either form of invasion. Peripheral nerve branches serve as a conduit for perineural tumor spread to the CNs and then into cranial structures through foramina in the skull base. There is no further anatomical barrier for tumor invasion to extend intracranially once the perineurium is involved.
Direct invasion is often seen with scalp head and neck NMSC. The scalp is formed of five principal layers: skin, subcutaneous tissue, aponeurosis, loose areolar tissue, and periosteum. The scalp forms a substantial barrier to tumor invasion, but sometimes all five layers are invaded by tumor cells. If tumor penetrates through the scalp, it can then invade the dura and other intracranial structures. Superficial-staged lesions based on the California staging system can be resected with full-thickness wide local scalp excision whereas intermediate or deep lesions may need a partial- or full-thickness craniotomy. The depth of invasion into the scalp and bony structures plays a vital role in intracranial extension. We observed that temple lesions may invade into the cranium even with small-sized primary tumors. The relatively high vascular architecture of the temple may contribute to this invasion pattern. The other face subsite with poor prognosis and a high rate of recurrence for skin cancers with skull base invasion is the external ear canal as the skin is very thin in this area and there are many potential pathways for tumor invasion with fissure lines and cranial/sensory nerves.
PNS of skin cancer is an uncommon entity with the incidence approximately 2% in BCCs and 3% in squamous cell carcinomas.11 The primary clinical presenting symptoms are usually paresthesia or anesthesia on the face, which correlates with the involved nerve branch. Unfortunately, early diagnosis is usually challenging because of a silent period at early stages. The maxillary division of trigeminal nerve (V2) is the most commonly involved nerve branch owing to its large anatomical size.12 Weakness of mastication muscles or facial asymmetry may be the first sign of PNS in cases of motor nerve involvement. Approximately 25% of patients with PNS have multiple CNs involved when it is diagnosed. Many patients are diagnosed with Bell palsy or trigeminal neuralgia due to the lack of an obvious index lesion, but then later are found to have PNS.13 This misdiagnosis has an important influence on the patient survival. The atypical features of facial paralysis including gradual-onset, partial paralysis, or a history of hemifacial spasm before the paralysis should raise the index of suspicion for PNS. Many patients have greater than a 6-month history of facial numbness or weakness prior to the diagnosis of PNS.12 13
Peri-neural invasion (PNI) can be micro- or macroscopic. Small nerves are invaded by tumor and are only detectable on microscopy, which is also called incidental PNI.14 When clinical or imaging findings suggest nerve invasion, this situation is termed clinical PNI or PNS. PNS is associated with a higher rate of locoregional recurrence and reduced OS when compared with incidental PNI5 15 however, the detection of either form declares more aggressive tumor behavior with a poor prognosis and increased risk of loco-regional recurrence. The clinical importance of nerve diameter in PNI is still unclear, but many authors advocate that a diameter ≥0.1mm is associated with a higher rate of locoregional recurrence.16 17 PNS is more frequent seen in male patients and is associated with large, midface tumors in the H-zone.18 19 Other risk factors for PNS include recurrent tumors, poor differentiation, and immunosuppression. PNS is usually observed as a contiguous retrograde (toward to the brain) spread, but anterograde (toward to the skin) spread is also described. This type of tumor spread pattern is helpful to differentiate from metastasis via hematogenous or lymphatic route.1 20
Orbital structures also play an important role as an invasion pathway to the skull base for NMSCs. The orbital apex has a critical anatomical architecture that has connections to many intracranial spaces via multiple foramina. Multiple CN involvement and a severe headache can be observed in case of bulky tumor invasion to the orbital apex or cavernous sinus that can cause orbital apex syndrome or cavernous sinus syndrome. Also, PNS is possible between CNV and CNVII via many anatomical connections both peripherally and centrally. The greater superficial petrosal nerve (a branch of CN VII) functions as a potential pathway for this type of tumor spread as it courses just inferior to Meckel cave and then joins with the deep petrosal nerve and forms the Vidian nerve. The Vidian nerve then travels into the pterygopalatine fossa in association with V2, so tumor that has invaded the pterygopalatine fossa has a potential route to travel toward either Meckel cave (CN V) or the Vidian canal (CN VII). The auriculotemporal nerve also serves as a potential pathway for PNS between CNV and CNVII. It is the first branch of mandibular branch of the trigeminal nerve (V3) and has multiple connections to the facial nerve in the parotid gland.
Prognosis due to PNS has been described.21 Williams et al described the zonal classification system for PNS that divides nerve invasion on the basis of anatomical boundaries. Zone I PNS is clinical disease extending up to a CN foramina. Generally, zone I disease is resectable without significant morbidity. Zone II PNS indicates advanced-stage disease in which a nerve is involved at the skull base at the foramina. Zone II PNS is also usually resectable, but more extensive surgeries are needed for complete tumor resection, such as a lateral temporal bone resection or an infratemporal fossa approach to the skull base with a foramina drill out. Zone III PNS is very advanced disease with intracranial involvement extending beyond the respective ganglia, which can be treated with surgical resection in conjunction with neurosurgery and adjuvant radiation, or definitive chemoradiation. Some authors argue against surgical resection and raise concerns that there is the potential for tumor spillage and microscopic spread of disease, which distorts the radiation fields when the natural anatomical barriers are disarticulated and the ganglion is resected. Warren et al documented improved survival outcomes for zone I disease in comparison with zones II and III disease.22 23
MRI is the most sensitive imaging test to determine PNS and skull base invasion.24 A CT scan is often complementary at the skull base and evaluates bony structures and neural foraminas. There are many useful imaging findings that indicate PNS: asymmetric enlargement of the nerve, obliteration of perineural fat planes, widening of nerve foramina, and denervation change in the relevant muscles.8 These criteria are helpful for clinicians to raise suspicion for PNS. Detailed evaluation with an experienced head and neck radiologist is useful for advanced-stage head and neck NMSC that have invaded the skull base.
The initial treatment for NMSC with skull base invasion is critical due to poor disease prognosis and limited salvage treatment options. The primary approach is surgical resection to negative margins, if possible, and adjuvant radiation, which has been shown to be the best chance for cure. Surgical resection is challenging, particularly in patients with zone III disease and extensive direct invasion, because of the extensiveness of the tumors and the complex anatomical structure of the skull base; however, we have shown these patients have reasonable long-term survival outcomes. Recurrences are almost always associated with poorer prognosis and worse survival outcomes.
The rate of nodal involvement in patients with PNS is approximately 9 to 16%,25 26 and the role of elective neck dissection in patients with PNS is controversial. Some centers prefer a routine elective neck dissection in patients with PNS due to the risk of occult cervical metastasis, but others prefer close follow-up with a therapeutic neck dissection for a recurrence. Further research is needed to evaluate this topic and the role of elective neck dissection in this scenario.
There are limited data for survival outcomes of head and neck NMSCs that invade the skull base. In addition, the reported data are heterogeneous and include a mix of skull base invasion cases for skin cancers, sinonasal malignancies, melanomas, parotid tumors, and other primary sites from the head and neck. Also, there are no standardized inclusion/exclusion criteria for these studies. This situation makes it hard to compare survival outcomes. Raza et al recently reported a retrospective series of 24 patients treated at MD Anderson with skull base invasion of NMSCs who were treated with a craniofacial resection.27 The median OS was 43.2 months with 37% of patients surviving 5 years. Our results are similar, with our median OS of 55.5 months and 52.6% of patients surviving 5 years; however, our cohort included all patients with skull base invasion and included all treatment types.
This study is limited because the data were retrospectively obtained. For future analysis, we have started prospectively enrolling patients and collecting data, with the goal of further understanding advanced-stage NMSC tumors that have invaded the skull base.
Conclusion
Nonmelanoma head and neck skin cancers invade the skull base, and this significantly impacts morbidity and mortality. The routes of skull base invasion are described, and zone II and CN V perineural tumor spread are associated with improved survival outcomes, as are BCCs. Nonsurgical therapy was rarely used and is associated with a reduction in DSS and OS.
This article has been updated as per the erratum published on December 26, 2016; doi: 10.1055/s-0036-1597845. Figs. 3, 4, 5, and 6 have been corrected.
References
- 1.Mendenhall W M, Amdur R J, Hinerman R W. et al. Skin cancer of the head and neck with perineural invasion. Am J Clin Oncol. 2007;30(1):93–96. doi: 10.1097/01.coc.0000251224.16075.60. [DOI] [PubMed] [Google Scholar]
- 2.Sewell D A, Lai S Y, Weber R S. Philadelphia, PA: WB Saunders/Elsevier; 2003. Non-melanoma skin cancer of the head and neck; pp. 117–121. [Google Scholar]
- 3.Buzzell R A. Carcinogenesis of cutaneous malignancies. Dermatol Surg. 1996;22(3):209–215. doi: 10.1111/j.1524-4725.1996.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 4.New York, NY: Springer; 2010. AJCC Cutaneous squamous cell carcinoma and other cutaneous carcinomas; pp. 301–314. [Google Scholar]
- 5.Balamucki C J, Mancuso A A, Amdur R J. et al. Skin carcinoma of the head and neck with perineural invasion. Am J Otolaryngol. 2012;33(4):447–454. doi: 10.1016/j.amjoto.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Long S D, Kuhn M J, Wynstra J H. Intracranial extension of basal cell carcinoma of the scalp. Comput Med Imaging Graph. 1993;17(6):469–471. doi: 10.1016/0895-6111(93)90065-u. [DOI] [PubMed] [Google Scholar]
- 7.Panizza B, Solares C A, Redmond M, Parmar P, O'Rourke P. Surgical resection for clinical perineural invasion from cutaneous squamous cell carcinoma of the head and neck. Head Neck. 2012;34(11):1622–1627. doi: 10.1002/hed.21986. [DOI] [PubMed] [Google Scholar]
- 8.Williams L S, Mancuso A A, Mendenhall W M. Perineural spread of cutaneous squamous and basal cell carcinoma: CT and MR detection and its impact on patient management and prognosis. Int J Radiat Oncol Biol Phys. 2001;49(4):1061–1069. doi: 10.1016/s0360-3016(00)01407-3. [DOI] [PubMed] [Google Scholar]
- 9.Gaddikeri S, Bhrany A, Anzai Y. Perineural invasion of skin cancers in the head and neck: an uncommon phenomenon revisited. Otolaryngology. 2014;4:169. doi: 10.4172/2161-119X.1000169. [DOI] [Google Scholar]
- 10.Donald P J, Boggan J, Farwell D G, Enepekides D J. Skull base surgery for the management of deeply invasive scalp cancer. Skull Base. 2011;21(6):343–350. doi: 10.1055/s-0031-1284216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hassanein A M Proper S A Depcik-Smith N D Flowers F P Peritumoral fibrosis in basal cell and squamous cell carcinoma mimicking perineural invasion: potential pitfall in Mohs micrographic surgery Dermatol Surg 200531(9 Pt 1):1101–1106. [DOI] [PubMed] [Google Scholar]
- 12.Boerman R H, Maassen E M, Joosten J. et al. Trigeminal neuropathy secondary to perineural invasion of head and neck carcinomas. Neurology. 1999;53(1):213–216. doi: 10.1212/wnl.53.1.213. [DOI] [PubMed] [Google Scholar]
- 13.Warner G C, Gandhi M, Panizza B. Slowly progressive cranial nerve palsies. Med J Aust. 2006;184(12):641–643. doi: 10.5694/j.1326-5377.2006.tb00423.x. [DOI] [PubMed] [Google Scholar]
- 14.McCord M W, Mendenhall W M, Parsons J T, Flowers F P. Skin cancer of the head and neck with incidental microscopic perineural invasion. Int J Radiat Oncol Biol Phys. 1999;43(3):591–595. doi: 10.1016/s0360-3016(98)00474-x. [DOI] [PubMed] [Google Scholar]
- 15.Jackson J E, Dickie G J, Wiltshire K L. et al. Radiotherapy for perineural invasion in cutaneous head and neck carcinomas: toward a risk-adapted treatment approach. Head Neck. 2009;31(5):604–610. doi: 10.1002/hed.20991. [DOI] [PubMed] [Google Scholar]
- 16.Ross A S, Whalen F M, Elenitsas R, Xu X, Troxel A B, Schmults C D. Diameter of involved nerves predicts outcomes in cutaneous squamous cell carcinoma with perineural invasion: an investigator-blinded retrospective cohort study. Dermatol Surg. 2009;35(12):1859–1866. doi: 10.1111/j.1524-4725.2009.01354.x. [DOI] [PubMed] [Google Scholar]
- 17.Veness M J. Defining patients with high-risk cutaneous squamous cell carcinoma. Australas J Dermatol. 2006;47(1):28–33. doi: 10.1111/j.1440-0960.2006.00218.x. [DOI] [PubMed] [Google Scholar]
- 18.Lawrence N, Cottel W I. Squamous cell carcinoma of skin with perineural invasion. J Am Acad Dermatol. 1994;31(1):30–33. doi: 10.1016/s0190-9622(94)70131-8. [DOI] [PubMed] [Google Scholar]
- 19.Moore B A, Weber R S, Prieto V. et al. Lymph node metastases from cutaneous squamous cell carcinoma of the head and neck. Laryngoscope. 2005;115(9):1561–1567. doi: 10.1097/01.mlg.0000173202.56739.9f. [DOI] [PubMed] [Google Scholar]
- 20.Solares C A, Lee K, Parmar P, O'Rourke P, Panizza B. Epidemiology of clinical perineural invasion in cutaneous squamous cell carcinoma of the head and neck. Otolaryngol Head Neck Surg. 2012;146(5):746–751. doi: 10.1177/0194599811434897. [DOI] [PubMed] [Google Scholar]
- 21.Galloway T J, Morris C G, Mancuso A A, Amdur R J, Mendenhall W M. Impact of radiographic findings on prognosis for skin carcinoma with clinical perineural invasion. Cancer. 2005;103(6):1254–1257. doi: 10.1002/cncr.20913. [DOI] [PubMed] [Google Scholar]
- 22.Warren T A, Panizza B, Porceddu S V. et al. Outcomes after surgery and postoperative radiotherapy for perineural spread of head and neck cutaneous squamous cell carcinoma. Head Neck. 2016;38(6):824–831. doi: 10.1002/hed.23982. [DOI] [PubMed] [Google Scholar]
- 23.Warren T A, Nagle C M, Bowman J, Panizza B J. The natural history and treatment outcomes of perineural spread of malignancy of the head and neck. J Neurol Surg B Skull Base. 2016;77(2):107–112. doi: 10.1055/s-0036-1579777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balamucki C J, DeJesus R, Galloway T J. et al. Impact of radiographic findings on for prognosis skin cancer with perineural invasion. Am J Clin Oncol. 2015;38(3):248–251. doi: 10.1097/COC.0b013e3182940ddf. [DOI] [PubMed] [Google Scholar]
- 25.Lin C, Tripcony L, Keller J, Poulsen M, Dickie G. Cutaneous carcinoma of the head and neck with clinical features of perineural infiltration treated with radiotherapy. Clin Oncol (R Coll Radiol) 2013;25(6):362–367. doi: 10.1016/j.clon.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Mendenhall W M, Ferlito A, Takes R P. et al. Cutaneous head and neck basal and squamous cell carcinomas with perineural invasion. Oral Oncol. 2012;48(10):918–922. doi: 10.1016/j.oraloncology.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 27.Raza S M, Ramakrishna R, Weber R S. et al. Nonmelanoma cutaneous cancers involving the skull base: outcomes of aggressive multimodal management. J Neurosurg. 2015;123(3):781–788. doi: 10.3171/2014.10.jns141037. [DOI] [PubMed] [Google Scholar]


