Abstract
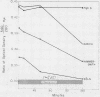
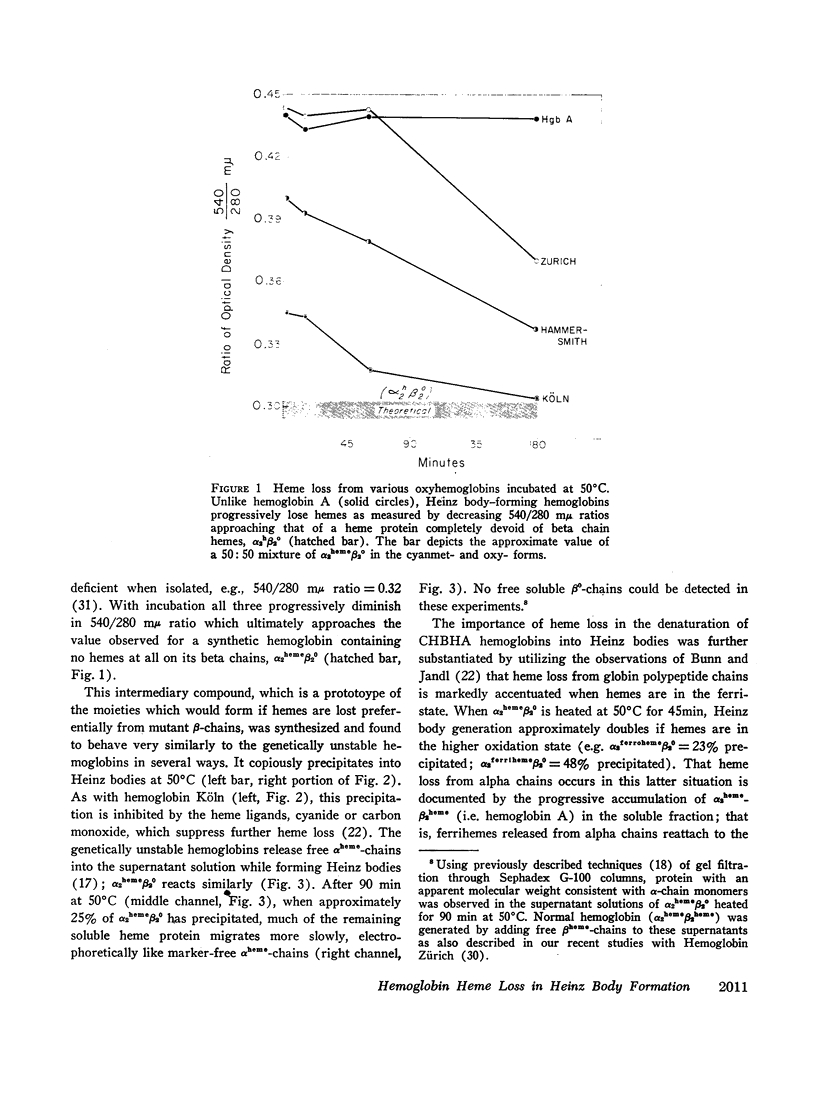
A number of mutant hemoglobins are inordinately unstable, denaturing in circulating red cells into Heinz bodies, resulting in congenital Heinz body hemolytic anemia (CHBHA). We have emphasized that most such hemoglobins involve amino acid substitutions at sites neighboring the heme group of the β-polypeptide chain, and have shown that heme binding to globin is diminished thereby. Thus, hemes were progressively lost from four unstable hemoglobins (Köln, Hammersmith, San Francisco, and Zürich) as they precipitated into Heinz bodies at 50°C.
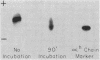
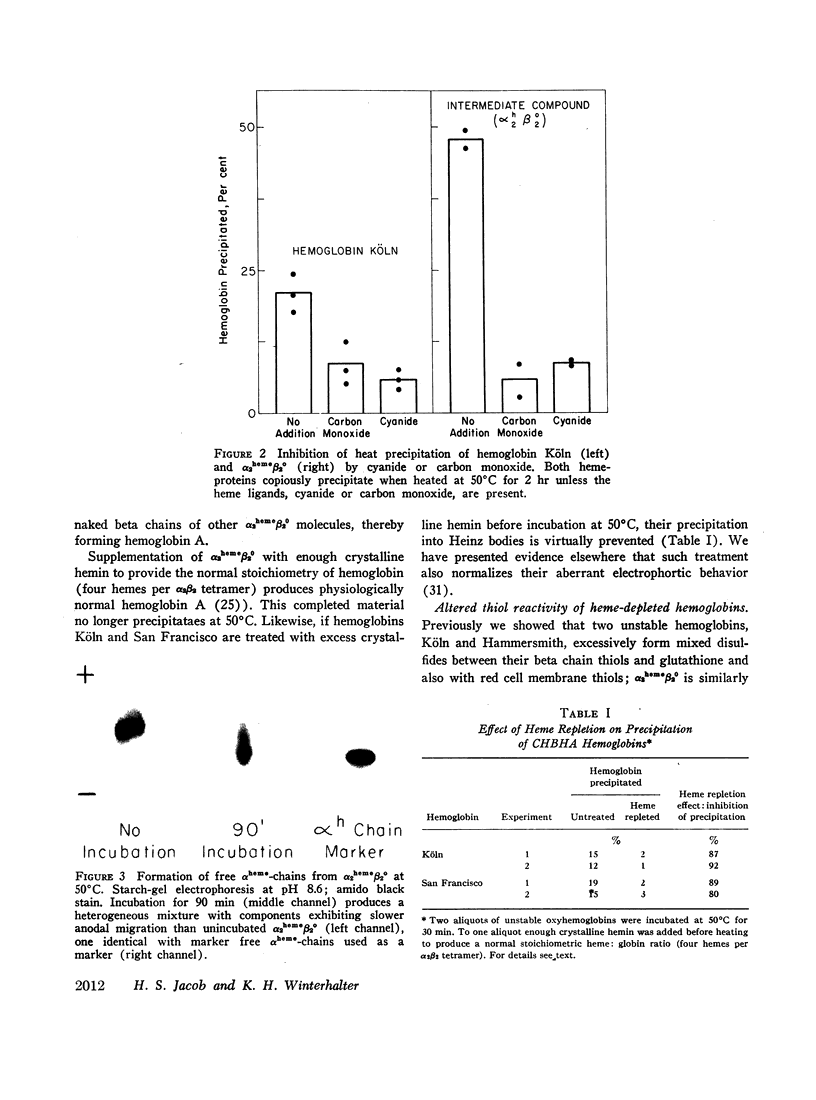
The role of heme loss, especially from beta chains, in Heinz body formation was supported by studies with a hemoglobin synthesized to contain hemes only on its alpha chains (α2hemeβ20). The behavior of this compound, postulated to be an intermediary in the formation of Heinz bodies, mimicked that of the genetically unstable hemoglobins in several ways: (a) it precipitated at 50°C into typical coccoid Heinz bodies; (b) as also observed with CHBHA hemoglobins this denaturation was virtually prevented by the heme ligands, cyanide or carbon monoxide, which inhibit further heme loss; it was potentiated by oxidation of hemes to the ferri- state, which accentuates heme loss; (c) the thiol groups of α2hemeβ20 were hyperreactive, forming mixed disulfides with glutathione and membrane sulfhydryls at rates similar to those of CHBHA hemoglobins and 10 or more times that of normal hemoglobin A; (d) heme repletion of the protein molecules by the addition of crystalline hemin to either α2hemeβ20 or to the genetically unstable hemoglobins, prevented their precipitation into Heinz bodies and normalized their aberrant electrophoretic behaviors; and (e) during Heinz body formation at 50°C both α2hemeβ20 and the genetically unstable hemoglobins released free αheme-chains into solution, suggesting that the bulk of the whitish, Heinz body precipitate is naked β8-chains.
We conclude that heme loss from mutant beta chains is an early step in Heinz body formation in several of the unstable hemoglobinopathies. The resulting hemedepleted compounds, of which synthetic α2hemeβ20 is a prototype, are unstable, cleaving into β0-chain precipitates (the bulk of the Heinz body material) and soluble, free αheme-chains (demonstrated previously in hemolysates from many patients with CHBHA).
Full text
PDF


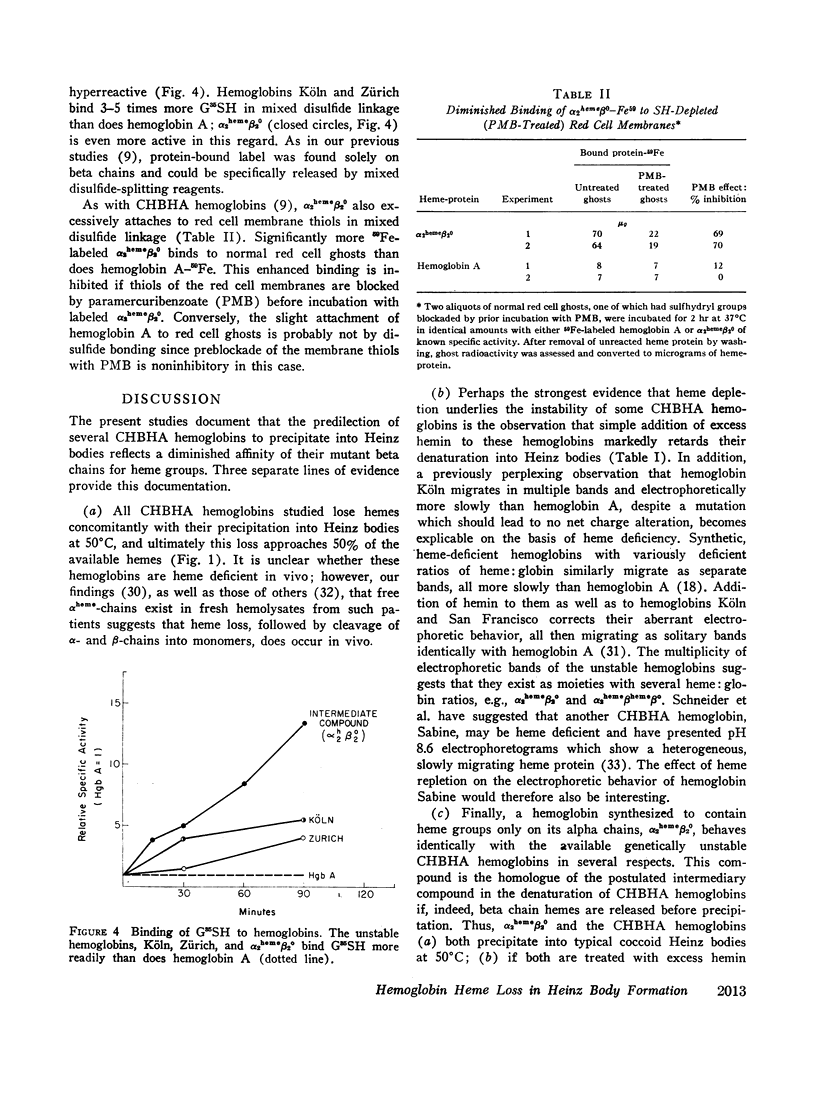



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beretta A., Prato V., Gallo E., Lehmann H. Haemoglobin Torino--alpha-43 (CD1) phenylalanine replaced by valine. Nature. 1968 Mar 16;217(5133):1016–1018. doi: 10.1038/2171016a0. [DOI] [PubMed] [Google Scholar]
- Bradley T. B., Jr, Wohl R. C., Rieder R. F. Hemoglobin Gun Hill: deletion of five amino acid residues and impaired heme-globin binding. Science. 1967 Sep 29;157(3796):1581–1583. doi: 10.1126/science.157.3796.1581. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Jandl J. H. Exchange of heme among hemoglobin molecules. Proc Natl Acad Sci U S A. 1966 Sep;56(3):974–978. doi: 10.1073/pnas.56.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn H. F., Jandl J. H. Exchange of heme among hemoglobins and between hemoglobin and albumin. J Biol Chem. 1968 Feb 10;243(3):465–475. [PubMed] [Google Scholar]
- Carrell R. W., Lehmann H., Hutchison H. E. Haemoglobin Köln (beta-98 valine--methionine): an unstable protein causing inclusion-body anaemia. Nature. 1966 May 28;210(5039):915–916. doi: 10.1038/210915a0. [DOI] [PubMed] [Google Scholar]
- DACIE J. V., GRIMES A. J., MEISLER A., STEINGOLD L., HEMSTED E. H., BEAVEN G. H., WHITE J. C. HEREDITARY HEINZ-BODY ANAEMIA. A REPORT OF STUDIES ON FIVE PATIENTS WITH MILD ANAEMIA. Br J Haematol. 1964 Jul;10:388–402. doi: 10.1111/j.1365-2141.1964.tb00715.x. [DOI] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Dacie J. V., Shinton N. K., Gaffney P. J., Jr, Lehmann H. Haemoglobin Hammersmith (beta-42 (CDI) Phe replaced by ser). Nature. 1967 Nov 18;216(5116):663–665. doi: 10.1038/216663a0. [DOI] [PubMed] [Google Scholar]
- FANELLI A. R., ANTONINI E., CAPUTO A. Studies on the structure of hemoglobin. I. Physicochemical properties of human globin. Biochim Biophys Acta. 1958 Dec;30(3):608–615. doi: 10.1016/0006-3002(58)90108-2. [DOI] [PubMed] [Google Scholar]
- FRICK P. G., HITZIG W. H., BETKE K. Hemoglobin Zurich. I. A new hemoglobin anomaly associated with acute hemolytic episodes with inclusion bodies after sulfonamide therapy. Blood. 1962 Sep;20:261–271. [PubMed] [Google Scholar]
- HUEHNS E. R., SHOOTER E. M. HUMAN HAEMOGLOBINS. J Med Genet. 1965 Mar;2(1):48–90. doi: 10.1136/jmg.2.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrkal Z., Vodrázka Z. A study of the conformation of human globin in solution by optical methods. Biochim Biophys Acta. 1967 Apr 11;133(3):527–534. doi: 10.1016/0005-2795(67)90557-0. [DOI] [PubMed] [Google Scholar]
- JACOB H. S., JANDL J. H. Effects of sulfhydryl inhibition on red blood cells. I. Mechanism of hemolysis. J Clin Invest. 1962 Apr;41:779–792. doi: 10.1172/JCI104536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB H. S., JANDL J. H. Effects of sulfhydryl inhibition on red blood cells. II. Studies in vivo. J Clin Invest. 1962 Jul;41:1514–1523. doi: 10.1172/JCI104607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANDL J. H., ENGLE L. K., ALLEN D. W. Oxidative hemolysis and precipitation of hemoglobin. I. Heinz body anemias as an acceleration of red cell aging. J Clin Invest. 1960 Dec;39:1818–1836. doi: 10.1172/JCI104206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob H. S., Brain M. C., Dacie J. V. Altered sulfhydryl reactivity of hemoglobins and red blood cell membranes in congenital Heinz body hemolytic anemia. J Clin Invest. 1968 Dec;47(12):2664–2677. doi: 10.1172/JCI105950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob H. S., Brain M. C., Dacie J. V., Carrell R. W., Lehmann H. Abnormal haem binding and globin SH group blockade in unstable haemoglobins. Nature. 1968 Jun 29;218(5148):1214–1217. doi: 10.1038/2181214a0. [DOI] [PubMed] [Google Scholar]
- Jacob H., Winterhalter K. Unstable hemoglobins: the role of heme loss in Heinz body formation. Proc Natl Acad Sci U S A. 1970 Mar;65(3):697–701. doi: 10.1073/pnas.65.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleihauer E. F., Reynolds C. A., Dozy A. M., Wilson J. B., Moores R. R., Berenson M. P., Wright C. S., Huisman T. H. Hemoglobin-Bibba or alpha-2-136Pro-beta 2, an unstable alpha chain abnormal hemoglobin. Biochim Biophys Acta. 1968 Jan 22;154(1):220–222. doi: 10.1016/0005-2795(68)90274-2. [DOI] [PubMed] [Google Scholar]
- LABBE R. F., NISHIDA G. A new method of hemin isolation. Biochim Biophys Acta. 1957 Nov;26(2):437–437. doi: 10.1016/0006-3002(57)90033-1. [DOI] [PubMed] [Google Scholar]
- LANGE R. D., AKEROYD J. H. Congenital hemolytic anemia with abnormal pigment metabolism and red cell inclusion bodies: a new clinical syndrome. Blood. 1958 Oct;13(10):950–958. [PubMed] [Google Scholar]
- Miwa S., Kato H., Saito M., Chiba A., Irisawa K., Ohyama H. Congenital hemolytic anemia with abnormal pigment metabolism and red cell inclusion bodies. Report of a case and review of literature. Nihon Ketsueki Gakkai Zasshi. 1965 Oct;28(5):593–608. [PubMed] [Google Scholar]
- Perutz M. F., Lehmann H. Molecular pathology of human haemoglobin. Nature. 1968 Aug 31;219(5157):902–909. doi: 10.1038/219902a0. [DOI] [PubMed] [Google Scholar]
- Rachmilewitz E. A., Peisach J., Bradley T. B., Blumberg W. E. Role of haemichromes in the formation of inclusion bodies in haemoglobin H disease. Nature. 1969 Apr 19;222(5190):248–250. doi: 10.1038/222248a0. [DOI] [PubMed] [Google Scholar]
- Ranney H. M., Jacobs A. S., Udem L., Zalusky R. Hemoglobin Riverdale-Bronx an unstable hemoglobin resulting from the substitution of arginine for glycine at helical residue B6 of the B beta polypeptide chain. Biochem Biophys Res Commun. 1968 Dec 30;33(6):1004–1005. doi: 10.1016/0006-291x(68)90413-0. [DOI] [PubMed] [Google Scholar]
- Rieder R. F., Oski F. A., Clegg J. B. Hemoglobin Philly (beta 35 tyrosine phenylalanine): studies in the molecular pathology of hemoglobin. J Clin Invest. 1969 Sep;48(9):1627–1642. doi: 10.1172/JCI106128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkind R. A. Heinz body anemia: an ultrastructural study. II. Red cell sequestration and destruction. Blood. 1965 Oct;26(4):433–448. [PubMed] [Google Scholar]
- Schneider R. G., Ueda S., Alperin J. B., Brimhall B., Jones R. T. Hemoglobin sabine beta 91 (f 7) leu to pro. An unstable variant causing severe anemia with inclusion bodies. N Engl J Med. 1969 Apr 3;280(14):739–745. doi: 10.1056/NEJM196904032801402. [DOI] [PubMed] [Google Scholar]
- WINTERHALTER K. H., HUEHNS E. R. PREPARATIONS, PROPERTIES, AND SPECIFIC RECOMBINATION OF ALPHA-BETA-GLOBIN SUBUNITS. J Biol Chem. 1964 Nov;239:3699–3705. [PubMed] [Google Scholar]
- Winterhalter K. H., Amiconi G., Antonini E. Functional properties of a hemoglobin carrying heme only on alpha chains. Biochemistry. 1968 Jun;7(6):2228–2232. doi: 10.1021/bi00846a027. [DOI] [PubMed] [Google Scholar]
- Winterhalter K. H., Anderson N. M., Amiconi G., Antonini E., Brunori M. Functional properties of hemoglobin Zürich. Eur J Biochem. 1969 Dec;11(3):435–440. doi: 10.1111/j.1432-1033.1969.tb00793.x. [DOI] [PubMed] [Google Scholar]
- Winterhalter K. H., Deranleau D. A. The structure of a hemoglobin carrying only two hemes. Biochemistry. 1967 Oct;6(10):3136–3143. doi: 10.1021/bi00862a022. [DOI] [PubMed] [Google Scholar]
- Winterhalter K. H. Sequence of linkage between the prosthetic groups and the polypeptide chains of haemoglobin. Nature. 1966 Aug 27;211(5052):932–934. doi: 10.1038/211932a0. [DOI] [PubMed] [Google Scholar]