Abstract
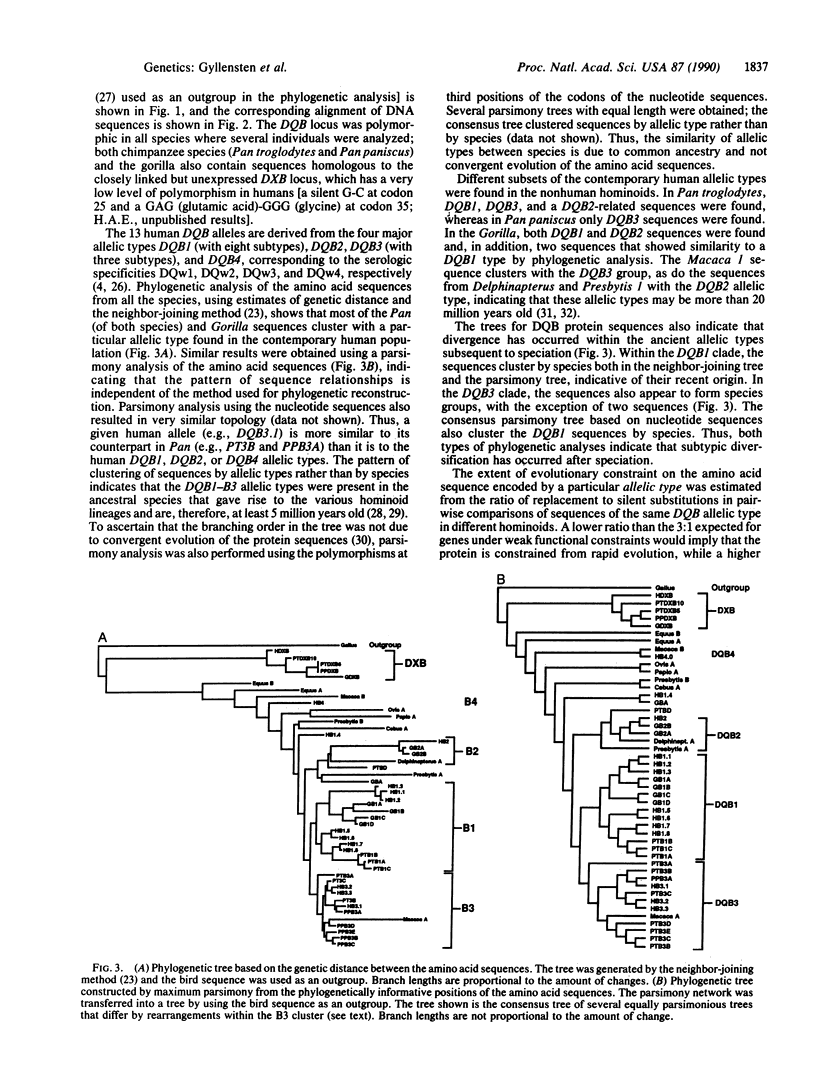
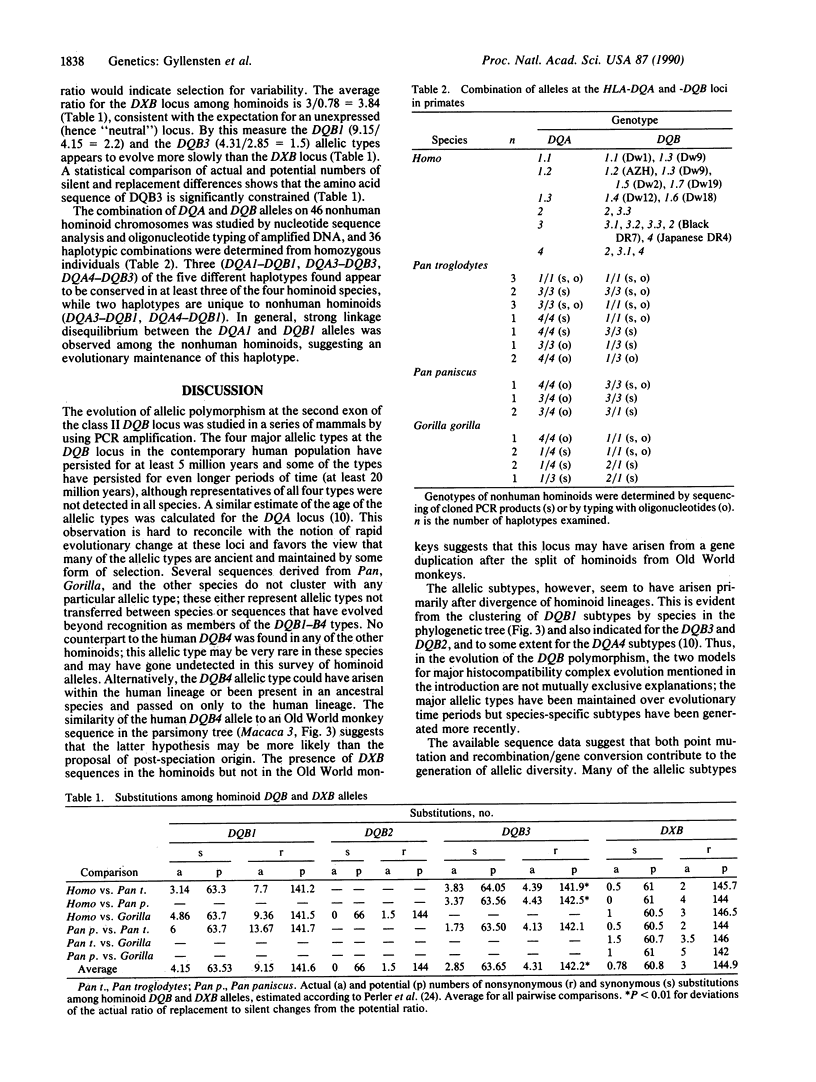
The allelic diversity at HLA class II loci either arose after the divergence of hominoid lineages or, alternatively, the polymorphism was present before speciation and has been maintained by selection. Here, we report the use of oligonucleotide primers to amplify, by the polymerase chain reaction, and sequence the polymorphic second exon of the DQB locus from 11 species, spanning more than 40 million years of mammalian evolution. Phylogenetic analysis reveals that of the four human DQB allelic types (DQB1-B4), three (DQB1-3) were found in chimpanzee and gorilla and two (DQB3 and -4) were identified in the rhesus monkey, suggesting that some of these types are 5-20 million years old. The ratio of replacement to silent substitutions was calculated between members of the same allelic type from different species. These results suggest that the evolution of the DQB3 allelic type is more constrained than that of the DQB1 allelic type; both evolve more slowly than the DXB locus, a linked but presumably nonexpressed locus. Further, the clustering of allelic subtypes by species in the phylogenetic tree indicates that allelic diversification has occurred subsequent to the divergence of hominoids. Finally, some haplotype combinations of DQA and DQB alleles are common to several hominoid species and may have been maintained for at least 5 million years.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arden B., Klein J. Biochemical comparison of major histocompatibility complex molecules from different subspecies of Mus musculus: evidence for trans-specific evolution of alleles. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2342–2346. doi: 10.1073/pnas.79.7.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourlet Y., Béhar G., Guillemot F., Fréchin N., Billault A., Chaussé A. M., Zoorob R., Auffray C. Isolation of chicken major histocompatibility complex class II (B-L) beta chain sequences: comparison with mammalian beta chains and expression in lymphoid organs. EMBO J. 1988 Apr;7(4):1031–1039. doi: 10.1002/j.1460-2075.1988.tb02910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. H., Jardetzky T., Saper M. A., Samraoui B., Bjorkman P. J., Wiley D. C. A hypothetical model of the foreign antigen binding site of class II histocompatibility molecules. Nature. 1988 Apr 28;332(6167):845–850. doi: 10.1038/332845a0. [DOI] [PubMed] [Google Scholar]
- Bugawan T. L., Horn G. T., Long C. M., Mickelson E., Hansen J. A., Ferrara G. B., Angelini G., Erlich H. A. Analysis of HLA-DP allelic sequence polymorphism using the in vitro enzymatic DNA amplification of DP-alpha and DP-beta loci. J Immunol. 1988 Dec 1;141(11):4024–4030. [PubMed] [Google Scholar]
- Figueroa F., Günther E., Klein J. MHC polymorphism pre-dating speciation. Nature. 1988 Sep 15;335(6187):265–267. doi: 10.1038/335265a0. [DOI] [PubMed] [Google Scholar]
- Giles R. C., Capra J. D. Structure, function, and genetics of human class II molecules. Adv Immunol. 1985;37:1–71. doi: 10.1016/s0065-2776(08)60337-5. [DOI] [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Ancient roots for polymorphism at the HLA-DQ alpha locus in primates. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9986–9990. doi: 10.1073/pnas.86.24.9986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn G. T., Bugawan T. L., Long C. M., Erlich H. A. Allelic sequence variation of the HLA-DQ loci: relationship to serology and to insulin-dependent diabetes susceptibility. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6012–6016. doi: 10.1073/pnas.85.16.6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. C. Molecular evolution. How old is a polymorphism? Nature. 1988 Apr 14;332(6165):588–590. doi: 10.1038/332588b0. [DOI] [PubMed] [Google Scholar]
- Hughes A. L., Nei M. Nucleotide substitution at major histocompatibility complex class II loci: evidence for overdominant selection. Proc Natl Acad Sci U S A. 1989 Feb;86(3):958–962. doi: 10.1073/pnas.86.3.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson A. K., Hyldig-Nielsen J. J., Servenius B., Larhammar D., Andersson G., Jörgensen F., Peterson P. A., Rask L. Class II genes of the human major histocompatibility complex. Comparisons of the DQ and DX alpha and beta genes. J Biol Chem. 1987 Jun 25;262(18):8767–8777. [PubMed] [Google Scholar]
- Klein J. Origin of major histocompatibility complex polymorphism: the trans-species hypothesis. Hum Immunol. 1987 Jul;19(3):155–162. doi: 10.1016/0198-8859(87)90066-8. [DOI] [PubMed] [Google Scholar]
- Lawlor D. A., Ward F. E., Ennis P. D., Jackson A. P., Parham P. HLA-A and B polymorphisms predate the divergence of humans and chimpanzees. Nature. 1988 Sep 15;335(6187):268–271. doi: 10.1038/335268a0. [DOI] [PubMed] [Google Scholar]
- Li W. H., Tanimura M., Sharp P. M. An evaluation of the molecular clock hypothesis using mammalian DNA sequences. J Mol Evol. 1987;25(4):330–342. doi: 10.1007/BF02603118. [DOI] [PubMed] [Google Scholar]
- McConnell T. J., Talbot W. S., McIndoe R. A., Wakeland E. K. The origin of MHC class II gene polymorphism within the genus Mus. Nature. 1988 Apr 14;332(6165):651–654. doi: 10.1038/332651a0. [DOI] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Nathenson S. G., Geliebter J., Pfaffenbach G. M., Zeff R. A. Murine major histocompatibility complex class-I mutants: molecular analysis and structure-function implications. Annu Rev Immunol. 1986;4:471–502. doi: 10.1146/annurev.iy.04.040186.002351. [DOI] [PubMed] [Google Scholar]
- Perler F., Efstratiadis A., Lomedico P., Gilbert W., Kolodner R., Dodgson J. The evolution of genes: the chicken preproinsulin gene. Cell. 1980 Jun;20(2):555–566. doi: 10.1016/0092-8674(80)90641-8. [DOI] [PubMed] [Google Scholar]
- Pilbeam D. The descent of hominoids and hominids. Sci Am. 1984 Mar;250(3):84–96. doi: 10.1038/scientificamerican0384-84. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sakoyama Y., Hong K. J., Byun S. M., Hisajima H., Ueda S., Yaoita Y., Hayashida H., Miyata T., Honjo T. Nucleotide sequences of immunoglobulin epsilon genes of chimpanzee and orangutan: DNA molecular clock and hominoid evolution. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1080–1084. doi: 10.1073/pnas.84.4.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarich V. M., Wilson A. C. Immunological time scale for hominid evolution. Science. 1967 Dec 1;158(3805):1200–1203. doi: 10.1126/science.158.3805.1200. [DOI] [PubMed] [Google Scholar]
- Serjeantson S. W. The reasons for MHC polymorphism in man. Transplant Proc. 1989 Feb;21(1 Pt 1):598–601. [PubMed] [Google Scholar]
- Stewart C. B., Schilling J. W., Wilson A. C. Adaptive evolution in the stomach lysozymes of foregut fermenters. 1987 Nov 26-Dec 2Nature. 330(6146):401–404. doi: 10.1038/330401a0. [DOI] [PubMed] [Google Scholar]
- Todd J. A., Bell J. I., McDevitt H. O. HLA-DQ beta gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature. 1987 Oct 15;329(6140):599–604. doi: 10.1038/329599a0. [DOI] [PubMed] [Google Scholar]
- Trowsdale J., Young J. A., Kelly A. P., Austin P. J., Carson S., Meunier H., So A., Erlich H. A., Spielman R. S., Bodmer J. Structure, sequence and polymorphism in the HLA-D region. Immunol Rev. 1985 Jul;85:5–43. doi: 10.1111/j.1600-065x.1985.tb01129.x. [DOI] [PubMed] [Google Scholar]



